New directions in dialysis research include cheaper treatments, home based therapies and simpler methods of blood purification. These objectives may be probably obtained with innovations in the field of artificial kidney through the utilization of new disciplines such as miniaturization, microfluidics, nanotechnology. This research may lead to a new era of dialysis in which the new challenges are transportability, wearability and why not the possibility to develop implantable devices. Although we are not there yet, a new series of papers have recently been published disclosing interesting and promising results on the application of wearable ultrafiltration systems (WUF) and wearable artificial kidneys (WAK). Some of them use extracorporeal blood cleansing as a method of blood purification while others use peritoneal dialysis as a treatment modality (ViWAK and AWAK.) A special mention deserves the wearable/portable ultrafiltration system for the therapy of overhydration and congestive heart failure (WAKMAN). This system will allow dehospitalization and treatment of patients with less comorbidity and improved tolerance. On the way to the wearable artificial kidney, new discoveries have been made such as a complete system for hemofiltration in newborns (CARPEDIEM). The neonate in fact is the typical patient who may benefit from miniaturization of the dialysis circuit.
This review analyzes the rationale for such endeavour and the challenges to overcome in order to make possible a true ambulatory dialysis treatment. Some initial results with these new devices are presented. We would like to stimulate a collaborative effort to make a quantum leap in technology making the wearable artificial kidney a reality rather than a dream.
Los nuevos enfoques en la investigación en diálisis incluyen el abaratamiento de los tratamientos, las terapias domiciliarias y métodos más sencillos de purificación sanguínea. Probablemente estos objetivos se consigan gracias a los avances en riñones artificiales mediante el uso de nuevas técnicas, como la miniaturización, los microfluidos o la nanotecnología. Esta línea de investigación podría llevarnos a una nueva era en el campo de la diálisis, en la que los nuevos retos serán la transportabilidad, la portabilidad y, por qué no, la posibilidad de desarrollar dispositivos implantables. A pesar de no haber alcanzado aún ese punto, recientemente se han publicado una serie de trabajos en los que los resultados sobre los sistemas de ultrafiltración portátiles y los riñones artificiales portátiles se revelan prometedores y de gran interés. Algunos de ellos recurren a la modalidad extracorpórea como método de purificación sanguínea, mientras que otros recurren a la diálisis peritoneal como modalidad de tratamiento (ViWAK, Vicenza Wearable Artificial Kidney, y AWAK, Automated Wearable Artificial Kidney). Merece mención especial el sistema de ultrafiltración portátil para la terapia de la sobrehidratación y la insuficiencia cardíaca congestiva (WAKMAN). Este sistema permitirá reducir el número de hospitalizaciones, el tratamiento de pacientes con menor comorbilidad y una mayor tolerancia. Durante la investigación en el riñón artificial portátil se han ido sucediendo nuevos avances, como el desarrollo de un sistema completo de hemofiltración para recién nacidos (CARPEDIEM, Cardio Renal Pediatric Dialysis Emergency Machine). El neonato, de hecho, es el paciente que más podría beneficiarse de la miniaturización del circuito de diálisis.
Esta revisión analiza los fundamentos de esta tendencia y los obstáculos que se le presentan a la hora de alcanzar un sistema de diálisis totalmente ambulatorio.
Se presentan los resultados iniciales de estos nuevos dispositivos. Con este trabajo nos gustaría promover un esfuerzo común para dar un salto cuantitativo tecnológico y hacer que el riñón artificial portátil sea una realidad y no una quimera.
