Haemodialysis (HD) patients are characterised by significant muscle loss. Recently, neuromuscular electrical stimulation (NMES) has emerged as a new therapeutic alternative to improve these patients’ physical condition. To date, no studies on the effects of NMES on body composition in HD patients have been published.
ObjectiveTo analyse the effect of NMES on muscle strength, functional capacity and body composition in our HD patients.
Material and methodsA 12-week, single-centre, prospective study. The patients were assigned to an electrical stimulation (ES) or control (CO) group. The ES group was subjected to intradialytic electrical stimulation of the quadriceps (Compex® Theta 500i), while the CO group received standard HD care. We analysed the following: (1) nutritional parameters; (2) muscle composition of the quadriceps; (3) maximum quadriceps extension strength (mes) and hand-grip (HG); (4) “sit to stand to sit” (STS10) and “six-minute walking test” (6MWT); (5) body composition (bioelectrical impedance analysis).
ResultsOf 20 patients, 55% were men. Mean age 67.7 years, 30.3 months in HD. Main aetiology: DM (35%). In the ES group were 13 patients, and 7 in the CO group. At the end of the study, an improvement was only observed in the ES group (*p<0.05): MES* (11.7±7.1 vs. 13.4±7.4kg), STS10 (39.3±15.5 vs. 35.8±13.7s) and 6MWT* (9.9%, 293.2 vs. 325.2m). Furthermore, increased quadriceps muscle area (QMA*: 128.6±30.2 vs. 144.6±22.4cm2) and lowered quadriceps fat area (QFA*: 76.5±26.9 vs. 62.1±20.1cm2) were observed. No relevant changes in body composition, nutritional parameters and dialysis adequacy were found.
Conclusions(1) NMES improved muscle strength, functional capacity and quadriceps muscle composition in our patients. (2) Based on the results obtained, NMES could be a new therapeutic alternative to prevent muscle atrophy and progressive physical deterioration. (3) However, future studies are necessary to establish the potential beneficial effects of NMES in HD patients.
Los pacientes en hemodiálisis (HD) se caracterizan por una gran pérdida muscular. Recientemente, la electroestimulación neuromuscular (EENM) constituye una nueva alternativa terapéutica para mejorar la condición física de estos pacientes. No existen estudios acerca de la EENM sobre la composición corporal en HD.
ObjetivoAnalizar el efecto de la EENM sobre la fuerza muscular, capacidad funcional y composición corporal en nuestros pacientes en HD.
Material y métodosEstudio prospectivo unicéntrico (12 semanas). Los pacientes fueron asignados a grupo electroestimulación (EM) o control (CO). El grupo EM incluía un programa de electroestimulación cuadricipital intradiálisis (Compex® Theta 500i). El grupo C recibió cuidado habitual en HD. Analizamos: 1) parámetros nutricionales; 2) composición muscular del cuádriceps; 3) fuerza de extensión máxima del cuádriceps (FEMQ) y handgrip (HG); 4) sit to stand to sit (STS10), six-minutes walking test (6MWT) y 5) composición corporal (bioimpedancia eléctrica).
ResultadosDe un total de 20 pacientes, el 55% fueron hombres. Edad media: 67,7 años, con 30,3 meses en HD. Principal etiología: DM (35%). Hubo 13 pacientes en EM y 7 en el grupo CO. Al final del estudio, únicamente EM presentó mejoría en (*p<0,05): FEMQ* (11,7±7,1 vs. 13,4±7,4kg), STS10 (39,3±15,5 vs. 35,8±13,7s) y 6MWT* (9,9%; 293,2 vs. 325,2m). Igualmente, el grupo EM incrementó el área muscular (AMQ*: 128,6±30,2 vs. 144,6±22,4cm2) y disminuyó el área grasa cuadricipital (AGQ*: 76,5±26,9 vs. 62,1±20,1cm2). No se observaron cambios relevantes en el resto de la composición corporal, parámetros nutricionales ni adecuación dialítica.
Conclusiones1) La EENM mejoró la fuerza muscular, la capacidad funcional y la composición muscular del cuádriceps de nuestros pacientes. 2) Con los resultados obtenidos, la EENM podría ser una nueva alternativa terapéutica para evitar la atrofia muscular y el deterioro progresivo de la condición física de estos pacientes. 3) No obstante, serían necesarios futuros estudios para establecer los potenciales efectos beneficiosos de la EENM en los pacientes en HD.
