Nonalcoholic fatty liver disease or metabolic-associated fatty liver disease (MAFLD) is a common condicion with increasing prevalence and incidence, specially in patients with type 2 diabetes mellitus (T2DM). Both cardiovascular and renal disease are clearly increased in these patients, particularly in those with diabetic nephropathy. In the liver-heart-kidney-metabolic axis, the common pathophysiological basis of MAFLD, cardiovascular disease (CVD), chronic kidney disease (CKD), and T2DM is the same. The clinical relationship between all of them is clear and is multidirectional: MAFLD may precede the development of cardiovascular and renal disease, and may also worsen the prognosis of these complications once developed. In this review we emphasize the importance of targeting MAFLD in Diabetic kidney disease, with the goal of detecting high-risk patients in order to improve their prognosis.
La incidencia y prevalencia de hígado graso no alcohólico o enfermedad hepática metabólica (EHmet) está en aumento y es mayor en pacientes con diabetes mellitus tipo 2 (DM2). El riesgo cardiovascular y renal está claramente incrementado en estos pacientes, especialmente cuando se desarrolla nefropatía diabética. El eje cardio-reno-hepato-metabólico, conformado por la enfermedad cardiovascular (ECV), la enfermedad renal crónica (ERC), la EHmet y la DM2, tiene una base fisiopatogénica común. La relación clínica entre todos los componentes es inevitable y multidireccional, pudiendo la EHmet preceder al desarrollo de complicaciones cardiovasculares y renales, y también empeorar el pronóstico de las mismas una vez desarrolladas. En esta revisión enfatizamos la importancia de buscar y tratar la EHmet en pacientes con ERC de la DM2 con el objetivo de identificar pacientes de mayor riesgo y de mejorar su pronóstico.
Non-alcoholic fatty liver disease (NAFLD) is defined as excessive fat accumulation in the liver (steatosis) demonstrated by imaging or biopsy, in the absence of dangerous alcohol consumption (≥30 g/day in males or ≥20 g/day in females), consumption of drugs that cause steatosis, and in the absence of genetic or hereditary diseases causing steatosis. Therefore, it is a disorder generally associated with metabolic risk factors. In fact, a change of nomenclature to Metabolic-associated fatty liver disease (MAFLD) has been proposed.1
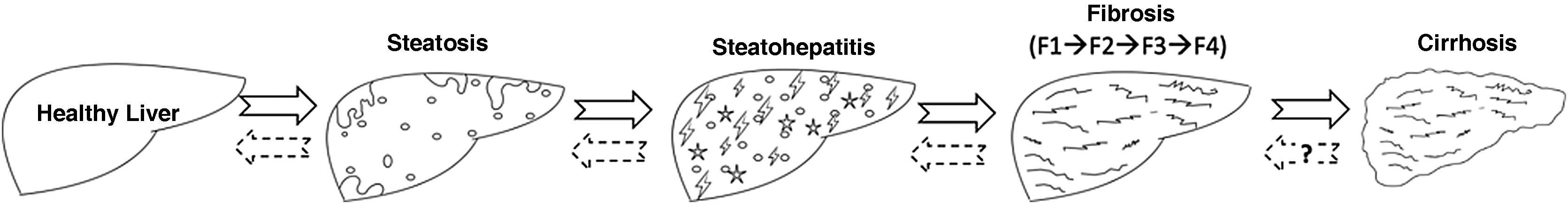
MAFLD is actually a generic term that encompasses different stages of disease severity ranging from simple steatosis to cirrhosis and in between the non-alcoholic steatohepatitis (NASH) and different stages of fibrosis (stages F0 to F4). The term NASH is therefore only one phase of MAFLD, namely the fatty inflammation phase (steatohepatitis). This phase implies a more accelerated progression of MAFLD, as it is where fibrogenic mechanisms are stimulated causing fibrosis at different stages, ultimately leading to cirrhosis. The natural history of the disease is shown in Fig. 1.
Histologic evolution of nonalcoholic fatty liver disease or metabolic-associated fatty liver disease (MAFLD). The different phases of MAFLD can be reversible if the necessary measures are taken in time, although complete reversibility is more controversial in the cirrhosis phase.
Source: own elaboration.
