La metformina es un fármaco ampliamente utilizado en sujetos con diabetes mellitus y su eficacia para descender la glucemia y la hemoglobina A1C (HbA1C) es notable. Sin embargo, en algunos pacientes, sobre todo en los que presentan comorbilidades, puede provocar una acidosis láctica grave que origina una elevada morbimortalidad. El tratamiento de esta complicación se basa en la utilización de medidas de soporte y, en los casos más graves, en procedimientos de depuración extrarrenal, como la hemodiálisis o la hemodiafiltración continua.
Metformin is an antihyperglycemic agent commonly used in diabetic patients. It is very effective and is able to reduce the plasma glucose and HbA1C. However, in some patients, specially those with comorbidities, metformin can provoke severe lactic acidosis with high morbimortality. Treatment of the lactic acidosis induced by metformin is based on the use of supportive general measures; in severe cases, procedures of extrarrenal purification like hemodialysis or continuous hemodiafiltration have been successfully used.
INTRODUCTION
Metformin is the main biguanide, widely used in diabetes mellitus treatment.1 It is a glycaemia-lowering agent that promotes glucose entering the tissues and reduces hepatic gluconeogenesis and glucose synthesis. It can reduce fasting and postprandial glycaemia and haemoglobin A1C (HbA1C), with weight loss and an improved lipid profile.1 Studies such as the UKPDS also show that it reduces morbidity and mortality in patients with type 2 diabetes.2 It is well absorbed through oral administration and it is mainly excreted through the kidney, which is why it can accumulate in cases of renal function deterioration. Its most frequent secondary effects are gastrointestinal, such as diarrhoea, abdominal pain and vomiting. However, a small percentage of patients may suffer a severe complication: metformin-induced lactic acidosis,3-5 especially subjects with comorbidities, mainly liver, heart and severe kidney diseases. Therefore, it is contraindicated in patients with chronic kidney disease.
We describe 5 patients with severe metformin-induced lactic acidosis, analysing the influence that kidney disease could have on its pathogenesis and evolution, as well as the role that extrarenal depuration techniques can have on its treatment.
CASE REPORTS
Case 1
A 78-year-old woman with history of high blood pressure, type 2 diabetes mellitus, generalised osteoarthritis, congestive heart failure (class II on the New York Heart Association) and chronic atrial fibrillation. She was being treated with metformin (850mg/8hr), glipizide (5mg/day), lercanidipine (10mg/day), lisinopril and hydrochlorothiazide (20/12.5mg/day), acenocoumarol and occasionally ibuprofen. Two days before being admitted she was taking ibuprofen (600mg/8hr) due to bone pain. Her general health started to deteriorate, with vomiting and decreased level of consciousness.
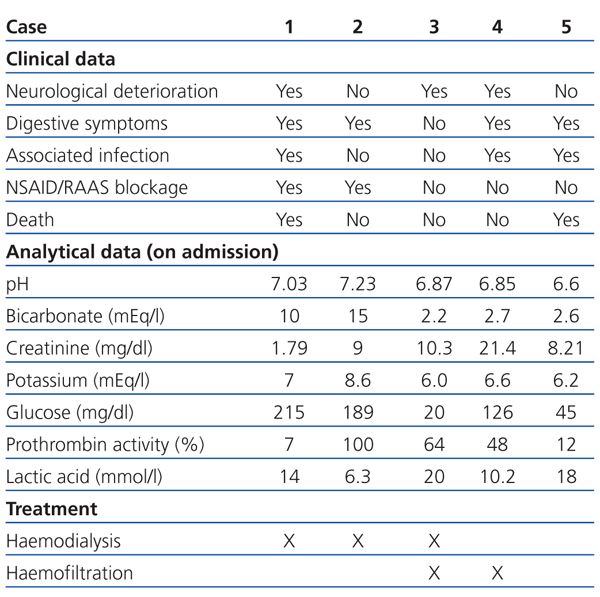
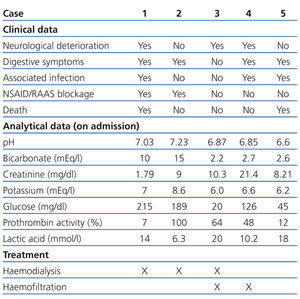
In the emergency department she was hypotensive, poorly perfused, and comatose (Glasgow coma score 6/15). Analytical tests showed plasma creatinine: 1.79mg/dl; glycaemia: 215mg/dl; and prothrombin activity: 7%. The arterial blood gas test showed pH: 7.03; bicarbonate: 10mEq/l; and plasma lactic acid: 14mmol/l (Table 1). The chest X-ray revealed infiltration in the left base.