Hemodialysis (HD) patients have a decrease in physical activity and deterioration in quality of life. Muscle weakness and low impaired daily activity are in part caused by cardiovascular comorbidity, malnutrition, chronic anaemia and inflammation, alterations in mineral bone metabolism, as well as abnormal urea metabolism.1,2
Changes in urea metabolism involve an alteration of type II muscle fibres and nerve endings in the form of myopathy; and it effects on the neuronal myelin sheath. Eventually, muscular atrophy occurs and the patient has symptoms of fatigue, weakness, cramps or myoclonus.3–5
Therefore, one of the fundamental aspects of renal patient care should be physical rehabilitation to preserve functional capacity and autonomy.6,7
Several studies have reported the beneficial effects of physical exercise during HD sessions2 on functional capacity, psychological status and quality of life.8
Some patients are unable to perform physical exercise programmes in HD. Therefore, there is interest in the role of neuromuscular electrical stimulation (NMES) as an effective alternative to physical exercise in HD sessions; however studies published to date are few and limited.9–13 NMES consists in stimulating a group of muscles using a low intensity electric currents through electrodes applied to the body surface; muscles contracts, just as it would with normal muscle activity.14,15 In the healthy population, this is often used to improve of physical fitness and muscle strength. It is also applied for muscle rehabilitation mainly in populations with severe neurological or post-traumatic movement disorders.16,17
However, there is not enough evidence on the favourable effect of NMES on the impairment of muscle strength or composition in HD patients. The aim of the present study was to analyse the effect of an NMES programme on muscle strength, functional capacity and body composition in our HD patients.
Material and methodsFrom the beginning of September to the end of November 2013 (12 weeks), a prospective, single centre study was conducted, after being approved by the local Ethics Committee and in accordance with the Declaration of Helsinki guidelines. The purpose was to evaluate the effect of an exclusive NMES programme on muscle strength, functional capacity and body composition of our HD patients.
The inclusion criteria was: signed informed consent, 18 years of age or older, on regular HD for more than 3 months at our site, and clinical and haemodynamic stability during the last 3 months. The exclusion criteria was: a recent cardiovascular event, presence of vascular access for HD in the lower limbs, on a pacemaker, unable to perform body composition analysis in the lower limbs, and refusal to sign informed consent.
Our patients are distributed into 6 groups of 10–12 patients each. All patients have a pre-established fixed number. These patient undergo a 4h sessions on alternate days (M–W–F or T–T–S), in morning, noon, and afternoon shifts. Our nursing staff performed the exclusive NMES programme and assessment of body composition. Since implementation of this additional activities entailed an increase in the daily care burden, patients on the midday schedule were excluded.
There were two groups of patients. Patients from the morning and afternoon shifts with even numbers in the fixed list were included in the control group (CO), and they received routine care. Patients with odd numbers from the morning and afternoon shifts received the electrical stimulation (EMS). The patients were asked to continue their regular daily physical activity, without prescribing any additional physical exercise programme.
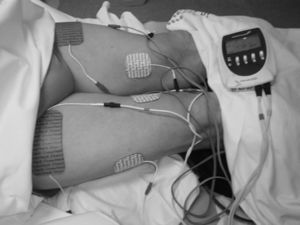
Neuromuscular electrical stimulationPatients assigned to the EMS group performed an NMES programme of the quadriceps muscles of both lower limbs as previously agreed with our the Rehabilitation Department. The device used was the Compex® Rehab Theta 500i model, equipped with various rehabilitation exercise programmes with different phases, types and current intensity. The electrical stimulation programme included (total time, intensity, contraction–relaxation phase time): a toning programme in the first week (25min, 8Hz, contraction 1.5s, phase 25min, relaxation 1.5s); one week of aerobic endurance (28min, 60Hz, contraction 1.5s, phase 8s, relaxation 0.75s); 2 weeks of rehabilitation–amyotrophy (30min, 25–40Hz, contraction 2s, phase 4s, relaxation 1s); 2 weeks of rehabilitation–hypertrophy (33min, 55Hz, contraction 1.5s, phase 6s, relaxation 1s); 3 weeks of muscle strengthening (35min, 9 peaks: 2–75Hz, phase 7s, relaxation 1.5s) and finally 3 weeks of strength-endurance (38min, 90Hz, contraction 1.5s, phase 4s, relaxation 0.75s). This was performed during the first 2 hours of each HD session, with a mean duration of 30–45min. Patients were in their usual supine HD position, with full extension of the lower limbs and minimal flexion (15°) of both knees resting over a soft pillow placed in the popliteal region. Each patient always had their own electrodes (5cm×10cm). These were placed just on the motor point of the muscle heads of the quadriceps (anterior rectus, internal and external vessel), guaranteeing maximum comfort and efficiency. When the patient noticed the passage of the electric pulse, they were asked to perform a voluntary contraction to achieve the maximum contraction of the muscle. The maximum intensity was achieved by encouraging the patient to resist the highest possible level of painless stimulation energy to achieve tolerable and effective muscle contraction (Fig. 1).