MAFLD is currently considered the most frequent liver disease in Western countries, with an overall prevalence of 25% in the general population and more than 60% in the diabetic patients, and it is a growing cause of cirrhosis and, eventually, liver transplantation.2,3 The incidence of MAFLD is increasing in parallel with the incidence of diabetes and obesity, closely related to insulin resistance. Currently, the prevalence of type 2 diabetes mellitus (T2DM) in adults in Spain (20–79 years) is 14.8%,4 while that of obesity and overweight is 16% and 35–40%, respectively.5 Taking into account that up to 40% of diabetic patients will develop diabetic nephropathy (DN) during their evolution,6 and that DN is the main cause of chronic kidney disease (CKD), it is obvious the magnitude of the problem and the need for action become clear. There is a close relationship between MAFLD and CKD, with increasing evidence that MAFLD can negatively influence the progression of kidney disease or even precede it. The aim of the present work is to summarize the main links between MAFLD and DN, and to propose a concerted algorithm to help identifying patients at higher risk and improve their prognosis.
Diagnosis of MAFLD and risk stratificationMost patients with MAFLD have no signs or symptoms of liver disease at the time of diagnosis. Fatty liver is usually an incidental finding on an image that was requested for another reason (ultrasound, computed tomography or magnetic resonance). However, the distinction between simple steatosis, steatohepatitis or the staging of the severity of fibrosis can only be evaluated with precisison by a liver biopsy. Since biopsy is an expensive and not risk-free technique, non-invasive markers of both steatosis and fibrosis have been developed. Unfortunately, we still do not have non-invasive markers that reliably detect steatohepatitis.
The main cause of overall mortality in MAFLD is cardiovascular disease, followed by extrahepatic neoplasms and, lastly, liver disease itself (decompensated cirrhosis and hepatocarcinoma).7 Mortality is directly related to liver fibrosis, which is the main predictor of poor prognosis in patients with MAFLD,8 therefore it is necessary to focus screening efforts on the subgroup of patients with steatosis and more fibrosis.9
The term significant fibrosis refers to the histological stage F2 or higher, and advanced fibrosis indicates stage F3 or higher. A multitude of non-invasive markers of fibrosis have been developed: indirect biomarkers, direct biomarkers, imaging techniques, “omics”, etc…. As it is not the purpose of this review to be exhaustive in the description of each one of them, let’s describe the most commonly used in real clinical practice:
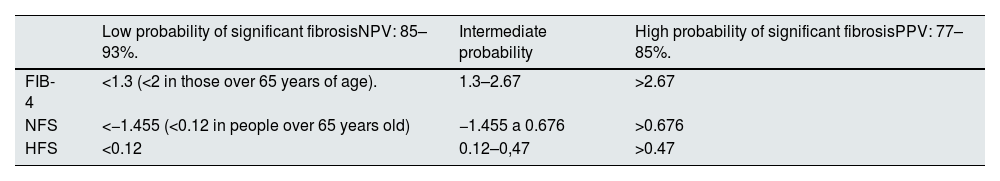
Indirect markers of liver fibrosis: transaminases do not correlate with the level of fibrosis or the severity of the disease. However, they are useful when used in conjunction with other variables in mathematical formulas that give us a fibrosis score. The most commonly used indexes in clinical practice are the FIB-4 (including age, AST, ALT, platelets),10NAFLD fibrosis score or NFS (age, T2DM/altered fasting basal glucose, AST/ALT, platelets, albumin)11 and Hepamet fibrosis score (HFS) (age, AST, T2DM, platelets, albumin, HOMA-IR).12 In general, they have a high negative predictive value (NPV) that allows to rule out the presence of advanced fibrosis quite reliably (Table 1). This characteristic, together with their low cost and high availability, makes them the first-line test to identify patients at lower risk of fibrosis and reduce the number of scans required. Furthermore, the combined use of HFS, NFS and FIB-4 increases the ability to discriminate patients without advanced fibrosis, making them the methods of choice in primary care or in non-liver specialized clinics.9
Cut-off points of the main noninvasive fibrosis indexes to rule out or detect significant fibrosis.
| Low probability of significant fibrosisNPV: 85–93%. | Intermediate probability | High probability of significant fibrosisPPV: 77–85%. | |
|---|---|---|---|
| FIB-4 | <1.3 (<2 in those over 65 years of age). | 1.3–2.67 | >2.67 |
| NFS | <−1.455 (<0.12 in people over 65 years old) | −1.455 a 0.676 | >0.676 |
| HFS | <0.12 | 0.12–0,47 | >0.47 |
FIB-4: Fibrosis-4-Index; HFS: hepamet fibrosis score; NFS: NAFLD fibrosis score; NPV: negative predictive value; PPV: positive predictive value.