INTRODUCTION
Patients with end stage kidney disease (ESKD) are progressively increasing and the demand for renal replacement therapies is expanding1. Hemodialysis and peritoneal dialysis represent reliable forms of therapy leading to significant and longlasting survival times2,3. Nevertheless, hemodialysis is still performed intermittently leading to a significant degree of unphysiology due to fluid and electrolyte shifts during intra and interdialytic periods. Peritoneal dialysis has been the only therapy truly mimicking the kidney since it provides a continuous renal replacement therapy with well tolerated fluid removal and smooth correction of uremic abnormalities. A gretaer interst has been risen recently to more frequent extracorporeal dialysis sessions and particularly to long nightly therapies leading to a better control of hypertension, phosphate derangements and uremic intoxication. While Dialysis therapy is a routine for developped geographical areas, it still represent a challenge for less advanced populations and nations4. At the same time, even where dialysis is performed with advanced technology in well equipped dialysis centers, patients’ life is often suboptimal and significant limitations are evident in day by day life5-9.
Last but not least, in some countries, dialysis therapy is only provided if private insurance or support is available to pay for the expenses of the care giver10,11. In summary, while we consider dialysis a well established routine therapy, in some areas of the planet this therapy is not or partially accessible, it is expensive and not affordable, it may be well performed but it still limits patient’s mobility and normal life. What will therefore be the challenge for the years to come towards a next generation of artificial kidney therapy? We suggest that dialysis should be made simple and therefore largely accessible, cheap and therefore accessible to all who need it and finally portable or wearable and therefore feasible without limiting patients mobility. This last aspect may be linked to the previous ones since mobility means ambulatory care, home care and ultimately self care. This in turn may means a simpler and cheaper care reducing expenses due to large dialysis centers and concentrating the expenditure on the single patient and his clinical needs.
FINANCIAL ISSUES IN DIALYSIS
The financial burden of chronic diseases is a major problem of all health care public services. The growing demand of dialytic services for end stage kidney patients is facing a true problem in countries where such therapies cannot be made available to all. Large areas in the planet may require a rethinking of the way renal replacement is conceived possibly through a cheaper and simpler dialysis care. Self administered care would certainly provide cost sparing while large hospital could only act as reference centers for initiation and management of complications. Peritoneal dialysis may represent a potential therapy with such characteristics although the fluid supply may become very expensive in areas where production is impossible or difficult. Other solutions may be represented by innovative dialytic approaches that require less water such as sorbent therapies or recirculation dialysis in small portable or wearable devices12. This technology is yet to come but it might represent the target for research in the coming years. Thus an effort should be made to create new, innovative and cost-effective dialysis devices with the potential for large scale application with a reasonnable cost-effectiveness. Such innovations may provide daily or even continuous dialysis, without imposing an unbearable burden on already scarce financial healthcare resources.
DIALYSIS THERAPY FOR EVERYBODY
The structure of the population is changing both in developed and less advanced societies. In the so-called developed world, elderly people are often alone and abandoned to a self care in all aspects. The traditional families have been disrupted by the large quest for better and rewarding jobs that led young members of the family moving far from the original familiar nucleus. In less advanced societies, the family is still compact but the increased need for supplies and funds has made all the young members of the family searching for a job and leaving the house. In this circumstances, health care providers are in deep troubles trying to provide services to lonely and elderly patients who may require costly and complicate treatments. A simpler dialysis therapy would allow these patients to deliver therapy to themselves with less problems. An easy treatment with limited technological complications may contribute to self care leading to cost saving and expanded application of therapies at home13.
DIALYSIS SCHEDULES
In spite of new trials going on on daily and nocturnal hemodialysis, the routine of current renal replacement therapy is basically intermittent with three sessions/week. This pattern still presents limitations in terms of quality of life, morbidity and mortality. During the last few years, a growing body of literature of several hundreds of peer-reviewed publications indicates that more frequent and prolonged dialysis treatment is associated with strikingly improved outcomes14-18. In healthy individuals blood is filtered by the native kidneys 168 hours a week. Obviously, filtering the blood only for 12 hours per week with dialysis (as typically prescribed in the US) is both un-physiologic and most inadequate, resulting in poor quality of life and morbidity- mortality rates.