Neuromuscular electrical stimulation (EMS) of the quadriceps muscles of both lower limbs. Electrodes on the motor point of the muscle heads of the quadriceps (anterior rectus, internal and external vessel). Patients in supine position, with full extension of the lower limbs and minimal flexion (15°) of both knees by means of a soft pillow placed in the popliteal region.
Coinciding with the usual quarterly medical follow-up visits on days that patients did not have HD, the following variables were analysed both at baseline and at the end of the study.
Demographic, biochemical data, nutritional and anthropometric parametersDemographic variables included age, sex, renal aetiology, Charlson's comorbidity index and length of time in HD. Similarly, the main biochemical data on HD, nutritional parameters (albumin, prealbumin, triglycerides, total cholesterol and its fractions, ferritin and C-reactive protein) and HD adjustment (Kt/V by 2nd generation Daugirdas method, interdialytic weight gain) were collected.
The total (QTA), muscle (QMA) and fat (QFA) areas of both quadriceps were obtained through the Gurney and Jelliffe anthropometric formulas18: QTA=[Muscle contour (cm)]2/4π; QMA=[(Muscle contour (cm)−π×Muscle skin fold (cm)2]/4π; AGC=QTA−QMA. Muscle contour was estimated in its reference anatomical position with a flexible and inextensible tape measure, and expressed in centimetres, without squeezing the area's soft tissues. The skin fold of both quadriceps was used for subcutaneous adipose tissue assessment. Callipers were used to estimate the skin fold thickness, i.e., a double layer of skin and underlying adipose tissue, always avoiding to include the muscle at the mid-longitudinal point of the line joining the inguinal fold and the proximal edge of the kneecap, on the anterior side of the thigh, with the patient's feet on the floor and forming an angle of 90° with the knees.19
Muscle strength and functional capacityA Jamar type standardised dynamometer (Hand-grip dynamometer) (HG) was used in the dominant arm to assess upper limb muscle strength (SH 5001, Seahan Corporation, Korea). This was performed with the subject in standing position with the arms extended along the body. The dynamometer was given to them in both arms, telling them to exert the greatest possible force without resting the arm on their body. The arm with the greatest strength was considered as the dominant arm.20
A Kern-type standardised traction dynamometer (Kern CH50 50KG dynamometer) was used to assess muscle strength of the lower limbs. The maximal quadriceps muscles extension strength (MQES) of the left leg was estimated. The patient remained seated in a fixed chair so the back was supported on the backrest with the hip and knee at 90°. In this position an inextensible strap was placed at the level of the distal third of the tibia and the subject was asked to exert the greatest possible force to perform the extension of the limb without holding the chair with his/her arms.21
The values of both the anthropometric and muscular strength variables were the mean of 3 consecutive measurements obtained by the same investigator to minimise errors.
The tests used to assess functional capacity were the 6-minute walk test (6MWT) and the sit-to-stand-to-sit test 10 (STS10). The 6MWT was performed with monitoring vital signs and the oxygen saturation by pulse oximetry. It consisted on measuring the maximum distance covered during a 6min period at an active pace, along a 20m corridor near the HD unit. At the end of the test period, the total distance travelled was recorded by means of a standardised odometer.22 The STS10 test consisted in getting up and sitting down 10 consecutive times as quickly as possible, starting from a sitting position with the arms close to the chest from a 44.5cm high and 38cm deep chair against the wall to avoid the risk of falls. The time in seconds it took to perform the exercise was recorded.23
Body composition analysis (electrical bioimpedance)Body composition analysis was performed by electrical bioimpedance using the OMRON BF-400 standardised device (Omron Healthcare UK LTD, Japan). It was performed with the subject barefoot, in standing uniform support and completely static, with both arms close to the body, on the device platform 15 minutes after finishing the third weekly session of HD. A second measurement was repeated, under the same conditions, 5min later, the mean was the value reported. The following parameters were analysed: total weight (TW), fat weight (FW), lean weight (LW), total body water (TBW) and abdominal fat percentage (AF%). FW and LW were estimated using the Hume formula24: FW=[TW (kg)×AF%)]/100; LW=TW (kg)−FW. TBW was calculated using the estimated Watson formula.25
Statistical analysis was performed with SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed by mean and standard deviation. Qualitative variables were expressed in percentage. Comparison of the quantitative data from the same group at the end of the study was performed using the Wilcoxon test for non-parametric related variables and the qualitative data using the McNemar test, considering statistically significant the ratios with a value of p≤0.05.
