Controversy persists about the role of hepatitis C as a risk factor for developing kidney disease in the general population. Some authors have evaluated the effect of antiviral therapy for HCV on the risk of kidney disease.
Study Aims and DesignA systematic review of the published medical literature was performed to assess whether antiviral therapy for HCV has an independent impact on kidney survival in the adult general population. A random effects model was used to generate an overall estimate of the risk of kidney disease after anti-HCV therapy across the published studies. Meta-regression and stratified analysis were also carried out.
ResultsFifteen studies were eligible (n=356, 285 patients) and separate meta-analyses were conducted according to the outcome. Pooling studies based on viral responses (n=7; 34,763 individual patients) demonstrated a relationship between sustained viral response and lower frequency of kidney disease; the overall estimate for adjusted risk of kidney disease was 2.50 (95% CI, 1.41; 4.41) (p=0.0016) and between-study heterogeneity was found (p-value by Q test=0.004). Aggregation of studies comparing treated vs untreated cohorts (n=8, n=333,312 patients) revealed an association between anti-HCV therapy and lower risk of kidney disease. The overall estimate for adjusted risk of kidney disease across the eight studies was 0.39 (95% CI, 0.25; 0.612) (p=0.0001). Meta-regression showed that the effectiveness of antiviral therapy in reducing the frequency of kidney disease diminishes as cirrhosis (p=0.02) and HBV infection (p=0.0001) increase among HCV-infected individuals.
ConclusionsAntiviral therapy for HCV lowers the risk of kidney disease among HCV-infected individuals. Studies to understand the mechanisms underlying this association are ongoing.
Sigue existiendo controversia acerca del rol de la hepatitis C como factor de riesgo de desarrollo de enfermedades renales en la población general. Algunos autores han evaluado el efecto de la terapia antiviral para el VHC en el riesgo de enfermedad renal.
Objetivos y diseño del estudioSe realizó una revisión sistemática de la literatura médica publicada, para evaluar si la terapia antiviral para el VHC tenía un impacto independiente en la supervivencia renal en la población adulta general. Se utilizó un modelo de efectos aleatorios para generar una estimación general del riesgo de enfermedad renal tras la terapia anti-VHC, entre los estudios publicados. También se realizaron análisis de meta-regresión y estratificados.
ResultadosQuince estudios resultaron elegibles (n=356, 285 pacientes), realizándose meta-análisis separados con arreglo al resultado. Los estudios agrupados basados en las respuestas virales (n=7; 34.763 pacientes individuales) demostraron una relación entre la respuesta viral sostenida y la menor frecuencia de enfermedad renal; la estimación general para el riesgo ajustado de enfermedad renal fue de 2,5 (95% IC, 1,41; 4,41) (p=0,0016), encontrándose una heterogeneidad entre estudios (valor p con prueba Q=0,004). La suma de estudios comparativos de cohortes tratadas vs no tratadas (n=8, n=333.312 pacientes) reveló una asociación entre la terapia anti-VHC y el menor riesgo de enfermedad renal. La estimación general para el riesgo ajustado de enfermedad renal entre ocho estudios fue de 0,39 (95% IC, 0,25; 0,612) (p=0,0001). La meta-regresión reflejó que la efectividad de la terapia antiviral para reducir la frecuencia de enfermedad renal disminuye a medida que aumentan los casos de cirrosis (p=0,02) e infección por VHB (p=0,0001) entre los individuos infectados de VHC.
ConclusionesLa terapia antiviral para el VHC disminuye el riesgo de enfermedad renal entre los individuos infectados de VHC. Son continuos los estudios para comprender los mecanismos subyacentes a esta asociación.
Hepatitis C is one of the main causes of liver cirrhosis worldwide1. In addition to its effects in the liver, chronic hepatitis C virus infection shows extra-hepatic activity in various organs and tissues including kidneys1. Recent evidence suggests a link between hepatitis C virus infection and chronic kidney disease2. Chronic HCV and CKD are major public health issues all over the world; globally, an estimated 71 million people have chronic hepatitis C infection and the rate of CKD in the adult general population of industrialized countries is around 10-15%3.
Conventional risk factors for developing chronic kidney disease include diabetes mellitus, arterial hypertension, smoking, metabolic syndrome and increasing age; however, these factors do not fully explain the current frequency of chronic kidney disease in the adult general population of developed world. A systematic review with meta-analysis of cross-sectional/longitudinal studies (n=15; 2,299,134 unique patients) recently demonstrated a relationship between positive anti-HCV serologic status and increased risk of chronic kidney disease; the summary estimate for adjusted hazard ratio was 1.54 (95% Confidence Interval, 1.26; 1.87, P=0.0001)4.
Novel data show that sustained viral response with interferon-based regimens leads to an improvement of hepatic and extra-hepatic outcomes, such as reduction rates of stroke, acute coronary syndrome, and better kidney survival5. In a prospective French study, 668 cirrhotic patients (50.5%) underwent antiviral treatment for HCV and achieved SVR; they had a lower risk of cardiovascular (HR, 0.42; 95% CI, 0.25-0.69; P=0.001) and infectious (HR, 0.44; 95% CI, 0.29-0.68; P<0.001) events6. In a retrospective analysis performed at the VA Greater Los Angeles Healthcare System (n=523 HCV-infected patients), patients who reached SVR12 had a lower decline in GFR compared with those who did not achieve SVR12, 3.1mL/min+0.75mL/min per 1.73m2 vs. 11mL/min+2.81mL/min per 1.73m2 (P=0.002)7. However, these results have not been confirmed by others8.