As patients are shifted from the typical three-dialysis treatments per week regime to one of daily dialysis, significant improvements of the quality of life are reported (i.e., liberalization of the diet, fluid restrictions, etc.) alongside substantial reductions in medication consumption, complications, psychological symptoms, admissions and hospitalization14-18. The reported advantages of daily dialysis are improved volume control, elimination of the need for phosphate binders, no sodium retention, improvement in appetite and nutrition, less hypertension, decreased need for blood pressure drugs, no hyperkalemia, decreased expected morbidity and mortality, no hyperphosphatemia from bone disease, lesser degree of anemia, no metabolic acidosis cardiovascular disease and stroke, improved serum albumin. The implementation of daily dialysis encounters obstacles that make its accomplishment in a large scale practically impossible. Some of these are the inability or unwillingness of most patients to dialyze at home, the lack of manpower both in nurses and technicians to provide more treatments in the dialysis units, and the reluctance of governmental payers to shoulder the expense of additional procedures10,11. Thus, there is a growing need for a practical around-the-clock solution that will afford to ESKD patients the ability of receiving significantly increased dialysis dosages, while increasing efficiency and reducing the overall cost with less utilization of manpower.
THE NEW DEAL OF WEARABLE AND MINIATURIZED DIALYSIS DEVICES
Continuous dialysis allows significantly higher doses of therapy but is may be impossible to carry out with the current technology. Machines are heavy, attached to a wall electrical outlet and require many gallons of water. Thus a definite need for technological breakthroughs facilitating daily or continuous dialysis emerges clearly. Besides continuous ambulatory peritoneal dialysis this has not been explored thoroughly so far. Portable solutions for extracorporeal hemodialysis may render daily nocturnal therapy easy and reliable. However, this approach has failed so far because application on a large scale basis was mainly limited by complicated technologies and unreliable devices hardly usable by the average patient. New possibilities may arise from the development of a new generation of devices designed to be worn continuously and operated by a simple delivery system. These systems are commonly defined WAK (wearable artificial kidney) and have been recently experimented. We think that if research will focus on this cathegory of devices, dialysis therapy may become easier, cheaper and it may offer important clinical advantages due to continuity of therapy while offering at the same time mobility and greater chances of patients rehabilitation12,13,19-25.
TECHNICAL CHALLENGES FOR WAK DEVELOPMENT
The development of a wearable dialysis device (WDD) requires a deep knowledge of the common technical and clinical problems of ESKD patients together with an effort to think «out of the box» in order to pursue innovative pathways.
The first challenge is to develop an adequate vascular access that allows for blood flows in the range of 100 ml/min. This blood flow range is different from those required in chronic dialysis but it is sufficient for a therapy that operates continuously. Therefore, if a dual lumen catheter will be the solution, new design, biomaterials and skin exit site technologies can be conceived. Limitations of infection and clotting will be the top priorities in the search for the adequate vascular access. Easy connection and disconnection should also be one of the main features.
The circuit should be designed with minimum priming volume, possibly with antithrombogenic materials and definitely with an easy priming and blood returning procedure. Adequate safety measures should be present in the circuit including air detection, pressure sensors and visual and audible alarms.
The circuit should be operated by a remote control that allow the patient to accurately program and deliver the scheduled therapy. Prescription of operational parameters and delivery data display should be easily accessible via a customized easy software interface.
The dialyzer can be probably reduced in size down to one tenth of the normal dialyzers. It should provide a clearance in the range of 20 ml/min with ultrafiltration rates not higher than 5 ml/min. Again a non thrombogenic membrane would be ideal to minimize risks of clotting.
The whole device must be wearable and therefore, independent of the electrical outlet. A 24 hour working apparatus may consume large amounts of energy and therefore adequate energy sources must be available. Today however, light, energy-efficient and cost-effective batteries and fuel cells are available and they should be implemented in this wearable equipment for a continuous operation.
The amount of dialysate has to be minimal. Thus, the need for a small amount of dialysate that can be continuously regenerated and reused. A commercially available sorbent system used for many decades in dialysis, must be reconfigured and adapted as the purifying medium, facilitating the use of sterile and pure dialysate in quantity lower than 500 cc.