Direct markers of liver fibrosis and metabolomics: Direct markers of fibrosis are the measurement of the products of synthesis or degradation of collagen or extracellular matrix, and therefore have greater diagnostic precision than indirect markers, although their availability in laboratories is usually lower and their cost is higher because they are patented indexes. Enhanced Liver Fibrosis (ELF®) is a method based on the determination of hyaluronic acid (HA), aminoterminal procollagen III peptide (PIIINP) and tissue inhibitor of metalloproteinase I (TIMP-1) levels. Moreover, OwlLiver® is a lipidomic technique with a NPV of 75% for steatohepatitis and significant fibrosis. Both ELF® and OwlLiver® are often used in cases where first-line (indirect) markers offer doubts regarding the degree of liver fibrosis. For example, the use of ELF® as a second-line test after FIB-4 significantly reduces the rate of referrals to hepatology. Despite these good results, the low cost and easy accessibility of indirect markers have made them the most widely used in daily clinical practice.9
Hepatic elastography or liver stiffness: it could be included within the group of imaging techniques, because it is actually considered a modified ultrasound technique. It is based on measuring the stiffness of a tissue by emitting a mechanical pulse that produces an elastic wave that is transmitted through the tissue. The speed of propagation of the elastic wave is proportional to the stiffness of the tissue (the more fibrotic the tissue, the faster the wave propagates). The result is expressed in units of kilopascal (kPa). Transient elastography using FibroScan® in most algorithms is positioned as the second-line test to be performed in patients in whom advanced fibrosis could not be ruled out by simpler analytical methods. A liver stiffness value <8 kPa rules out advanced fibrosis with a NPV: 95–100%. Patients with ≥8 kPa should be studied in depth, sometimes with a liver biopsy.9
The metabolic heart-liver-kidney axisT2DM is a clear predisposing factor for MAFLD and also worsens its prognosis, because the progression of disease is more accelerated in diabetics than in the non-diabetic population. But MAFLD also predisposes to the development of T2DM and arterial hypertension, especially when fibrosis is more advanced.13 For this reason, the main scientific societies of hepatology and endocrinology fields recommend screening and stratification for MAFLD in all diabetic patients and to look for diabetes in all patients with hepatic steatosis.7,14–17
Numerous publications point to a cardiovascular risk (CVR), especially increased in patients with MAFLD. These patients are at increased risk of subclinical cardiovascular disease (CVD) (endothelial dysfunction, atherosclerosis, increased carotid intima-media thickness) and major cardiovascular events (ischemic heart disease, arrhythmias, heart failure with preserved ejection fraction, valvular heart disease, and stroke).18–20 This association seems to be independent of traditional cardiovascular risk factors and its magnitude increases with the severity of liver involvement. Nevertheless, MAFLD cannot yet be considered as an independent cardiovascular risk factor, but it is clearly an enhancer of cardiovascular risk and should be taken into account when assessing CVR in any patient.21
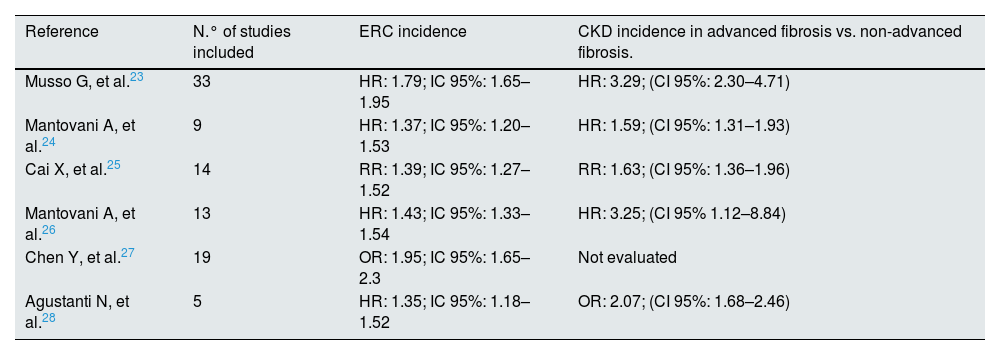
MAFLD also increases the risk of CKD, defined by a glomerular filtration rate less than 60 ml/min/1.73 m2 or urinary albumin/creatinine ratio (ACR) greater than 30 mg/g. There are many common aspects between MetEH and CKD, both pathophysiologically and clinically. It is widely known that the development of CKD in every diabetic patient leads to a parallel increase in cardiovascular risk and mortality, and in this context, again MAFLD could be seriously considered as an enhancer.22 In both pathologies, cardiovascular events are the main cause of mortality. To date, there are 6 published meta-analyses showing an evident relationship between MAFLD and CKD (Table 2).23–28 Although most are retrospective studies, and with heterogeneity in terms of populations, follow-up, definitions (MAFLD, CKD), it is clearly observed that patients with MetEH and preserved baseline renal function have an incidence between 1.5 and 1.95 times higher than the general population of presenting CKD in the following 5–10 years. This association persists after correcting for confounding variables that also predispose to CKD (age, gender, BMI, dyslipidemia, HTN, T2DM, smoking, basal renal function).