The aim of this study was to review the available evidence on the impact of antiviral therapies for HCV on the risk of kidney disease in the adult general population by performing a systematic review of the literature with a meta-analysis of clinical observational studies.
Material and methodsThis work is in agreement with the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (Supplemental File 1)9.
Search Strategy and Data ExtractionTwo authors (F.F., and V.D.) independently reviewed English-language citations from the National Library of Medicine's Medline database from 1989 through June 30, 2018. Data on HCV status were not available before 1989, when the first assay for HCV was manufactured. We conducted our search by four Medline databases engines (Embase, Grateful Med, Ovid, and PubMed). Our Medline search was limited to human studies. We applied the following algorithm in medical subject heading and in free text words: (“HEPATITIS C” or”HCV” or “HEPATITIS C VIRUS INFECTION” or “HCV INFECTION” or”ANTIBODY POSITIVE SEROLOGIC STATUS” or”ANTI-HCV”) and (“CHRONIC KIDNEY DISEASE” or “CKD” or “END-STAGE RENAL DISEASE” or “ESRD” or “GLOMERULONEPHRITIS” or”GN” or”LOW GLOMERULAR FILTRATION RATE” or”low GFR” or”KIDNEY DISEASE” or”KIDNEY EVENTS”) and (“INTERFERON” or”IFN” or”pegylated-INTERFERON” or”peg-IFN” or “RIBAVIRIN” or”RBV”) and (”DIRECT-ACTING ANTIVIRAL AGENTS” or “DAAs”) and (“SUSTAINED VIROLOGICAL RESPONSE” or”SUSTAINED VIRAL RESPONSE” or”SVR” or “CURE”) and (“HAZARD RATIO” or “HR” or “INTERNATIONAL RATE RATIO” or “IRR” or “ODDS RATIO” or”OR”). An additional search was performed with electronic searches of the Cochrane Library; manual searches of selected specialty journals were done to identify all pertinent literature. Reference lists from qualitative topic reviews and published clinical studies were also searched. It was previously demonstrated that a Medline search alone might not be sensitive enough10. Data on study design, study period, patient characteristics, HCV prevalence, antiviral therapy towards HCV, and kidney disease outcomes were abstracted. Authors of selected papers were contacted to obtain missing data and only data from individuals with known HCV status were included in the meta-analysis. Consensus was achieved for all data. Studies were compared to eliminate duplicate reports for the same patients, which included contact with investigators when necessary. Eligibility and exclusion criteria were pre-specified. Our search was limited to human studies that were published in the English literature.
Inclusion CriteriaStudies were included if they met the following inclusion criteria: (1) they presented original data from cohort and longitudinal studies; (2) the outcome of interest was clearly defined as the risk of occurrence (or deterioration) of kidney disease after antiviral therapy among HCV-positive patients; (3) they provided adjusted risk estimates and their confidence intervals. We considered both case-control studies and cohort studies as eligible for inclusion in the analysis. If data on the same population were duplicated in more than one study, the most recent study was included in the analysis.
Ineligible StudiesStudies were excluded if they reported inadequate data on the rate of kidney disease in the HCV- infected population after antiviral therapy towards HCV (e.g., incomplete information on HCV status or antiviral therapy or renal outcomes). Unpublished studies, studies that were only published in abstract form or as interim reports were excluded; letters and review articles were not considered for this systematic review.
Quality AssessmentThe quality of the 15 studies was appraised using a scale adapted from the ‘Newcastle/Ottawa Scale (NOS)’11. The Newcastle-Ottawa scale is a scoring system that assesses every aspect of an observational epidemiologic study from a methodological point of view. When a study included relevant information that could be associated with the NOS, one point was added. Seven items in cross-sectional studies and eight items in cohort and case-control studies that could be related to the NOS were identified. Therefore, cross-sectional studies assigned 8-10, 6-7, 4-5, or 0-3 points (stars) were evaluated as very good, good, satisfactory or unsatisfactory studies, respectively. Similarly, cohort/case-control studies with 7-9, 5-6, 4 and 0-3 points (stars) were identified as very good, good, satisfactory or unsatisfactory, respectively. We carried out subgroup analyses based on those studies provided with very good quality. Data extraction and quality scoring were performed independently by two reviewers (F.F. and V.D.) and the results were merged by consensus. The complete protocol for quality scoring is available on-line (see Supplemental File 2).
Outcomes measuresThe primary end point was to provide adjusted estimates of the risk (and 95% CIs) of kidney disease among HCV-infected population after antiviral therapy for HCV. Multivariate analysis was made to estimate the independent effect of anti-HCV therapy on the frequency of kidney disease after adjustment for potential confounders (covariates) (e.g., age, gender, race/ethnicity, diabetes mellitus, and others). Cox proportional hazard regression analysis was carried out to assess the effect of antiviral therapy for HCV per se on the risk of kidney disease after adjustment for differential follow-up time and distribution of potential confounders.