ResultsWe evaluated 41 out of the 63 patients of our dialysis unit, all were from morning and afternoon shifts. Nineteen patients were excluded. The main reasons for exclusion were: physical impossible to perform the body composition analysis in the lower limbs (5), hospitalisation (4), not giving informed consent (3), less than 3 months on our HD unit (3), vascular prostheses in lower limbs (2) and having a pacemaker (2). A total of 2 patients dropped out during the study (one death due to abdominal sepsis, one kidney transplant). Thus 20 patients completed the study. Of these, 13 were assigned to the EMS group, and 7 to the CO group. None of the patients had any relevant NMES-related adverse effects (lower limb pain, burning, or cramps) or haemodynamic instability episodes. 55% were men, with a mean age of 67.7±12.5 years and a mean HD vintage of 30.3±22.3 months. The mean Charlson index was 8.8±2.5. The etiologies of chronic kidney failure were: diabetes mellitus (35%), glomerular disease (15%), chronic pyelonephritis (15%), kidney disease of unknown origin (15%), hypertension (10%), polycystic kidney disease (5%) and others (5%).
No significant differences were found between the study groups in terms of demographic data, comorbidity and main aetiology of kidney disease at study baseline (Table 1). The data of muscle composition of the quadriceps are shown in Table 2. A significant increase in the QMA was observed only in the EMS group (128.6±30.3 vs. 144.6±22.4cm2; p 0.032). Also in EMS patients there was a significant decrease in the average value of QFA (76.5±26.9 vs. 62.1±20.1cm2; p<0.024). No significant changes were observed in QTA in any study group. The main biochemical data and dialysis parameters are shown in Table 3. No significant differences were observed in main nutritional biochemical parameters and the dialysis parameters. Medical treatment was not modified during the study, the mean dose of erythropoietic agents in EMS and CO groups were 25.6±11.6 vs. 26.2±12.4mcg darbopoietin/week and the treatment with native vitamin D (calcifediol 0.266mg/month) prescribed in any of our patients throughout the study was: 38.2 vs. 36.8% in EMS and CO groups, respectively.
Main demographic data.
| EMS group | CO group | p value | |
|---|---|---|---|
| Age (years) | 65.7 (12.8) | 71.6 (12.1) | 0.311 |
| Time in HD (months) | 33.9 (24.7) | 27.1 (21.5) | 0.466 |
| Gender, % (male) | 69.2 | 41.7 | 0.471 |
| Charlson index | 9.1 (2.3) | 9 (2.2) | 0.934 |
| HBP, % | 7.7 | 14.3 | 0.664 |
| DM, % | 38.5 | 28.6 | 0.762 |
| GLOM, % | 15.4 | 14.3 | 0.828 |
| BMI | 26.9 (3.5) | 25.2 (3.2) | 0.605 |
EMS (n=13) and CO (n=7) groups at study baseline.
Results expressed in mean (standard deviation) and percentage.
DM: diabetes mellitus; GLOM: glomerular disease; HBP: high blood pressure; BMI: body mass index; Stat. sign.: statistical significance.
Statistical significance: p<0.05.
Main muscular anthropometric data of the quadriceps.
| EMS group | CO group | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| Quadriceps total area, cm2 | ||||
| Quadriceps R | 205.6 (38.3) | 197.1 (33.8) | 179.6 (27.8) | 189.5 (38.4) |
| Quadriceps L | 204.8 (41.3) | 204.3 (37.1) | 179.7 (22.9) | 185.5 (30.9) |
| Mean QTA | 205.2 (39.1) | 200.7 (34.7) | 179.7 (24.9) | 187.7 (34.5) |
| Quadriceps muscle area, cm2 | ||||
| Quadriceps R | 129.2 (28.9) | 142.2 (19.8) | 84.3 (20.8) | 93.8 (32.1) |
| Quadriceps L | 128.1 (33.7) | 146.1 (28.6)* | 88.8 (18.4) | 92.7 (27.1) |
| Mean QMA | 128.6 (30.3)* | 144.6 (22.4)* | 86.5 (19.2) | 93.3 (29.4) |
| Quadriceps R | 76.2 (26.2) | 59.9 (21.2)* | 95.4 (13.2) | 95.6 (12.9) |
| Quadriceps L | 76.7 (29.1) | 64.2 (20.4) | 90.8 (10.1) | 93.2 (12.3) |
| Mean QFA | 76.5 (26.9) | 62.1 (20.1)* | 93.1 (11.3) | 94.4 (12.3) |
EMS (n=13) and CO (n=7) groups.