Meta-analyses that have analyzed the relationship between MALFD and CKD.
| Reference | N.° of studies included | ERC incidence | CKD incidence in advanced fibrosis vs. non-advanced fibrosis. |
|---|---|---|---|
| Musso G, et al.23 | 33 | HR: 1.79; IC 95%: 1.65–1.95 | HR: 3.29; (CI 95%: 2.30–4.71) |
| Mantovani A, et al.24 | 9 | HR: 1.37; IC 95%: 1.20–1.53 | HR: 1.59; (CI 95%: 1.31–1.93) |
| Cai X, et al.25 | 14 | RR: 1.39; IC 95%: 1.27–1.52 | RR: 1.63; (CI 95%: 1.36–1.96) |
| Mantovani A, et al.26 | 13 | HR: 1.43; IC 95%: 1.33–1.54 | HR: 3.25; (CI 95% 1.12–8.84) |
| Chen Y, et al.27 | 19 | OR: 1.95; IC 95%: 1.65–2.3 | Not evaluated |
| Agustanti N, et al.28 | 5 | HR: 1.35; IC 95%: 1.18–1.52 | OR: 2.07; (CI 95%: 1.68–2.46) |
CKD, chronic kidney disease; HR, hazard ratio; 95% CI, 95% confidence interval; OR, odds ratio; RR, relative risk.
*One out of 13 articles evaluated fibrosis by liver biopsy (ref. 26).
Furthermore, it seems that the increased risk of CKD is related more to the metabolic component than to the hepatic steatosis itself. Patients who meet the criteria for MAFLD have a higher incidence of CKD, but this is not true for patients with nonalcoholic steatohepatitis without metabolic syndrome.29
Similarly to what happened with cardiovascular involvement, renal impairment is higher as the liver fibrosis increases (however there are only limited studies showing data from liver biopsy).