Data Synthesis and AnalysisWe weighted the study-specific log hazard ratios by the inverse of their variance to obtain a pooled effect estimate and its 95% confidence intervals. For each study, we used the estimate of the effect measure that was adjusted for the largest number of confounders. We present both fixed-effects and random-effects pooled estimates but use and report the latter when heterogeneity was present. We used the random-effects approach, as described by DerSimonian and Laird12, Cochrane Q–test was used for quantifying the heterogeneity13. The I2 statistic, which is the percentage of total variation across studies due to heterogeneity rather than chance, was also calculated14. The null hypothesis of this test is the absence of heterogeneity. We explored the origin of heterogeneity by stratified analysis and meta-regression technique. Subgroup or stratified analysis and meta-regression were pre-specified. Stratified analysis was made by restricting the analysis to subgroups of studies defined by study characteristics (such as country of origin, response to antiviral therapy, and others). Meta-regression was performed in order to look at simultaneously the effects of multiple (continuous and categorical) covariates on the outcome of interest. We performed random-effects meta-regression using the method of moments or maximum likelihood approaches where appropriate. Publication bias was assessed by the Egger test for funnel-plot asymmetry. All analyses were done with the statistical package Comprehensive Meta-Analysis (CMA), version 2.0 (Biostat Inc., USA, 2005). The 5% significance level was adopted for α risk. Every estimate was given with its 95% Confidence Intervals.
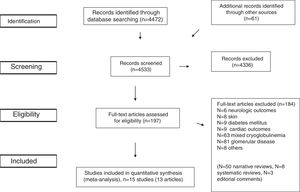
ResultsLiterature ReviewAs shown in Figure 1, we retrieved 4,533 articles and 197 full-text papers were assessed for eligibility. The list of the 197 full-text papers is reported in the Supplemental File 3. Fifteen studies met our inclusion criteria; these were published in 13 papers [Figure 1] and carried out in 3 continents (n=356,825 patients)15–27. Thus, some studies reported data on more than one kidney disease outcome, but each cohort was represented once in any meta-analysis. There was a 100% concordance between reviewers with respect to final inclusion and exclusion of studies reviewed based on the predefined inclusion and exclusion criteria.
We included studies where the diagnosis of HCV infection was done by testing for anti-HCV antibody in serum15,16,18,21,22,26,27. Information on HCV serological status was collected at the time of enrolment. Surveys based on the diagnosis of HCV infection by administrative data (ICD-9-CM codes) were also considered17,19,20,23–25.
Some authors defined the presence of kidney disease by ICD-9-CM codes17,19,20,23–25; others adopted assessment of eGFR15,16,18,21,22,26,27.
Many studies adopted the Cox proportional hazard model to examine the independent association of antiviral therapy with outcomes; one study used the multivariable Poisson regression model26. Conventional regression analyses were conducted in two papers16,18. Some authors have performed the propensity score methods to obtain better control for confounding19,24,25,27.
Patient characteristicsTables 1–4 report some salient demographic and clinical characteristics of subjects enrolled in the included studies. The mean age of patient cohorts ranged from 39.8 to 58 years. The gender distribution ranged from 49.6% to 97.1% male. Five studies were from Asia; six and four from U.S. and Europe, respectively. The average follow-up ranged between 1.8 to 8.2 years. Table 5 gave information on study design and anti-viral therapy.
Studies included in the meta-analysis (demographic and clinical characteristics at baseline) (SVR-based results).
| Authors | Country | Patients, n | Follow-up, yrs | Anti-HCV positive pts, n | Age, yrs | SVR |
|---|---|---|---|---|---|---|
| Arase Y, et al., 2011 | Japan | 650 | 6.5 | 650 | 57.4+11.7 | 210 (32%) |
| Ble M, et al., 2014 | Spain | 175 | 3.8 (1-9) | 175 | 55 (33-84) | 75 (42.8%) |
| Leone S, et al., 2016 | Italy | 340 | NA | 340 | 39.8(35.8-43.5) | 102 (30%) |
| Berenguer J, et al., 2017 | Spain | 1,625 | 5.4 (3.5-7.1) | 1,625 | 40 (37-43) | 628 (38.6%) |
| Mahale P, et al., 2017 (1) | USA | 31,143 | 5.1 (2.9-7.2) | 31,143 | 50.8 | 10,575(33.9%) |
| Kovari H, et al., 2017 | Switzerland | 626 | 8.2 | 626 | 49 (43-53)/50 (45-55)* | 345 (55.1%) |
| Satapathy S, et al.2018 | USA | 204 | 5.5 | 204 | 55+7 | 145 (71%) |
Studies included in the meta-analysis (demographic and clinical characteristics at baseline) (treated vs. untreated).
| Authors | Country | Patients, n | Follow-up, yrs | Anti-HCV positive pts, n | Age, yrs |
|---|---|---|---|---|---|
| Satapathy S, et al. 2012 | USA | 552 | 7 | 552 | 50+10.6 |
| Hsu Y, et al. 2014 | Taiwan | 2,822 | 3.78+2.2/3.59+2.1* | 2,822 | 54.9+8/55+8* |
| Chen Y, et al., 2015 | Taiwan | 8,747 | 3.2+2.1/4.7+1.8* | 8,747 | 54+11/55.7+15* |
| Hsu Y, et al., 2015 | Taiwan | 37,152 | 3.3+2.4/3.2+2.4* | 37,152 | 51.8/51.7* |
| Mahale P, et al., 2017 (2) | USA | 158,097 | 5.1 | 158,097 | 50.8/52.3* |
| Lin M, et al., 2017 | Taiwan | 2,583 | 3.8+2.7/3.6+2.6* | 2,583 | 58.1+10/58.4+11 |
| Park H, et al,, 2017 (1) | USA | 55,818 | 1.8 | 55,818 | 52 (47; 57)/54 (49; 58)/56 (51; 60)/55 (48; 59)** |
| Park H, et al., 2017 (2) | USA | 55,751 | 1.8 | 55,751 | 52 (47; 57)/54 (49; 58)/56 (51; 60)/55 (48; 59)** |
Studies included in the meta-analysis (demographic and clinical characteristics).
