In medicine, learning has been based, until now, on study and clinical practice. There is great concern for improving patient safety, reducing complications in invasive techniques, and reducing healthcare costs. This has led to the creation of simulators and experimental models for medical and surgical skills development in the teaching–learning process. Simulators have been widely introduced in surgical specialties. However, their use is not widespread in medical specialties using invasive techniques. This is the case for renal biopsy (RB), essential invasive technique in nephrology. It is a procedure that can result in patient morbidity and mortality and, although supervised by experienced physicians, is learnt on real patients.1
There are very few studies in the literature related purely to teaching RB.2–5 Mrug and Bissler2 performed RB simulation with ultrasound control on an ex vivo model, using pig or cow kidney inserted into a turkey.2 They obtained ultrasound images similar to those of real patients, and characteristics of needle penetration resistance comparable to those of a real model, in both muscular tissue and renal tissue. They also investigated3 the effect of the simulator on improving residents’ confidence at performing RB and on the rate of post-biopsy bleeding complications in the before and after the use of the simulator. They found a significant increase in trainee doctors’ self-assurance and a smaller reduction in haematocrit after the procedure.
That learning model is highly interesting and represents a great advance in training. However, its simulation of the RB technique is not entirely realistic: in real patients, the kidneys move with respiration; also, the model does not allow users to see the haemodynamic consequences of renal haemorrhage or to detect post-biopsy vascular complications.
We present a novel progressive learning method based on 2 simulation models – ex vivo and in vivo – designed for teaching RB without putting patients at risk.
Our project consisted of designing 2 anatomical simulators, inanimate and animate respectively, that nephrologists could use to learn the RB technique correctly as a step before performing the RB in patients. With the inanimate anatomical model, they could acquire dexterity and skill in performing real-time ultrasound-guided RB, and with the live animal model, which resembled as closely as possible the human kidney in the practice of ultrasound-guided RB, they could optimize their skills before performing RB on humans.
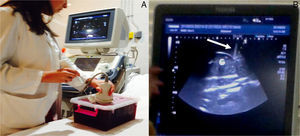
Ex vivo model: a commercially available silicon kidney (CAE Healthcare®, USA) that simulated very closely the renal ultrasound anatomy was submerged in a recipient filled with edible gelatin. The surface of the model was covered with latex to simulate the resistance of skin. We used a Xario SSA-660a ultrasound (Toshiba Medical Systems, Japan). Biopsy was performed using a real-time ultrasound-guided technique with a convex multifrequency probe (3.5–5mHz) (Fig. 1).
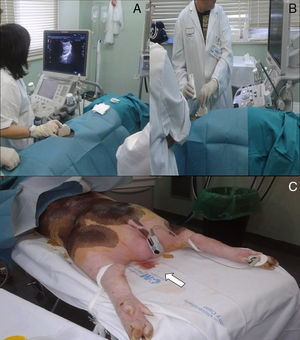
In vivo model: after obtaining the required regulatory approval for animal handling, the animal facilities of our hospital acquired a common piglet (40kg). The animal was anaesthetized and intubated by a veterinary in prone position, so that the performance of RB would be the same as in patients. With a sterile field, the inferior pole of the kidney was biopsied using the same technique and equipment as used in the inanimate model. To simulate patient apnoea, the respirator was stopped for 2–3s, during which the biopsy needle was shot. After the biopsy we performed, colour and pulsed Doppler ultrasound were performed to look for potential vascular complications from the technique (Fig. 2). Animal's vital signs were monitored throughout the procedure.
An automatic 14 gauge needle (ACECUT-TSK®, Japan), was used in both models.
This method of progressive learning was put into practice in the II Curso de Experto en Nefrología Diagnóstica e Intervencionista 2012–2013 (Expert in Diasnotic and Interventional Nephrology Course II), a qualification from the UAH (code EC36) and in the I Máster Universitario en Nefrología Diagnóstica e Intervencionista 2013–2014 (University Masters I in Diagnostic and Interventional Nephrology), a qualification from the UAH (code EF59). Here, we are presenting the results obtained.
Timeline of activitiesThe learning dynamic consisted of a brief review of the theory – the indications, contraindications, complications, and documentation necessary to perform RB – followed by a first attempt with the inanimate model and a subsequent attempt with the animal model.
With the inanimate model, the students learned:
- -
The materials necessary for RB and how automated punch-biopsy needles work.
- -
The appropriate use of ultrasound.
- -
Ultrasound imaging of the kidney and how to locate the puncture site.
- -
To efforts required to sample correctly one cylinder of renal tissue.
Once the students had acquired skill in controlling both needle and ultrasound on the inanimate model, they moved on to try the technique on the animal model. We chose a common pig as an alive model as the dimensions of the kidney and the ultrasound anatomy are very similar to those of a human.
With the animal model, the students learned:
- -
To prepare a sterile field for performing an invasive technique.
- -
To perform RB on a kidney that moves with respiratory movements.
- -
To identify and detect the haemodynamic complications that may occur in a severe haemorrhagic complication.
- -
To identify and detect post-biopsy vascular complications using 2-dimensional and Doppler ultrasound on the animal.
This teaching model was put into practice with 50 students (nephrology specialists) who participated in the 2 university courses mentioned above.