She was transferred to the intensive care unit (ICU) and underwent orotracheal intubation with mechanical ventilation. Vasoactive drugs and antibiotics were administered. A lumbar puncture was performed, and the result was compatible with pneumococcal meningitis. Haemodialysis was performed with bicarbonate solution. Her analytical tests then improved: plasma creatinine (Cr): 1.2mg/dl; and serum bicarbonate: 22mEq/l. However, the patient remained in a coma, with low-voltage EEG. She died 72 hours after being admitted.
Case 2
A 76-year-old woman with history of type 2 diabetes mellitus, dyslipidaemia, and depressive disorder. She was being treated with metformin (850mg/8hr), escitalopram (20mg/24hr), mirtazapine (30mg/24hr) and simvastatin (40mg/24hr). In an analytical test taken 6 months before, she had creatinine of 0.81mg/dl.
Two weeks before being admitted she started treatment with ibuprofen (600mg/8hr) for lower back pain. A week before, she had nausea, with vomiting and diarrhoea, which is why she went to the emergency department. In the examination she had dry skin and mucosa and the analytical tests showed: plasma Cr: 9mg/dl; glucose: 189mg/dl; urea: 196mg/dl; haemoglobin: 10.9g/dl; sodium: 125mEq/l; potassium: 8.6mEq/l; and lactic acid: 6.3mmol/l. The arterial blood gas test showed: pH: 7.23; and bicarbonate: 15mEq/l (Table 1). An abdominal ultrasound was performed showing bilateral ureterohydronephrosis secondary to bladder neoplasm. Given that the patient was haemodynamically unstable, haemodialysis was performed for 2 hours. She then had: Cr: 5.9mg/dl; potassium: 6.1mEq/l; pH: 7.39; and bicarbonate: 22mEq/l. Later, a bilateral nephrostomy was performed with improved clinical symptoms and analytical values normalised: Cr: 1.1mg/dl, pH: 7.31; and bicarbonate: 23mEq/l.
Case 3
A 47-year-old man, with long-standing type 2 diabetes, being treated with metformin (850mg/8hr) and glimepiride (4mg/day), an active smoker and drinker. He had had several episodes of acute non-calculous pancreatitis. One week before he was admitted, he showed signs of syncope while he was working in the field. He then presented with intense asthenia, hypoorexia, general discomfort, and postural hypotension. He arrived at the health centre with blood pressure of 70/35mm Hg and capillary glycaemia of 20mg/dl, and was subsequently administered IV glucagon. Hypotension persisted in the emergency department, with signs of extracellular volume depletion and tachypnoea. Analytical tests showed: plasma Cr: 10.3mg/dl; glycaemia: 287mg/dl; and lactic acid: 20mmol/l. The arterial blood gas test showed: pH: 6.87 and bicarbonate: 2.2mEq/l (Table 1). Orotracheal intubation was performed and he was admitted to the ICU with assisted ventilation and was administered inotropic agents. He later underwent haemodialysis for four hours, followed by continuous venovenous haemofiltration with improved analytical results. Upon discharge he had Cr: 1.20mg/dl; pH: 7.46; and bicarbonate: 24.6mEq/l.
Case 4
A 65-year-old man, with type 2 diabetes and history of revascularisation for ischaemic heart disease with mild systolic dysfunction and generalised atheromatosis. He was being treated with enalapril (40mg/day), atenolol (50mg/day), acetylsalicylic acid (300mg/day), fenofibrate (200mg/day) and a fixed combination of rosiglitazone/metformin (2/500mg/12hr). He was admitted to the emergency department due to acute deterioration of general health, decreased consciousness, and tachypnoea. Five days before admission he had presented with epigastralgia associated with nausea, vomiting and diarrhoea. He continued his usual treatment. The physical examination showed: blood pressure: 100/60mm Hg, oxygen saturation: 84% and auscultation found rales in the right lung base and abdominal vascular murmur.
Analytical tests showed: Cr: 21.4mg/dl; glucose: 126mg/dl; potassium: 6.6mEq/l; creatine phosphokinase (CPK): 315 with normal troponin I; and lactic acid: 10.2mmol/l (Table 1). Furthermore, leukocytes: 16 210/µl; haemoglobin: 13.3g/dl; and platelets 407x1000/µl. The chest X-ray showed condensation in the right base. He was transferred to the ICU, where he underwent orotracheal intubation, mechanical ventilation and treatment with vasoactive drugs. However, he underwent cardiac arrest and needed CPR. Treatment with continuous venovenous haemofiltration was started and maintained for five days with gradual improvement; he was discharged from the ICU with plasma Cr: 3.5mg/dl; potassium: 3.3mEq/l; pH: 7.39; and bicarbonate: 20.6mEq/l.