Baseline vs. end of study. Results expressed: mean (standard deviation) and percentage.
QFA: quadriceps fatty area; QMA: quadriceps muscle area; QTA: quadriceps total area; CO: control; R: right; EMS: electrical stimulation; L: left.
Statistical significance: *p<0.05, comparison of baseline vs. final means.
Main biochemical data, nutritional parameters and dialysis adjustment.
| EMS group | CO group | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| Glucose, mg/dL | 153.2 (51.6) | 155.8 (62.4) | 152.2 (32.1) | 155.4 (36.1) |
| Creatinine, mg/dL | 8.1 (2.3) | 8.2 (3.1) | 7.8 (2.3) | 7.8 (2.6) |
| K, mEq/L | 5.2 (0.8) | 5.5 (1.1) | 4.9 (0.2) | 5.2 (0.7) |
| Ca, mg/dL | 9.2 (0.4) | 9.2 (0.8) | 9.2 (0.4) | 9.3 (0.8) |
| P, mg/dL | 4.4 (1.2) | 4.3 (1.2) | 4.5 (1.4) | 4.2 (1.3) |
| i-PTH, pg/mL | 245.6 (189.6) | 262.3 (196.2) | 274.9 (110.7) | 238.6 (183.2) |
| 25-OH vitD, ng/mL | 23.3 (8.1) | 27.5 (13.1) | 28.2 (13.5) | 27.1 (7.2) |
| Albumin, g/dL | 3.7 (0.3) | 3.7 (0.2) | 3.8 (0.2) | 3.9 (0.2) |
| Prealbumin, mg/dL | 28.5 (10.5) | 27.1 (4.8) | 30.2 (8.9) | 33.1 (9.5) |
| Total cholesterol, mg/dL | 143.2 (57.2) | 148.1 (59.1) | 153.2 (34.4) | 149.9 (34.8) |
| HDL Cholesterol, mg/dL | 42.6 (11.1) | 41.2 (11.9) | 42.7 (10.3) | 41.2 (43.6) |
| LDL Cholesterol, mg/dL | 68.7 (42.3) | 63.4 (49.2) | 67.2 (65.4) | 65.9 (33.7) |
| Triglycerides, mg/dL | 154.1 (76.2) | 158.6 (73.6) | 158.3 (67.6) | 155.2 (73.4) |
| Blood count data | ||||
| Haemoglobin, g/dL | 10.4 (1.8) | 11.1 (0.9) | 10.5 (1.2) | 10.3 (0.7) |
| Ferritin, ng/mL | 401.6 (210.7) | 433.2 (267.8) | 434.1 (204.9) | 421.9 (300.1) |
| Hs-PCR, ng/L | 7.6 (1.5) | 8.2 (1.7) | 8.1 (1.8) | 8.3 (1.2) |
| Dialysis parameters | ||||
| SBP, mmHg | 155 (22.6) | 153 (20.2) | 157 (23.4) | 155 (19.8) |
| DBP, mmHg | 88.9 (12.6) | 87.2 (13.4) | 84.9 (13.2) | 81.4 (13.6) |
| Theoretical weight, kg | 71.9 (2.6) | 71.5 (2.3) | 69.5 (3.4) | 69.8 (3.5) |
| Interdialytic weight, kg | 2.6 (1.2) | 2.7 (1.4) | 2.5 (1.3) | 2.8 (1.5) |
| Dialysis dose, Kt/V | 1.63 (0.4) | 1.62 (0.7) | 1.63 (0.5) | 1.65 (0.6) |
EMS (n=13) and CO (n=7) groups. Baseline vs. end of study. Results expressed: mean (standard deviation) and percentage.