If we face the problem of MAFLD from the renal point of view, one of the few studies with ND confirmed by renal biopsy demonstrates that the existence of MAFLD worsens the prognosis of CKD,30 especially if there is more liver fibrosis estimated by non-invasive markers.
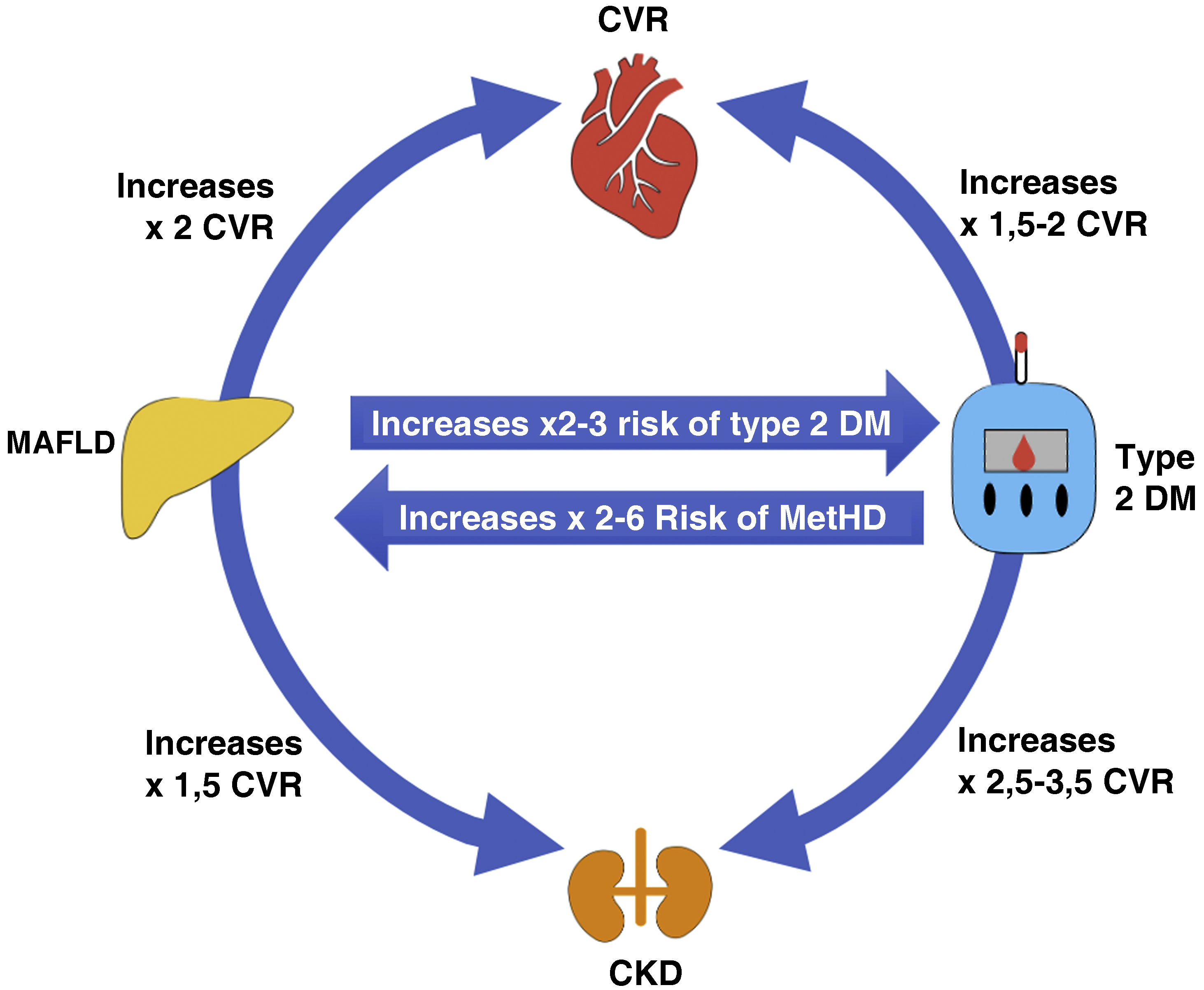
Fig. 2 illustrates a scheme of the interactions between MAFLD, T2DM, CVD and kidney disease. It can be seen how both MAFLD and DM2 increase CV and renal risk independently, and the risk increases when both entities coexist, thus both DM and MAFLD potentiate and multiply this risk.15,31,32
Interrelationship fatty liver, cardiovascular risk, diabetes and kidney disease.
T2DM, type 2 diabetes mellitus; CKD, chronic kidney disease; CVR, cardiovascular risk.
Source: modified from Targher et al.31
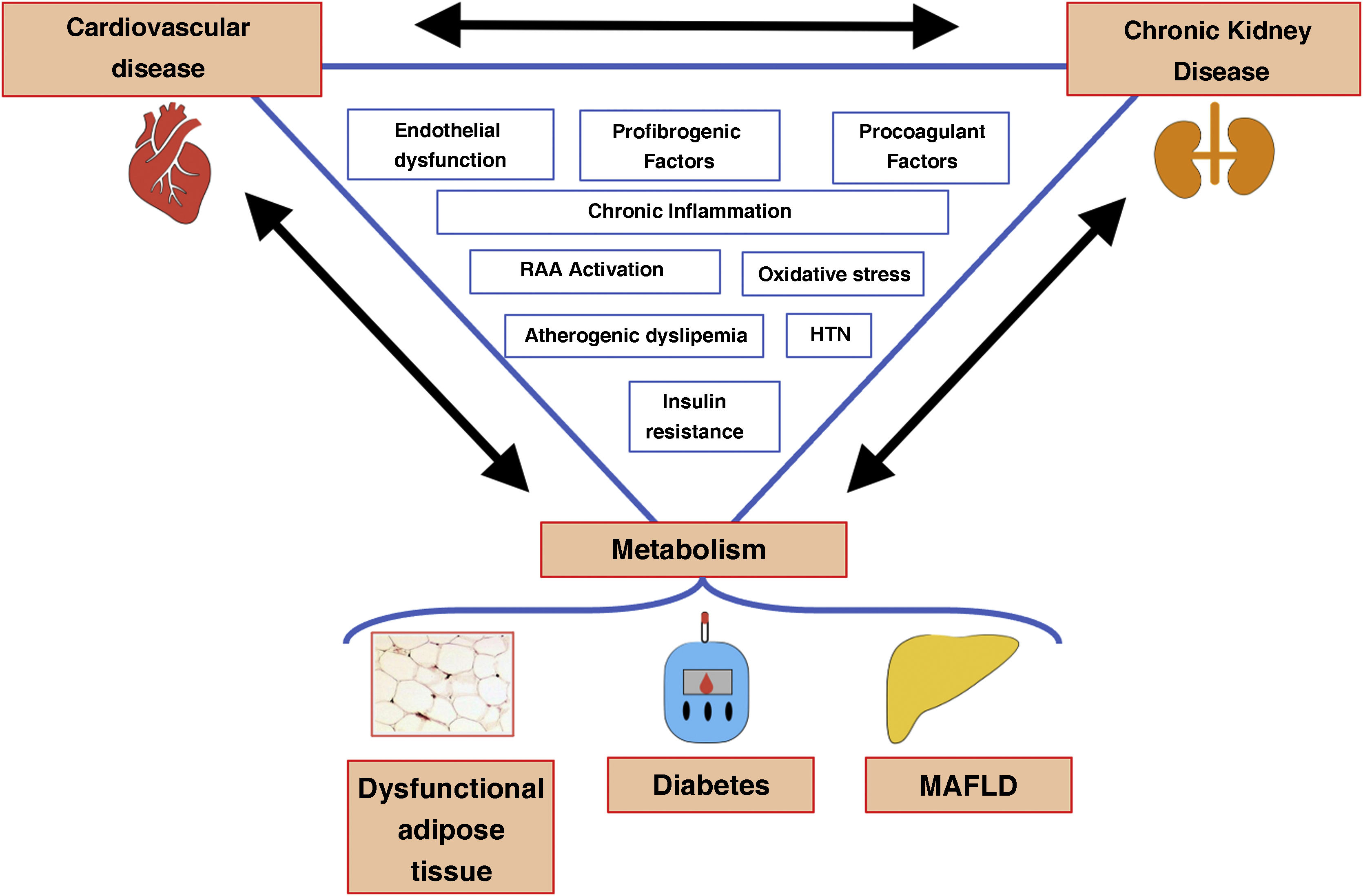
The hypothesis that MAFLD increases the incidence and prevalence of CKD is biologically conceivable. The main pathogenic link is chronic inflammation and peripheral insulin resistance. Although more prospective studies are needed, the available evidence suggests that fatty liver (especially if more fibrosis is present) exacerbates insulin resistance (IR) and worsens the progression of HTN and T2DM while promoting atherogenic dyslipidemia. In turn, IR interferes with adipose tissue and worsens its dysfunction, favoring the arrival of more fatty acids to the liver, which causes more IR as a vicious cycle.