The patient should be able to wear the device, and ambulate without impinging in his ability to perform activities of daily life. Thus, the need for lightweight and ergonomic design that would be unobtrusive and adapt to the body contour. In addition it may reduce the utilization of nursing resources and cost of treating ESKD patients. The WAK is intended to be used for continuous renal replacement therapy, 24 hours a day, 7 days a week. It should be able to deliver adequate doses of dialysis. The efficiency of the device may be such to almost normalize the volume status and the blood chemistry in uremic patients if the device is worn continuously.
The capability of the device to remove fluid steadily from the vascular space an amount of volume similar to that physiologically removed by normal kidneys gives the treating physician the ability to keep the patients euvolemic with this device, regardless of the amount of fluid they may ingest. Furthermore, the elimination of excess fluid may well results in a better control of hypertension. In addition, the sodium content of the ultrafiltrate is equal to that of the plasma. Thus, the removal of 1.5 to 2 liters of fluid a day will result in the removal of 13.5 to 18 grams of salt19-25. This alone would not only contribute to better control of hypertension, but also result in liberalizing salt intake for ESRD patients.
Improved quality of life and better nutrition are obviously the expected direct results of this new proposed treatment. The amounts of potassium and phosphorus removed might result in eliminating the restrictions on oral intake of both these elements, and the elimination of oral phosphate binders. Although the long-term effects of this technology expected on the well being or the patients can be significant, a well designed specific clinical research will be required to confirm the hypothesis.
These objectives should be achieved in designing any prototype of a WAK to be tested in clinical settings. Several innovations may come from a specific research or from a careful search in parallel fields or other areas of development. In some cases, a reevaluation of past projects and a complete appraisal of the history in this field may result important and helpful.
PREVIOUS EXPERIENCES
The evolution of dialysis has led to sophisticated devices and equipment with important technical features. However, in the early years of dialysis therapy some investigators tried a different pathway with the intent to develop a truly wearable or portable dialysis device, mimicking the native kidney, so that patients could be treated 24 hours a day, allowing a liberal diet and fluid intake. Although there are many reports of «wearable» haemodialysis devices, these generally refer to haemofiltration circuits, often using indwelling femoral arterial and venous catheters, and/or arterio-venous shunts26. The earliest attempts date back to Kolff’s team in the 1960s27. Many nephrologists have subsequently tried to create a truly wearable device, that would allow patients to carry out their normal daily living activities, or go to work, whilst being treated28-30. The early pioneers were confronted with many technical problems, including vascular access, anticoagulation, and both the size and reliability of any such device. Some of the earlier devices used an arterial blood supply, and those which worked only with venous blood access required a blood pump, and an electrical power source. Often these devices lacked safety features, being simply strapped to the thigh, with no alarms in case of disconnection, or air detection monitors, and with very basic volumetric controls. More sophisticated devices recycled ultrafiltrate by passage through sorbent cartridges22,23. However these pioneering attempts were very far from the development of truly wearable devices resulting in unacceptably large and heavy devices with insufficient clearance rates. Only recently with the development of miniature pumps, it has been possible to make the ideas of these early pioneers flourishing into potentially effective wearable haemodialysis and/or haemofiltration devices.
RECENT APPROACHES AND CLINICAL RESULTS
Based on the studies of the past and relying on the technology of today, new WAK devices have been developed.
We have recently reported our experience with both a wearable ultrafiltration device22, and also a wearable artificial kidney23. In both trials patients could walk and move around independently whilst being treated. In one of the studies patients walked out from the hospital, into a neighbouring park, whilst being treated. Therefore, the feasibility of the concept was proven in these pilot trials showing that this is a potential pathway for the future. In a recent conference on wearable and miniaturized devices held in Vicenza in October 2010, a series of experiences has been reported including our own approach.