Ca: calcium; CO: control; Kt/V: Daugirdas 2nd generation method; EMS: electrical stimulation; Hs-PCR: C-reactive protein; i-PTH: intact parathyroid hormone; K: potassium; P: phosphorus; DBP: diastolic blood pressure; SBP: systolic blood pressure; VitD: vitamin D.
No significant differences were found (baseline vs. final) between the groups studied.
Table 4 shows the results on muscle strength and functional capacity. We did not observe significant changes in muscle strength through the HG in any of groups at the end of the study. In contrast, it was observed a significant improvement in the MQES of the lower extremities in the EMS group (MQES 11.7±7.1 vs. 13.4±7.4kg, p 0.002), while in the CO group we did not observe significant changes at the end of the study (MQES 11.4±6.6 vs. 11.2±5.9kg, p 0.773).
Assessment of muscle strength and functional capacity.
| EMS group | CO group | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| HG, kg | 21.2 (9.3) | 21.6 (9.8) | 27.8±4.7 | 27.5±6.3 |
| QMES, kg | 11.7 (7.1) | 13.4 (7.4)* | 11.4±6.6 | 11.2±5.9 |
| 6MWT, m | 293.2 (163.9) | 325.2 (176.4)* | 348.7±93.4 | 354.3±80.4 |
| STS10, s | 39.3 (15.5) | 35.8 (13.7) | 38.1±13.3 | 39.1±13.2 |
EMS (n=13) and CO (n=7) groups. Baseline vs. end of study. Results expressed: mean (standard deviation) and percentage.
6MWT: 6min walk test; CO: control; EMS: electrical stimulation; MQES: quadriceps maximum extension strength; HG: hand grip, dominant arm; m: metres; s: seconds; STS10: sit to stand to sit test 10.
Statistical significance: *p<0.05, comparison of baseline vs. final means.
In the 6MWT, we observed a 9.9% increase in the distance covered at the end of the study in the EMS group (293.2±163.9 vs. 325.2±176.4m, p 0.018), whereas no changes were observed for the CO group (348.7±93.4 vs. 354.3±80.4m; p=0.753). In the STS10 test, a shorter performance time was obtained in the EMS group (39.3±15.5 vs. 35.8±13.7s; p 0.310) at the end of the study, although these differences did not reach the pre-established statistical significance. In contrast, in the CO the time used to complete the test was longer but the difference was not statistically different (38.1±13.3 vs. 39.2±13.3s; p 0.299).
Regarding the body composition no significant differences were found between the study groups. In the EMS group at the end of the study, there was a moderate increase in LW (56.2±10.9 vs. 57.8kg±11.6, p 0.247), as well as a certain decrease in FW (15.4±7.8 vs. 13.9kg±7.7; p 0.278), in the percentage of abdominal fat (21.3 vs. 19.5%; p 0.262) and in TBW (36.4±4.9 vs. 35.5±6.3 l; p 0.534) (Table 5).
Assessment of body composition by electrical bioimpedance.
| EMS group | CO group | |||
|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |
| TBW, L | 36.4 (4.9) | 35.5 (6.3) | 34.1 (4.8) | 34.4 (5.2) |
| Total weight, kg | 71.6 (9.8) | 71.7 (10.5) | 69.9 (11.1) | 69.7 (11.2) |
| Fat weight, kg | 15.4 (7.8) | 13.9 (7.7) | 18.5 (12.1) | 21.9 (15.1) |
| Lean weight, kg | 56.2 (10.9) | 57.8 (11.6) | 51.4 (15.4) | 47.8 (18.1) |
| Abdominal fat, % | 21.3 | 19.5 | 31.9 | 31.7 |
EMS (n=13) and CO (n=7) groups. Baseline vs. end of study. Results expressed: mean (standard deviation) and percentage.
TBW: total body water; EMS: electrical stimulation; CO: control; kg: kilograms.
No significant differences were found (baseline vs. final) between the groups studied.