| Authors | Gender, males | Hypertension | Diabetics, n | Cirrhotics, n |
|---|---|---|---|---|
| Arase Y, et al. | 405 (62.3%) | 152 (23%) | 149 (22.9%) | 650 (100%) |
| Satapathy S, et al. (2012) | 377 (68.3%) | 217 (39.3%) | 105 (19%) | 33 (5.9%) |
| Hsu Y, et al. (2014) | 1,838 (65%) | 1,212 (42.9%) | 2,822 (100%) | 646 (22.9%) |
| Ble M, et al. | 129 (73.7%) | 48 (27%) | 62 (35%) | 16/96 (17%) |
| Chen Y, et al. | 4,343 (49.6%) | 3,825 (43.7%) | 2,897 (33.1%) | 1,535 (17.5%) |
| Hsu Y, et al. (2015) | 21,890 (58.9%) | 8,036 (21.6%) | 5,153 (13.8%) | 5,792 (15.5%) |
| Leone S, et al. | 262 (77.1%) | 66 (19.4%) | NA | 54 (15.9%) |
| Berenguer J, et al. | 1,219 (75%) | NA | 47 (2.9%) | 445 (38.6%) |
| Mahale P, et al (1) | 29,980 (96.3%) | 14,397 (46.2%) | 5,436 (17.5%) | 7,850 (26.4%) |
| Mahale P, et al. (2) | 156,253 (97.1%) | 82,081 (51.5%) | 33,135 (20.7%) | 37,842 (24.8%) |
| Lin M, et al. | 1,937 (63.2%) | 1,829 (59.7%) | 1,295 (42.2%) | 340 (11.1%) |
| Park H, et al. (1) | 33,667 (60.3%) | 20,954 (37.5%) | 9,136 (16.3%) | 2,459 (4.4%) |
| Park H, et al. (2) | 33,667 (60.3%) | 20,954 (37.5%) | 9,136 (16.3%) | 2,459 (4.4%) |
| Kovari H, et al. | 462 (73.8%) | NA | NA | NA |
| Satapathy S, et al. (2018) | 138 (68%) | 85 (42%) | 52 (25%) | 0 |
Studies included in the meta-analysis (demographic and viral characteristics).
| Authors | HBV | HIV | Caucasian, n | HCV genotype 1, n |
|---|---|---|---|---|
| Arase Y, et al. | 0 | 0 | 0 | 389 (59.8%) |
| Satapathy S, et al. (2012) | NA | 42 (7.6%) | 22 (4%) | NA |
| Hsu Y, et al. (2014) | 0 | NA | 0 | NA |
| Ble M, et al. | NA | NA | NA | 153 (87%) |
| Chen Y, et al. | 0 | NA | 0 | NA |
| Hsu Y, et al. (2015) | 0 | NA | 0 | NA |
| Leone S, et al. | 175 (51.5%) | 340 (100%) | 319 (93.8%) | 125 (36.7%) |
| Berenguer J, et al. | 55 (3.4%) | 1,625 (100%) | NA | 805 (49.5%) |
| Mahale P, et al. (1) | 321 (1%) | 736 (2.4%) | 17,075 (54.8%) | 21,498 (69%) |
| Mahale P, et al. (2) | 2,037 (1.3%) | 5,086 (3.2%) | 72,197 (44.9%) | 87,979 (54.7%) |
| Lin M, et al. | 0 | 0 | 0 | NA |
| Park H, et al. (1) | 1,193 (2.1%) | 1,187 (2.1%) | NA | NA |
| Park H, et al. (2) | 1,193 (2.1%) | 1,187 (2.1%) | NA | NA |
| Kovari H, et al. | 34 (5.4%) | 626 (100%) | NA | 204 (32.5%) |
| Satapathy S, et al. (2018) | 0 | 0 | 149 (73%) | NA |
Studies included in the meta-analysis (design and therapy).