The WUF
The number of patients with symptomatic congestive heart failure continues to increase in North American and Europe. As cardiac output falls, the natural compensatory response to arterial underfilling is an increase neuro-hormonal activation, which paradoxically can lead to further reduction in cardiac output, compromising renal and gut blood flow31. This may result in deteriorating renal function, and diuretic resistance. Peritoneal and hemodialysis have therefore been advocated as useful adjunctive treatments in severe cases of congestive heart failure, and other fluid retention states, refractory to diuretic therapy32. In the early 1980s Kramer, and colleagues described a simple ultrafiltration device, designed to remove fluid from fluid overloaded intensive care patients33. This required arterial access, which drove the flow through the hemofilter by hydrostatic pressure, leading to ultrafiltration. However it took more than 25 years before specific devices designed for ultrafiltration of patients with refractory heart failure were available34. However, this machine was designed to be used intermittently in hospitalized bed-bound inpatients. To create a truly wearable device, that would allow patients to ambulate, whilst being treated, and so offer the possibility of outpatient therapy, other workers pursued Kramer’s original concept. To allow mobility, patients required a dual lumen central venous access catheter, coupled to a miniaturized blood pump, with accurate battery powered mini-pumps to regulate the ultrafiltration flow, and a heparin infusion for anticoagulation35. A standard commercially available high flux polysulfone hemofilter (Medica, Medolla, Italy) was strapped to a belt, which was worn around the waist. The total weight of the device was 2.5 lbs (1.135 kg). The first human study using this wearable hemofiltration device as a continuous ambulatory ultrafiltration device was recently reported22, with six volume overloaded patients treated for 6 hours (figure 1). Blood flow through the device was around 116 ml/min with an ultrafiltration rate ranging from 120 to 288 ml/hr, leading to an average of 151 mmol of sodium removed during the treatment. More importantly during the study, all patients maintained cardiovascular stability. As one of the main problems with traditional intermittent hemodialysis treatments, is intradialytic hypotension, which may cause further ischemic renal injury36. Thus, this device by being designed to operate continuously, can remove fluid at a slower hourly rate, compared to standard intermittent hemodialysis, such that refilling of the plasma volume from the extravascular spaces can be maintained, so avoiding episodes of cardiovascular instability. By returning heart failure patients back to the summit of their Starling curve, cardiac output improves, with a reduction in arterial underfilling, so patients become diuretic responsive once again. Potentially, development of this device would allow patients with symptomatic congestive heart failure to be managed as outpatients, or day ward attendees. An evolution of this concept has been presented by the Vicenza group in a recent conference: the project has been called WAKMAN. The system is based on a miniaturized circuit for ultrafiltration mounted on a specific vest that includes a filter/pump unit, safety controls, a remote control unit, fluid disposal bags and long lasting batteries (figure 2). This system can operate for 8 to 24 hours and it can be easily worn by the patient A small dual lumen catheter is connected to the circuit and a blood flow between 50 and 80 ml/min is generally obtained. An ultrafiltration rate between 2 and 10 ml/min can be achieved making the prescription for fluid removal flexible and personalized according to patient needs.
The WAK
Only recently, has a study been published of a truly wearable haemodialysis device23. In this preliminary trial, the device was worn by eight chronic haemodialysis patients, who were treated for times varying between 4 and 8 hours. The device was worn on a belt around the waist, and weighed around 5 kg. The blood and other pumps were powered by standard batteries. Fluid removal was accurately controlled by an ultrafiltration pump, and as with a conventional haemodialysis machine, there were safety features to stop blood flow in case or air entry, or disconnection. The device was connected using the patient’s standard vascular access, so via fistula needles in some patients and by central venous access catheter in others. In one case, the arterial needle became disconnected, whereas the blood pump in a conventional haemodialysis machine, would have continued to pump, with this device, the arterial disconnect was sensed, and the blood pump stopped. This allowed almost immediate re-insertion of the dialysis needle, with no significant loss of blood, and the treatment promptly resumed. In two cases, as the heparin infusion was reduced, prior to the planned termination of treatment, clotting occurred. Thus, as with standard intermittent haemodialysis, adequate anticoagulation is required for this device.