At the molecular level, in both MAFLD and CKD there is a release into the bloodstream of proinflammatory cytokines (TNF-α, IL-1, IL-6, IL-17, IL-22, IL-23, C-Reactive-Protein). On the other hand, there is a decrease in anti-inflammatory molecules (adiponectin, TGF-beta, IL-4, IL-10), as well as an increase in procoagulant (PAI-1, fibrinogen, factor VII) and pro-fibrogenic (FGF-21, TGF-beta) factors, oxidative stress and endothelial injury factors that clearly increase the risk of CVD and renal function deterioration.32,33
It should be emphasized the importance of oxidative stress in the pathogenesis of MAFLD, since it accelerates the transition from steatohepatitis to fibrosis. Similarly, in ND, free radical production is increased by hyperglycemia, and this results in the release of proinflammatory cytokines, growth factors and transcription factors that gradually cause changes in renal structure and function that eventually lead to CKD.
There are other molecules involved in the interelationship between MAFLD and CKD (adiponectin, fetuin-A, leptin, resistin, visfatin, aldosterone, uric acid), although the mechanisms are not yet entirely clear and an analysis of the evidence is beyond the scope of this review. A summary of the complex interaction between the components of the metabolic heart-liver-kidney axis is shown in Fig. 3.22,33,34
Role of noninvasive markers in the identification of high risk patientsThe fact that patients with more advanced liver disease have a an increased risk of CKD and CVD makes it necessary to focus our efforts on the subgroup of patients with steatosis and more pronounced fibrosis.