| Authors | Study design | Outcome | Population, type | Antiviral therapy, type |
|---|---|---|---|---|
| Arase Y, et al. | Retrosp, Co | CKD | Inner city residents | IFN (Recombinant-IFNα2a or IFNα2b, Natural-IFNα or IFNβ) alone or with ribavirin |
| Satapathy S, et al. (2012) | Retrosp, Co | CKD | Metropolitan area residents | IFN-based therapy |
| Hsu Y, et al. (2014) | Case Control | ESRD | National health insurance program Users | Peg-IFN plus RBV |
| Ble M, et al. | Retrosp, Co | CKD | Liver transplant recipients | Peg-IFNα2a or Peg-IFNα2b plus RBV |
| Chen Y, et al. | Case Control | CKD | National health insurance program Users | IFN (IFNα, Peg-IFNα-2a, Peg- IFNα-2b)alone or plus RBV |
| Hsu Y, et al. (2015) | Case Control | ESRD | National health insurance program Users | Peg-IFN (α2a or 2b)plus RBV |
| Leone S, et al. | Retrosp, Co | CKD | HIV/HCV co-infected patients | Peg-IFN (or standard IFN) plus RBV |
| Berenguer J, et al. | Prosp, Co | ESRD andCRF | HIV/HCV co-infected patients | Peg-IFNα2a or Peg-IFNα2b or Standard IFNα plus RBV |
| Mahale P, et al. (1) | Retrosp, Co | GN | Veterans | Any IFN alone or plus RBV |
| Mahale P, et al. (2) | ||||
| Lin M, et al. | Case control | ESRD | National health insurance program Users | Peg-IFN alone or plus RBV |
| Park H, et al. (1) | Retrosp, Co | CKD | Health insurance program Users | 1) IFNα, IFNβPeg-IFNα-2a, Peg- IFNα-2b(alone or plus RBV)2) pegIFN/RBV and DAAs3) DAAs with or without RBV |
| Park H, et al. (2) | GN | |||
| Kovari H, et al. | Prosp, Co | CKD | HIV/HCV co-infected patients | 1) PegIFN plus RBV2) PegIFN plus RBV and DAAs |
| Satapathy S, et al. (2018) | Retrosp, Co | ESRD | Liver transplant recipients | 1) IFN-based therapy2) IFN-based therapy plus DAAs3) DAAs |
Arase Y, et al. [15] HR adjusted for age, gender, body mass index, eGFR, HCV viral load, HCV genotype, AST, ALT, platelet count, type of IFN, uric acid, triglycerides, total cholesterol, diabetes mellitus, hypertension, frequency of contrast imaging per year, antiviral combination with ribavirin
Satapathy S, et al. [16] OR adjusted for arterial hypertension, body weight, HCV RNA, age, gender, HIV status, diabetes mellitus, intravenous drug abuse, alcohol abuse, race, HCV genotype 1.
Hsu Y, et al. [17] HR adjusted for age, gender, arterial hypertension, hyperlipidemia, chronic obstructive lung disease, peripheral arterial occlusive disease, cirrhosis, thyroid disorder, aspirin, NSAID, ACEi, ARB, statin, metformin and insulin consumption
Ble M, et al. [18] RR adjusted for age, gender, arterial hypertension, eGFR, diabetes, cirrhosis, donor age, liver fibrosis, time since EOT, CNI levels 1yr after treatment, fibrosis
Chen Y, et al. [19] HR adjusted for age, gender, comorbidities (diabetes mellitus, arterial hypertension, coronary artery disease, hyperlipidemia, cirrhosis), geographic region of residence, socio-economic status, urbanization level, enrollee category, number of medical visits, Charlson comorbidity index score
Hsu Y, et al. [20] HR adjusted for demographic characteristics (age, gender), comorbidities (arterial hypertension, hyperlipidemia, diabetes mellitus, chronic lung disease, peripheral artery disease, cirrhosis, thyroid disorder), Charlson index, hospital level, use of aspirin and NAIDs
Leone S, et al. [21] HR adjusted for baseline covariates (age, calendar year, mode of HIV transmission, HCV genotype, HBsAg status), time-updated covariates (body mass index, systolic blood pressure, diastolic blood pressure, cirrhosis, cholesterol, tryglicerides, CD4 T-cell count, HIV RNA, number of AIDS events, antiretroviral therapy use-abacavir, tenofovir, ritonavir, atazanavir, lopinavir, indinavir), diabetes mellitus, chronic vascular disease
Berenguer J, et al. [22] HR adjusted age, gender, previous AIDS-defining conditions, HIV transmission category (injection drug use or not), nadir CD4 T-cell count, combination antiretroviral therapy, HIV RNA status, liver fibrosis-4 score, HCV genotype, and cumulative exposure to selected antiretroviral drugs
Mahale P, et al. [23] HR adjusted for age, gender, race/ethnicity, period of military service, number of outpatient visits, BMI, smoking, alcohol intake, arterial hypertension, diabetes
Lin M, et al [24] HR adjusted for propensity score (including gender, age, income, urbanization level, hospital level, medical history, medications)
Park H, et al. [25] HR adjusted for age, gender, diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, ACEI use, ARB use, contraindications to IFN-based therapy, hepatitis A, hepatitis B, alcohol/drug abuse disorders, cirrhosis, decompensated cirrhosis, hepatocellular carcinoma
Kovari H, et al. [26] IRR adjusted for HIV acquisition category, age, HIV-RNA, smoking, alcohol use, active intravenous drug use, and duration of HIV and HCV infection, HIV type 1 RNA
Satapathy S, et al. [27] HR adjusted for propensity score (including age at liver transplant, gender, race/ethnicity, eGFR 3 month after transplant, body mass index, arterial hypertension, diabetes mellitus, proteinuria degree, LDL-cholesterol value, use of mTOR inhibitor or tacrolimus for immunosuppression after LT)
As listed in Table 6, there was large heterogeneity in the definition of the outcome. The majority of studies addressed the incidence of kidney disease after antiviral therapy among patients with HCV infection15–17,19–27. One report evaluated the impact of antiviral therapy on the progression of kidney disease among HCV-infected individuals18.