Case 5
An 87-year-old woman, with history of high blood pressure and long-standing type 2 diabetes mellitus. Furthermore, she presented with chronic atrial fibrillation and severe pulmonary blood pressure (for which she was being anticoagulated with acenocoumarol) and polymyalgia rheumatica. She was taking administered deflazacort (6mg/day), metformin (850mg/8hr), repaglinide (2mg/8hr), candesartan/hydrochlorothiazide (16/12.5mg/day), omeprazole (20mg/day), torasemide (5mg/day) and nifedipine (30mg/day). Three months before her plasma Cr was 1.22mg/dl. She presented with significant diarrhoea, vomiting and disorientation for two days before admission. Capillary glycaemia was determined at 45mg/dl. In the emergency department she had blood pressure: 101/79mm Hg; temperature: 38ºC; and oxygen saturation: 98%. She presented with dry mucosa and slightly decreased consciousness with a Glasgow coma score of 13.
Analytical tests showed: Cr: 8.21mg/dl; glucose: 262mg/dl; potassium: 6.2mEq/l; leukocytes: 19 220/µl; haemoglobin: 9.9g/dl; and lactic acid: 18mmol/l. Prothrombin activity was 12%. The arterial blood gas test showed: pH: 6.6; and bicarbonate: 2.6mEq/l. Given that admission to ICU was rejected due to associated comorbidities, treatment with IV bicarbonate and inotropic agents were indicated, but her health did not improve. The patient died 10 hours after admission.
DISCUSSION
Metformin is a glycaemia-lowering agent that is widely used to treat diabetic patients, as a monotherapy or with associated anti-diabetic agents and even insulin. Clinical practice guidelines especially recommend its use in obese type 2 diabetic patients and have even started to recommend it in subjects with an altered fasting blood glucose.6,7 Following oral administration, peak metformin plasma levels are reached after 2.5 hours. Its protein binding is insignificant, its distribution volume is high and it is mainly excreted by the kidney. These characteristics promote drug elimination using extrarenal depuration techniques.
Metformin’s main secondary effects are gastrointestinal (anorexia, nausea, vomiting, diarrhoea and abdominal pain) and, occasionally, induces skin disorders or hypersensitivity reactions.1 Metformin-induced lactic acidosis is a rare complication, but it is potentially very severe and its incidence is estimated to be 3 cases per 100 000 person-years.8 Its cause is not well known, although it seems to alter the mitochondrial oxidative metabolism on one hand, and increase the intestinal lactic acid production and decrease intestinal glucose absorption.9-11 However, a recent systematic review of the Cochrane library concluded that there is no evidence showing that metformin is clearly associated with an increased risk of lactic acidosis.12
The following predisposing factors to metformin-induced lactic acidosis development should be highlighted: volume depletion, renal function deterioration, severe liver diseases, congestive heart failure or use of drugs that interfere with renal autoregulation, such as renin-angiotensin-aldosterone system inhibitors or non-steroidal anti-inflammatory drugs.3 Among our patients, two cases had volume depletion with acute renal failure, three had taken renin-angiotensin-aldosterone system inhibitors and one received non-steroidal anti-inflammatory drugs.
Mortality was related with a lower blood pH and higher plasma concentrations of lactic acid or metformin,13 although other authors found a relationship with coagulation disorders.5 Indeed, in our study, the two patients that died had significant coagulation disorders and the other patients made favourable progress, having normal coagulation parameters.
Initial treatment is based on haemodynamic and respiratory stability. Furthermore, the metabolic disorder must be corrected, although using bicarbonate sodium is controvertial,14 given that it could cause the haemoglobin disassociation curve to shift to the left, a sodium overload, and occasionally, rebound metabolic alkalosis.14,15
For the most severe cases, extrarenal depuration techniques have been used, i.e. intermittent haemodialysis and continuous haemofiltration.16 Haemodialysis using a bicarbonate solution is the most rapid way to correct acidosis, but continuous techniques should be considered for patients with haemodynamic instability.9 A combination of the two techniques can also be used depending on the patient’s evolution.17
The benefits of haemodialysis seem to be related to the correction of metabolic acidosis and drug elimination. Prolonged sessions are recommended to increase clearance.5,15,18
We performed haemodialysis on two patients, one was treated with continuous haemodiafiltration and another one underwent both techniques. Each technique was chosen in accordance with the haemodynamic stability of each patient; continuous techniques were preferred for patients that presented with haemodynamic instability. Despite treatment, prognosis was poor for 40% of our patients, probably related to the severe associated diseases that they presented.
To conclude, diagnosis should be suspected in those subjects treated with the drug who present with severe lactic acidosis. Special caution should be taken for patients with severe associated diseases such as liver or renal failure, volume depletion or concomitant treatment with renin-angiotensin-aldosterone system inhibitors or non-steroidal anti-inflammatory drugs.
Table 1. Clinical and analytical data, and treatment received by patients