ResultsTwenty-five students from each course carried out RB on both models with the method described. After a mean of 2.6±0.8 punctures (range 1–4) on the inanimate model, the students acquired sufficient skill to control the ultrasound and the automated punch device. Practicing RB on the animal model allowed students a more realistic experience than with the inanimate model, in terms of the look and feel of a native kidney RB in a living being. The resistance of the skin, the depth of the organ, the movement of the kidney with respiratory movements, and the ultrasound imaging of the animal model were very similar to those of a human. In the biopsy in the pig, the students witnessed the most common complication of RB: macroscopic haematuria (Fig. 2). The animal did not develop haemorrhagic shock. As in humans, we performed 2-dimensional and Doppler ultrasound of the biopsied kidney. In doing this, the students witnesses the development of post-biopsy arteriovenous fistula, perirenal haematoma, and intrarenal haematoma. In the satisfaction questionnaire of the course, practicing RB on simulators received a score of 4.8 out of 5.
DiscussionThe use of simulators and models for learning was first used in aviation, with the aim that pilots would acquire sufficient dexterity and skill to fly an aeroplane without a passenger load, to improve flight safety and reduce accidents. In medicine, the use of simulators to learn various techniques was first used in the specialty of anaesthetics; however, it is the surgical specialties that have widely incorporated it in teaching and learning endoscopic and open surgery. Currently, simulators represent a valuable tool for surgeons to develop their surgical skills, to record surgeons’ psychomotor behaviour, and even as a method for innovation of surgical techniques.6–9
Teaching on patients is being increasingly questioned, not just for ethico-legal reasons, but also for economic reasons and due to the lack of time available for undisturbed teaching in areas of service overload. Simulators allow doctors to be “in the situation” without the stress of potential patient complications due to their actions. The safe environment in which learning is developed is more comfortable for both the doctors teaching and those who are learning. Furthermore, it allows a better use of material resources and reduced time dedicated to procedures done by trainee doctors. Finally, it allows self-learning and repeated practice without risk to the patient.
Currently, there is a great variety of types of simulator. They range from explanatory videos and computer programmes to cadavers, mannequins, and animal models.
The use of animal models is not free from controversy. The main barrier is the ethical aspect. The considerations about animal rights, the administrative procedures, and the required permits are amongst the obstacles to this method. Furthermore, the practice must be done in an animal laboratory equipped with a veterinarian and anaesthetist that are accredited in animal handling. Despite this list of disadvantages, there are multiple advantages, given that in animals the procedure has the same look and feel as when working with human tissues.
Renal biospy is an invasive technique in the specialty of nephrology. The most feared complication is bleeding, as this can be life threating. Since its introduction by Iversen and Brun in 1951, the percutaneous RB technique has remained practically unchanged.10 However, significant technological advances have been made that have led to more safety and efficacy in this technique, such as improving the punch needles, from the old and bloody Vin Silverman needles to the current automated punch models, which are much safer. Another great technological advance has been the use of ultrasound equipment to locate and guide the puncture device, in real time. Until a few years ago, renal puncture was blind, with the consequent high rate of blank samples and complications. With the emergence of imaging techniques (ultrasound and computed tomography), the drawbacks of blind RB have been eliminated to a great extent. Real-time ultrasound-guided RB is now an established technique.1,11,12 Compared with CT, ultrasound presents obvious advantages. In addition to having no radiation risk for the patient, it is more available, the biopsy can be performed “at the bedside”, it is cheaper, and it does not require contrast. Finally, it allows continuous visualization of the needle position in the renal parenchyma and in the desired renal zone, because it is not harmful to the professional using it. The time for performing a biopsy is also shorter, being around 30min with CT and 10–15min with ultrasound.
Real-time ultrasound-guided biopsy requires a degree of expertise in the use of ultrasound, as at times selecting and locating the puncture site and visualizing the point of the needle as it enters the kidney can be difficult (obese, senile, or uncooperative patients, and small or cystic kidneys). With the incorporation of ultrasound in RB, the current rate for obtaining sufficient material for diagnosis is over 90% in most series. The diagnostic yield depends on the ability of professional to control the needle and position it in the most superficial point possible to get a predominantly cortical sample. The incidence of complications from biopsy has been reduced from around 10% with the blind technique to between 2% and 6% with ultrasound guidance. The reported mortality is less than 0.1%: not an insignificant figure for being an exclusively diagnostic technique.
Since its original description, RB technique has been learnt on patients. It is easy to recognize not only that the ability to perform ultrasound-guided RB takes time, but also that this approach is quite unsafe for the patient. Therefore, having simulators available for use would be ideal. There have been interesting initiatives on this subject,2–5 which have partially recreated the conditions of RB, being performed only on inanimate models.
This study presents a novel methodology for learning real-time ultrasound-guided RB in 2 models (inanimate and live) which allows teaching of the RB technique on 2 levels (beginner and advanced) for both residents and nephrology specialists. With its drawbacks, above all bureaucratic, the animal model allows learning in a realistic setting, very similar to that experienced with patients, but without harming them. Thereby, the Hippocratic aphorism “First, do no harm” is met, and learning can be enjoyed.
ConclusionsThe use of simulators for learning RB technique could mean shorter training times, improved training quality, and increased patient safety.
Please cite this article as: Rivera Gorrín M, Correa Gorospe C, Burguera V, Ortiz Chercoles AI, Liaño F, Quereda C. Innovando en la docencia de la biopsia renal ecodirigida. Nefrologia. 2016;36:1–4.