The dialysate was continuously regenerated by passage through three sorbent canisters, containing urease, activated charcoal, and both hydroxyl zirconium oxide and zirconium phosphate. Dialysate was regularly tested for ammonia, to ensure that the canisters had not become saturated. Similarly the dialysate was tested to ensure sterility.
Blood and dialysate flows were much slower, than traditional thrice weekly intermittent haemodialysis, being 59 and 47 ml/min, respectively. As expected, minute by minute small solute clearances were also much lower than those of intermittent haemodialysis, with an average whole blood urea clearance of 23 ml/min and creatinine clearance of 21 ml/min respectively. Although these minute by minute clearances are low, and similar to those achieved by continuous arterio-venous dialysis circuits in the intensive care setting37, the device was designed to be worn for protracted periods. If this wearable haemodialysis device could be worn daily, then this would potentially provide an estimated urea clearance (Kt/V) of almost 6.0, well above that for conventional thrice weekly intermittent haemodialysis.
In addition to urea and creatinine clearances, beta-2 microglobulin clearance was also assessed. Beta-2 microglobulin clearance was some 50% of that of urea and 55% for creatinine, respectively. Recent re-evaluation of the HEMO study, has suggested the importance of beta-2 microglobulin clearance in predicting patient survival38. As the adequacy of haemodialysis therapy, is not simply a matter of small solute clearance. The beta-2 microglobulin clearances observed would suggest that the relative clearances for so called «middle molecules» is somewhat greater than that for conventional intermittent haemodialysis. This is probably due to a degree of internal haemodiafiltration within the dialyzer, due to the pressures generated by the blood pump. The blood pump differed in design to that of a conventional haemodialysis machine blood pump, in that instead of being a roller pump, which occluded the dialysate tubing, this blood pump comprised two chambers, one for blood and one for dialysate. Such that when the blood chamber was full the dialysate chamber was empty and vice versa. This resulted in a different pattern of pulsatility and pressure generation in terms of blood and dialysate flows through the dialyzer compared to standard dialysis, resulting in increased internal haemodiafiltration.
Obviously, this device is in its early stages of development, and clearances could potentially be improved by redesigning the blood pump, to increase the volume of blood pumped, or similarly by increasing the flows.
The ViWAK
Recently, the structure and operational characteristics of a new wearable system for continuous peritoneal dialysis for CKD patients have been described in a paper39 and called Vicenza wearable artificial kidney (ViWAK).
The ViWAK system consists of a double lumen peritoneal catheter; a dialysate outflow line; a miniaturized rotary pump; a circuit for dialysate regeneration featuring a water proof container with cartridges connected in parallel with a mixture of activated carbon and and polystyrenic resins; a filter for deaeration and micro-biological safety; a dialysate inflow line; a palm computer as a remote control.
The system has been tested circulating 12 liters of exhausted PD solution through the experimental adsorption unit at the rate of 20 ml/min. Creatinine, beta-2 microglobulin and angiogenin were measured before and after the adsorption unit at baseline, and after 4 and 10 hours of use.
The cartridges containing polystirenic resin completely removed beta-2 microglobulin and angiogenin from the fluid batch. Those with the ion exchange resin removed completely urea and creatinine. The final result was 11.2 liters of net solute clearance.
The system is designed to be used as follows: The peritoneal cavity is loaded in the morning with 2 liters of fresh PD solution. After 2 hours, when dialysate/plasma equilibration at approximately 50% has occurred, recirculation is activated for 10 hours at the rate of 20 ml/min. After this period, recirculation stops and glucose is optionally added to the peritoneal cavity to achieve ultrafiltration if needed. After 2 hours the fluid is drained and a 2 liters icodextrin exchange is performed overnight to achieve further ultrafiltration. The clearance provided by the minicycler is further increased by the 2 liter exchange and the overnight exchange.