Patients on HD are characterised by a decrease in the physical activity and a deterioration in quality of life. High cardiovascular comorbidity, malnutrition, chronic anaemia and inflammation, HD-related sedentary lifestyle, as well as the abnormalities proper to urea metabolism could be, among others, some of the various factors that will lead to a marked muscle weakness and functional impotence throughout their permanence in HD.1,2
Metabolic abnormalities associated to uraemia mainly affect type II muscle fibres above all, and musculoskeletal nerve endings in the form of myopathy and affect the neuronal myelin sheath, that will eventually lead to significant muscular atrophy with symptoms as diverse as fatigue, weakness, cramps, or myoclonus.3–5
Some of the different strategies used in the prevention and treatment of muscle loss in these patients have been the correction of metabolic acidosis with bicarbonate supplements, the use of anabolic hormones, adequate insulin regulation and physical exercise in HD.8,26–28 An effective therapeutic alternative that may reduce the progression of muscle deterioration is not yet available. One of the fundamental aspects in the care of renal patients should be adequate physical rehabilitation to preserve their functional capacity and autonomy.6,7
The role of NMES as an alternative therapy to physical exercise during HD sessions has received considerable interest. In some cases, due to its clinical characteristics and the associated comorbidity, patients are unable to perform physical exercise programmes. The few studies published in the literature regarding the role of NMES, mainly in patients with chronic heart failure or lung disease, show favourable effects on muscle composition and functional capacity.29–31 In addition, they appear to be easy to apply and safe without serious complications.14,16,32
After a thorough review of recent literature, we found only small studies published in relation to NMES in renal patients. The first study comparing the effects of NMES with the classic effects of physical exercise was conducted by Dobsak et al.9; this was a randomised, 20-week study with 3 comparative groups (physical exercise, NMES and control), both with an exclusive NMES programme and with an aerobic physical exercise programme using cycloergometers. Patients (n=32) in HD were able to improve muscle strength in lower limbs estimated by dynamometry, functional capacity (6MWT), quality of life (SF-36 health questionnaire) as well as dialysis parameters (Kt/V, urea reduction rate) with respect to a third control group without intervention. No significant differences were observed between the 2 exercise groups (exercise vs. NMSE) in the different aspects studied.
Similarly, Farese et al.,10 evaluated the effect of NMES and physical exercise on blood pressure control and dialysis parameters. Nine patients were randomly assigned to 3 different protocols. During 9 consecutive HD sessions (3 weeks), each group underwent, on a different weekday, either a physical exercise programme using cycloergometers or a lower limb NMES programme or did not perform an intervention. The authors observed a significant increase in blood pressure and higher urea and phosphorus levels coming out in the dialysed fluid in those sessions in which patients performed NMES and physical exercise as compared to the sessions without intervention. No relevant changes were observed in plasma concentrations of these solutes or in dialysis parameters (Kt/V, urea reduction rate). The parameters of muscle strength, functional capacity and quality of life were not evaluated.
In Spain, it is worth to comment on the only 3 published studies on NMES associated with exercise. Our group obtained similar results to the mentioned in terms of muscle strength, functional capacity and quality of life after an exclusive 12-week NMES programme in 38 HD.11 Another study of 12 weeks duration, NMES associated with mainly aerobic physical exercise using cycloergometers in 11 patients in HD shows favourable results on safety, efficacy and tolerability of NMES during the HD sessions.12 Similarly, Contreras Martos et al.,13 in a group of 11 HD patients, also showed positive results after a 5-week strength-resistance programme associated with NMES of both quadriceps during HD sessions.
In the present study, we have observed an improvement in muscle strength and functional capacity in our HD patients after an exclusive NMES programme. These results can be basically superimposed to those obtained published studies; however, our study adds data of interest in relation to changes in muscle composition after the NMES programme. Our results are similar to those obtained by Dobsak et al.,9 in a population with similar demographic characteristics, through the evaluation of identical muscle strength and functional capacity tests; although in our study only both quadriceps were stimulated and it did not include stimulation of the calves. The present study did not evaluate parameters of quality of life; we, in a previous study11 observed a significant improvement in health-related quality of life through the EuroQoL-5D standardised test. As an important difference with respect to the Dobsak et al.9 and Farese et al.10 studies, it should be mentioned that our study did not specifically analyse either the blood pressure control or dialysis parameters nor the elimination of various solutes, so we cannot provide results on these matters. Nevertheless, we did not observe significant changes in the control of blood pressure, biochemical parameters, or routine parameters of dialysis adequacy. In our modest opinion, it would very unlikely to observe any changes in these parameters.