All studies included in the meta-analysis (outcome and definition criteria).
| Authors | Outcome | Definition |
|---|---|---|
| Arase Y, et al. | CKD | eGFR <60 mL/min/1.73m2 |
| Satapathy S, et al. | CKD | eGFR <60 mL/min/1.73m2or serum creatinine>1.5 mg/dL |
| Hsu Y, et al. (2014) | ESRD | Dialysis or renal transplant |
| Ble M, et al. | CKD | eGFR <60 mL/min/1.73m2 |
| Chen Y, et al. | CKD | Stage 1-5 CKD |
| Hsu Y, et al. | ESRD | Dialysis or renal transplant |
| Leone S, et al. | CKD | eGFR <60 mL/min/1.73m2 |
| Berenguer J, et al. | Renal events | Dialysis, renal transplant, chronic renal failure (requiring dialysis or not) |
| Mahale P, et al. (1) | Glomerular disease | * |
| Mahale P, et al. (2) | Glomerular disease | |
| Lin M, et al. | ESRD | New onset dialysis |
| Park H, et al. (1) | CKD | Stage 3-5 CKD |
| Park H, et al. (2) | Glomerular disease | * |
| Kovari H, et al. | Kidney events | Dialysis or renal transplant |
| Satapathy S, et al. | CKD | eGFR <30 mL/min/1.73m2 |
glomerular disease was defined either by the histologic finding of glomerulonephritis on kidney biopsy, or by the presence of at least two of the following clinical signs: proteinuria, hematuria, and reduced estimated glomerular filtration rate (eGFR) <60mL/min/1.73m2 without alternative cause for chronic kidney disease
The definition of kidney disease adopted by the authors was chronic kidney disease (n=7), end-stage renal disease (n=3), glomerular disease (n=3), and kidney events (n=2) (Table 6). Chronic kidney disease and end-stage renal disease were assessed according to the Kidney Disease Outcomes Quality Initiative/Kidney Disease: Improving Global Outcomes classification.
Summary estimate of outcome (SVR-based studies)Some studies (n=7) expressed their results according to the viral response after anti-viral therapy [Table 7]. Pooling studies based on viral responses (n=7; 34,763 unique patients) demonstrated that the risk of kidney disease was greater in patients who did not obtain than those who obtained sustained viral response after anti-HCV treatment; the summary estimate for adjusted risk was 2.5 (95% CI, 1.41; 4.41) (P=0.0016) and between-studies heterogeneity was found (P value by Q test=0.004). According to the Egger's regression intercept, no publication bias occurred (P=0.58). The quality scores ranged between 4 and 8 stars.
Summary estimate for adjusted effect estimate among various subgroups of interest (studies based on viral response: No-SVR vs. SVR).
| N | Adjusted effect estimate (random-effects model) | Q value (by Chi-squared test) | I2 | |
|---|---|---|---|---|
| No-SVR vs. SVR(all studies) | 7 | 2.50 (1.41; 4.41)(P=0.0001) | 19.07(P=0.004) | 14.2 |
| Studies adopting HR | 6 | 2.46 (1.34; 4.53)(P=0.003) | 18.6(P=0.002) | 19.2 |
| Outcome: CKD (stage 3-5) | 3 | 2.36 (1.22; 4.57)(P=0.001) | 2.77 (NS) | 0.73 |
| Outcome: CKD (stage 4-5) | 2 | 6.88 (2.75; 17.2)(P=0.0001) | 1.06 (NS) | 22.1 |
| Studies from Europe | 4 | 2.3 (1.29; 4.13)(P=0.005) | 2.76 (NS) | 12.3 |
| Studies from US | 2 | 3.26 (0.5; 21.3)(P=0.21) | 12.9 (P=0.003) | 32.3 |
| Studies with quality score>6 | 2 | 1.36 (1.04; 1.78)(P=0.02) | 0.9 (NS) | 12.5 |
| Studies with quality score <6 | 5 | 3.01 (1.61; 5.62)(P=0.005) | 8.26 (NS) | 14.2 |
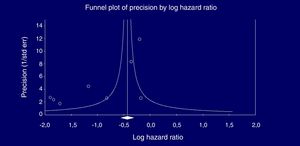
Eight studies (n=333,312 patients) compared patient cohorts who received vs. those who did not receive antiviral treatment for HCV. The summary estimate for adjusted risk of lower incidence of kidney disease across the eight studies was 0.39 (95% CI, 0.25; 0.612) (P=0.0001) [Table 8]. Tests for homogeneity of the adjusted risk across the eight studies gave a Q value of 52,608 (P=0.0001), I2=86.6; the homogeneity assumption was rejected. The funnel plot concerning the publication bias is reported in Figure 2. The Egger test showed publication bias (P=0.015). The quality scores ranged between 5 and 8 stars.