Therefore, the system operates 24h/day and provides creatinine and beta-2 microglobulin clearance in the range of 15-16 l/day, corresponding to a weekly clearance of 100-110 liters40.
The patient reduces the number of exchanges compared to CAPD and uses less fluid then in APD. Furthermore, the palm pilot allows for prescription and assessment of the therapy providing information on of cartridge saturation, flow and pressure conditions and offering the possibility of remote wireless control of operations.
Some problems still remain to be solved in the present configuration including the addition of an injection system for glucose and bicerbonate when needed, a system to reduce fibrin delivery to the sorbent and finally a more complex misture of sorbents to make sure a complete removal of small molecules including urea is achieved.
In conclusion, the wearable peritoneal dialysis system may become a possible alternative to APD or CAPD reducing the time dedicated to perform exchanges and improving peritoneal dialysis adequacy and patient’s rehabilitation.
THE CARPEDIEM PROJECT
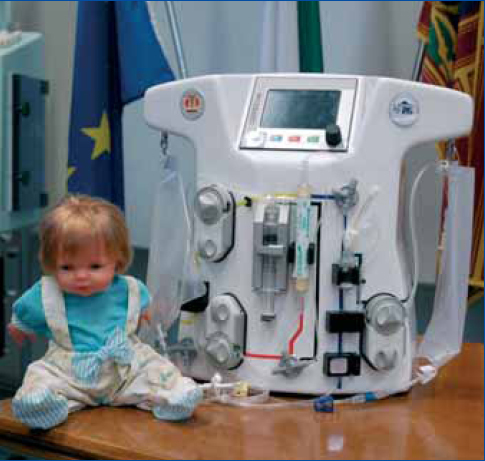
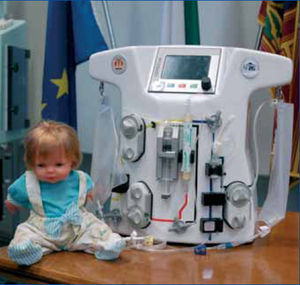
CARPEDIEM stays for Cardio Renal Pediatric Dialysis Emergency Machine and it is indeed a newly conceived hemofiltratio/dialysis equipment miniaturized to a point that it is perfectly suitable for an accurate and safe renal replacement therapy in newborns and children with a body weight ranging from 2 to 10 Kg (figure 3). The system operates with a hemofiltration circuit featuring a very low priming volume (15 ml for the whole circuit including the hemofilter). Low blood flows (20 to 80 ml/min) and very low ultrafiltration rates (UFR = 1-8 ml/min) are performed with an accuracy of fluid balance of 0.1 ml/min. Dedicated technology has been developed for this purposes. This is a typical example of a side result in the research on miniaturization and wearable devices. Nanotechnology, micromechanics and microfluidics should be the sciences of the future to help further development of this field in the years to come.
CONCLUSIONS
New systems for blood purification must fulfil specific requirements: they must be affordable such to allow a large application even in financially limited environments; they must be simple in order to allow for an easy and effective self care; they must be portable or wearable in order to permit patient’s mobility and rehabilitation. Once a system has these characteristics, the advantages of a continuous regime of treatment becomes evident in terms of efficiency and tolerance. Recent experiments show that the technology of a wearable artificial kidney is becoming a reality although many improvements may still be required. Technical challenges should be overcome by research and innovation. This approach will help to develop new strategies also in other areas such as pediatric dialysis and the treatment of the critically ill patient41,42. Miniaturization and non thrombogenic surfaces will probably represent the most important advances required for the immediate future. We are well aware of the many problems in this field, but scientific advances will probably solve the problems one by one. Even a trip of a thousand miles starts with a single step.
Figure 1. The first patient treated in Vicenza with the wearable ultrafiltration system.
Figure 2. WAKMAN project: a complete system for the ambulatory treatment of congestive heart failure.
Figure 3. The Cardio Renal pediatric Dialysis Emergency Machine (CARPEDIEM).