The multiple beneficial effects of physical exercise at cardiovascular, psychological, muscular or skeletal level have been widely described. At muscular level, there is an increase in strength, resistance and size of the group of muscles being exercised, this is accompanied of t changes in the body composition in the form of decrease of abdominal fat, increase of lean mass and muscle tissue, decrease of skin folds or increased muscle diameter.33,34 This muscular adaptation to physical exercise and the changes in body composition have also been described after the use of NMES globally.35,36 However, NMES is based on the application of low-frequency repetitive pulses through surface electrodes; this achieves immediate local activation and recruitment of small muscle fibres of the different muscle groups.14–16,32 Actually, this local muscle activation and recruitment could explain, in some patients with marked muscle atrophy and functional impotence, the significant improvement in muscle strength exclusively in both quadriceps (MQES) and the absence of changes in HG, a marker of upper limb muscle strength in elderly patients.7,20
Local application of NMES to certain groups of muscles, may also justify the absence of significant changes in the biochemical and nutritional parameters, as well as the absence of changes in body composition estimated by electrical bioimpedance. To produce significant changes in biochemical parameters and overall body composition it would be necessary the electrical stimulation of the upper limbs in combination with the rest of the abdominal musculature or electrical stimulation programmes combined with physical exercise, so a greater number of muscles are recruited with a more generalised effect.
The increase of muscle strength in both lower limbs was associated to a significant increase of the muscular area of the quadriceps, as well as in a significant decrease of fat. The mechanisms involved in this structural muscle change are: Increased oxygen supply to tissues, greater production of vascular endothelial growth factors (vascular endothelial growth factor), increased synthesis of some proteins related to muscle metabolism such as insulin growth factor-1 or the inhibition of myostatin, as well as the decrease in certain proinflammatory cytokines such as interferon γ or interleukin 6 caused by repeated and continuous electrical stimulationl.37–40
Despite these significant muscle findings, no significant changes were observed in the total area. These finding may be attributed, to the short duration of the specific programme of muscular hypertrophy (only 2 weeks), to the level of contraction intensity used throughout the study, as well as to the high muscle atrophy; however, we cannot either rule out errors in the measurements. Perhaps a longer-term NMES programme or perhaps the use of supplementary examinations not based on anthropometric muscle estimation formulas, such as ultrasound, computed tomography or muscle magnetic resonance imaging, intended to measure muscle and body composition changes, could more accurately detect total muscular anthropometric changes.
Both the 6MWT and the STS10 are functional tests widely used in clinical practice as indicators of quadriceps muscle strength.7,22,23 On the other hand, the quadriceps is the largest muscle in the lower limbs. Functionally, it is a powerful knee joint extender, hip flexor and knee stabiliser necessary for walking. Thus it participates in walking, running and jumping. The application of an NMES programme resulted in a significant increase in the distance covered in the walk test in the EMS group alone. This finding demonstrates the fundamental role of NMES in the strengthening of the lower limbs. Interestingly, we obtained no significant changes in the STS10 test; this could be attributed to the great variation of results obtained in the test, the difficulty in actually performing the STS10 in this particular type of patients in HD, as well as the limited number of patients. However, unlike the CO group, which used more time, the results of the EMS group showed shorter time to perform the test. This illustrates some extent, the favourable effect of the NMES programme on this functional test.
It is worth noting the efficacy, safety and the easy application and management of our NMSE programme during the HD sessions; we had no dropouts or unfavourable effects throughout the study.
Among the many limitations of our work, we should mention the absence of randomisation of the study groups, although this allocation was conditioned by the absence of external financing or additional resources, so it seemed reasonable, in the opinion of the authors, to assign the patients according to the daily nursing care burdens. Also noteworthy is the small sample size, which obliged the use of non-parametric tests, as well as the short duration of the NMES programme, although they are similar, in terms of number and duration, to those previously published. Unfortunately, we did not have more sophisticated methods (DEXA) in our unit for the evaluation of muscle composition. Thus there is still a need to perform better designed studies to establish the exclusive role of NMES in the long term and its potential beneficial effects in this type of patients.
In conclusion, intradialysis NMES of both quadriceps safely and effectively improved the muscle strength, functional capacity and muscle composition of our patients in HD. Pending future studies, NMES is a novel therapeutic alternative to improve the physical condition and muscle composition of these patients, especially in those where the performance of an intradialysis physical exercise programme is difficult or contraindicated.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To all the patients and nursing staff for their valuable collaboration in this research, inasmuch as they have made possible the development of the present work.
This research work was carried out within the framework of the Doctoral Programme in Medicine of the Universitat Autónoma de Barcelona [Barcelona Autonomous University] (UAB).
Please cite this article as: Esteve V, Carneiro J, Moreno F, Fulquet M, Garriga S, Pou M, et al. Efecto de la electroestimulación neuromuscular sobre la fuerza muscular, capacidad funcional y composición corporal en los pacientes en hemodiálisis. Nefrología. 2017;37:68–77.