Summary estimate for adjusted effect estimate among various subgroups of interest (Treatment vs. No-treatment studies).
| N | Adjusted effect estimate(random-effects model) | Q value(by Chi-squared test) | I2 | |
|---|---|---|---|---|
| Treatment vs. No-treatment(all studies) | 8 | 0.39 (0.259; 0.612)(P=0.001) | 52.608(P=0.0001) | 86.69 |
| Studies adopting aHR | 7 | 0.44 (0.25; 0.63)(P=0.0001) | 47.5(P=0.0001) | 87.36 |
| Case-control studies | 4 | 0.24 (0.15; 0.39)(P=0.0001) | 6.06(NS) | 50.4 |
| Outcome: CKD studies | 3 | 0.451 (0.226; 0.9)(P=0.024) | 6.47(P=0.039) | 69.1 |
| Outcome: GN studies | 2 | 0.82 (0.69; 0.96)(P=0.016) | 2.12(NS) | 0.00 |
| Outcome: ESRD studies | 3 | 0.213 (0.127; 0.35)(P=0.0001) | 3.87(NS) | 48.3 |
| Studies from Asia | 4 | 0.24 (0.15; 0.39)(P=0.0001) | 6.06(NS) | 50.4 |
| Studies from US | 4 | 0.706 (0.527; 0.946)(P=0.002) | 7.736(P=0.05) | 61.2 |
| Studies using ICD-9 code | 7 | 0.44 (0.25; 0.63)(P=0.001) | 47.5(P=0.0001) | 87.36 |
| Studies adopting IFN-based therapy | 6 | 0.29 (0.14; 0.59)(P=0.001) | 51.4(P=0.0001) | 90.2 |
| Studies with quality score>6 | 6 | 0.459 (0.29; 0.72)(P=0.001) | 35.09(P=0.0001) | 85.7 |
| Studies with quality score<6 | 2 | 0.29 (0.19; 0.43)(P=0.001) | 0.78(NS) | 0.00 |
| Studies using propensity score matching analysis | 3 | 0.528 (0.32; 0.85)(P=0.01) | 11.7(P=0.008) | 74.53 |
Stratified analyses are shown in Tables 7–8, no substantial difference in pooled adjusted effect estimates across designs was found even if the homogeneity assumption was rejected in some subsets.
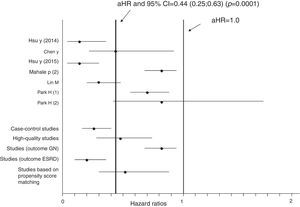
Aggregation of studies comparing treated vs. untreated cohorts (n=7; 321,522 unique patients) reported that the incidence of kidney disease was lower in patients who received antiviral therapy compared to those who had not received it, the summary estimate for adjusted HR of reduced incidence of kidney disease was 0.44 (95% CI, 0.25; 0.63) (P=0.0001) (Figure 3). Between-studies heterogeneity was noted (P value by Q test P=0.0001). According to the Egger's regression intercept, there was publication bias (P=0.037). Figure 4 shows the adjusted risk of lower incidence of CKD among HCV-infected patients receiving IFN-based therapy (n=6 reports).
Estimated HR and 95% confidence intervals for each study (cohorts who received antiviral therapy vs. those who did not; n=7 studies, n=321,522 unique patients)
The vertical line represents the pooled aHR of risk of kidney disease (random-effects model) after comparison.
Adjusted HR 0.44 (95% CI, 0.25; 0.63) (P=0.0001). Heterogeneity statistics: Q-value (by Chi-squared test)=47.5, df=6, P=0.0001; I2=87.4%.
We made meta-regression analysis in the subset of studies (n=8, n=333,312 unique patients) comparing patient cohorts who received antiviral therapy for HCV vs. those who did not [Table 2]. As listed in Table 9, meta-regression demonstrated a significant impact of frequency of cirrhosis (P=0.02), HBV infection (P=0.0001), arterial hypertension (P=0.002), DM (P=0.051), and gender (male) (P=0.05) on the outcome of interest (adjusted risk of reduced incidence of renal disease among HCV-positive patients after antiviral therapy ended). Other covariates included in the model (age, duration of follow-up, size, reference year) had no effect on the attitude of antiviral therapy in reducing the incidence of kidney disease among HCV-infected patients.
| Intercept | Regression Coefficient | Standard error | 95% CI | Z value | P value |
|---|---|---|---|---|---|
| Intercept | -1.8187 | 0.6105 | -3.01; -0.622 | -2.98 | 0.0029 |
| Cirrhosis | 0.0816 | 0.0354 | 0.012; 0.151 | 2.3 | 0.0212 |
| HBV | 0.9066 | 0.2131 | 0.48; 1.324 | 4.25 | 0 |
| Males | -0.0365 | 0.0192 | -0.074; 0.0001 | -1.91 | 0.0568 |
| Diabetics | -0.0138 | 0.0071 | -0.027; 0.001 | -1.95 | 0.051 |
| Hypertension | -0.0437 | 0.0145 | 0.0153; 0.072 | 3.02 | 0.0025 |
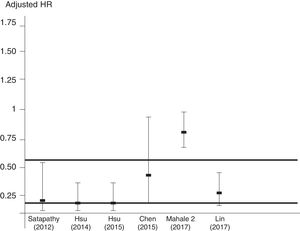
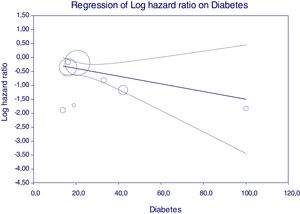
Figure 5 reports that the adjusted HR of lower incidence of kidney disease after antiviral therapy among HCV-infected patients (when adjusted for the other covariates) becomes more important when the frequency of diabetes increases.
DiscussionIn the last decade, numerous authors have addressed the relationship between hepatitis C and kidney disease. The REVEAL-HCV cohort study, a community-based prospective study conducted in Taiwan, is the latest report on the topic- the adjusted prevalence odds ratio of CKD (defined by consecutive proteinuria or an estimated glomerular filtration rate under 60mL/min/1.73m2) was 4.99 (95% CIs, 2.25; 11.06) for those with high HCV RNA and genotype 22. As mentioned above, HCV infection and CKD are commonly observed in a large proportion of the adult general population; it is difficult, however, to establish whether a simple association exists or whether additional pathogenic mechanisms directly or indirectly link chronic HCV infection to CKD.
The recent publication of reports evaluating the impact of antiviral therapies for HCV upon renal survival gave us the opportunity to deepen the correlation between HCV and CKD. The current meta-analysis (n=15 studies, n=356,825 patients) suggests that antiviral therapy per se favours renal survival in the HCV-infected population. The summary estimate for the adjusted risk of kidney disease was 0.43 (95% CI, 0.26; 0.69) (P=0.0001) from surveys (n=8) comparing patients who received vs. those who did not receive antiviral treatment. These conclusions appear reliable despite some between-studies heterogeneity occurred in some comparisons.
There is growing evidence in medical literature suggesting that HCV infection is not a liver-focused disease but a systemic illness giving several extra-hepatic (including kidney) manifestations. Kidneys appear an important target of the extra-hepatic activity of HCV; HCV can give glomerular damage through activation and deposition of cryoglobulins28. Also, HCV may cause tubulo-interstitial damage via a direct cytopathic effect or antibody immune complex29. Additionally, non-immunological pathways (oxidative stress, pro-inflammatory cytokines, liver or peripheral insulin resistance, among others) help the development of renal disease by vascular injury30–33. Endothelial dysfunction at kidney level can be promoted by viral replication in the atherosclerotic plaque34.
Thus, the results generated by this systematic review and meta-analysis lend further support to the notion that HCV is a risk factor for the acquisition (or progression) of CKD in the adult general population. Prior studies have found a consistent relationship between anti-HCV positive serologic status and risk of CKD in the developed world; and these have been systematically reviewed 4 but the activity of anti-HCV viral therapy on the outcomes has not been reviewed. The effect of anti-HCV antiviral therapy on the risk of renal disease in the general population has not been reviewed to date.
The findings obtained in the current meta-analysis are in keeping with those obtained by other investigators who addressed the influence of HCV eradication on extra-hepatic outcomes including chronic kidney disease. In a retrospective cohort of patients with stage 3 CKD, regression models reported that sustained viral response was associated with a 9.3 (95% CI, 0.44 to 18) mL/min per 1.73m2 increase in eGFR during the 6-month post-treatment follow-up period35.
Some authors have already suggested that IFN-based treatments of chronic hepatitis C promote a long-term inhibition of oxidative stress with concomitant improvement of necro-inflammatory activity and fibrosis36,37. In other words, it remains unclear whether the benefit on hepatic or extra-hepatic outcomes conferred from IFN can be attributed to the antiviral activity per se or to the immunomodulatory/antiproliferative properties of IFN. The impact of the newer direct-acting antiviral agents remains to be addressed.
The results from the current meta-analysis present some limitations. First, many reports show retrospective and cohort design- from a theoretical point of view, a randomized controlled trial with placebo gives the best evidence on the efficacy of an intervention. However, a large sample and a long follow-up are needed to perform a RCT in this setting as the frequency of events is low; also, the current availability of safe and effective drugs (DAAs) for the treatment of HCV makes the randomisation to placebo not ethically acceptable. Secondly, multivariate analysis was performed in all studies but residual confounding (confounding remaining after adjustment) cannot be excluded as full information was not given on various confounders. Data on HCV virology, life style, illicit drug abuse, and family history were often missed. Thirdly, our stratified analysis and meta-regression was not able to capture fully the heterogeneity among studies we have observed. The great heterogeneity observed in some comparisons suggested that all the studies included in the analysis are not functionally identical and this precluded the adoption of a fixed-effects model. Moreover, individual data from each study (‘meta-analysis at patient level’) were not available; thus, it was impossible to perform our own adjustments even if the studies enrolled in this meta-analysis adjusted for numerous factors (‘covariates’) that could prove to be potential confounders. Finally, as with all meta-analyses, this study has the potential limitation of publication bias; under publication bias, only results showing either statistical significance and/or the desired direction of the effects are published. In this case, the published literature is merely a selective (and too optimistic) part of all existing knowledge38. Our approach to avoid the publication bias is to rely on the retrieval of data from as many sources as possible. However, we have not included trials published as abstracts; evidence presented in abstract format is often without high quality and can give greater treatment effect39.
In conclusion, this meta-analysis shows the reduction of kidney complications among HCV-infected patients who attained viral response after therapy. We need studies provided with prospective design and uniform outcomes; the purpose of antiviral therapy in the current era of DAAs is to treat not only liver disease but also extra-hepatic complications (including kidney outcomes), irrespective of liver disease staging. Early initiation of antiviral therapy to reduce the exposure of HCV among infected individuals is encouraged.
FundingNo sources of funding were used for the preparation of this manuscript
Conflict of interest statementFabrizio Fabrizi: consultant or advisor to AbbVie and Merck & Co