Non-invasive fibrosis markers not only serve to stratify the severity of hepatic lesions, but also have been shown to be useful as markers of cardiovascular and renal risk. We have previously discussed the relationship between advanced fibrosis measured by noninvasive markers of fibrosis and cardiovascular events.18 Similarly, from the nephrology point of view, there are also prospective and retrospective studies showing that high FIB-4 values35–38 increase the risk of CKD in both diabetic and non-diabetic populations. Similar results have been obtained with the NFS index.39–41 Liver elastography using FibroScan® is also capable of predicting renal function deterioration in patients with MAFLD with or without T2DM.42,43 Thus, the meta-analysis by Ciardullo et al. detected an association between elevated FibroScan® values and an increased albuminuria/creatininuria ratio (OR: 1.98; 95% CI: 1.29–3.05; p = 0.002), and an increased incidence of CKD (OR: 2.49; 95% CI: 1.89–3.29; p < 0.001).
The main reason for the use of noninvasive markers of fibrosis in patients with CKD is to identify patients at higher risk of progression of both hepatic and renal disease in order to optimize their treatment and improve their prognosis.
Current management of MAFLDThe treatment has two objectives: to improve both the hepatic and cardiovascular prognosis. Hence the importance of working in a multidisciplinary manner. It is important to insist that efforts should be directed towards the detection and treatment of the advanced stages of MAFLD (advanced fibrosis), since these are the ones that truly increase the risk of cardiovascular and renal disease, in addition to the risks that liver disease itself entails.
Lifestyle modificationLifestyle modification (Mediterranean diet, physical exercise) is the cornerstone in the treatment of these patients. A weight loss of 3–5% improves steatosis, a 7–10% improves steatohepatitis and with ≥10% of weigth loss improvement in liver fibrosis is achieved.15 Weight loss also improves the prognosis of metabolic comorbidities and cardiovascular risk. Moreover, liver biopsy studies have shown that improvement in liver fibrosis obtained with lifestyle modifications were independently associated with improved renal function in patients with MAFLD.44 Physical exercise, even in the absence of weight loss, improves MAFLD. It is recommended to combine aerobic with anaerobic exercise, of a duration of 150–300 min per week if moderate intensity or 75–150 min per week if the exercise is vigorous.45
Needless to say, it is necessary to promote alcohol abstinence and smoking cessation in these patients.
PharmacotherapyCurrently there is no approved drug for treatment of MAFLD, so there are multiple clinical trials under development. There are excellent reviews on molecules and drugs that could be future therapies for MAFLD,46,47 but a detailed explanation is beyond the scope of this article.
The fundamental concept of a possible pharmacological approach is to keep in mind that patients with MAFLD are at increased risk of renal and cardiovascular disease and also, at increased hepatic risk. With these assumptions in mind, good control of hypertension, dyslipidemia and diabetes is especially important in these patients. Statins are safe in patients with MAFLD. A logical approach would be to prescribe drugs that serve to achieve two goals simultaneously: decrease cardiovascular risk and decrease hepatic risk. This would include agents currently under investigation against the metabolic target, against apoptosis, against inflammation and oxidative stress, or against fibrosis. The option offered by new antidiabetic agents, such as glucagon-like-peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (iSGLT-2) inhibitors or even GLP1-GIP dual agonists, is currently of great interest because these agents not only exert hepatoprotective actions, but also offer clear benefits at the renal and cardiovascular levels.48,49
A subanalysis of the CANVAS and CANVAS-R study (CANagliflozin cardioVascular Assessment Study [CANVAS] Program) has recently been published, showing the benefit of iSGLT2 on noninvasive markers of fibrosis in patients with MAFLD and T2DM, and that was independent of glycemic control and weight loss. Taking into account this results, this drugs are a very promising tool in patients with MAFLD and CKD.50
Proposed co-management algorithmIn view of the studies published to date on the relationship between MAFLD and CKD, there is no doubt about the need for a double and reciprocal evaluation: a periodic evaluation of renal function in patients with MAFLD (due to their higher risk of developing CKD) and, a liver evaluation in patients with CKD, especially those with diabetic nephropathy.
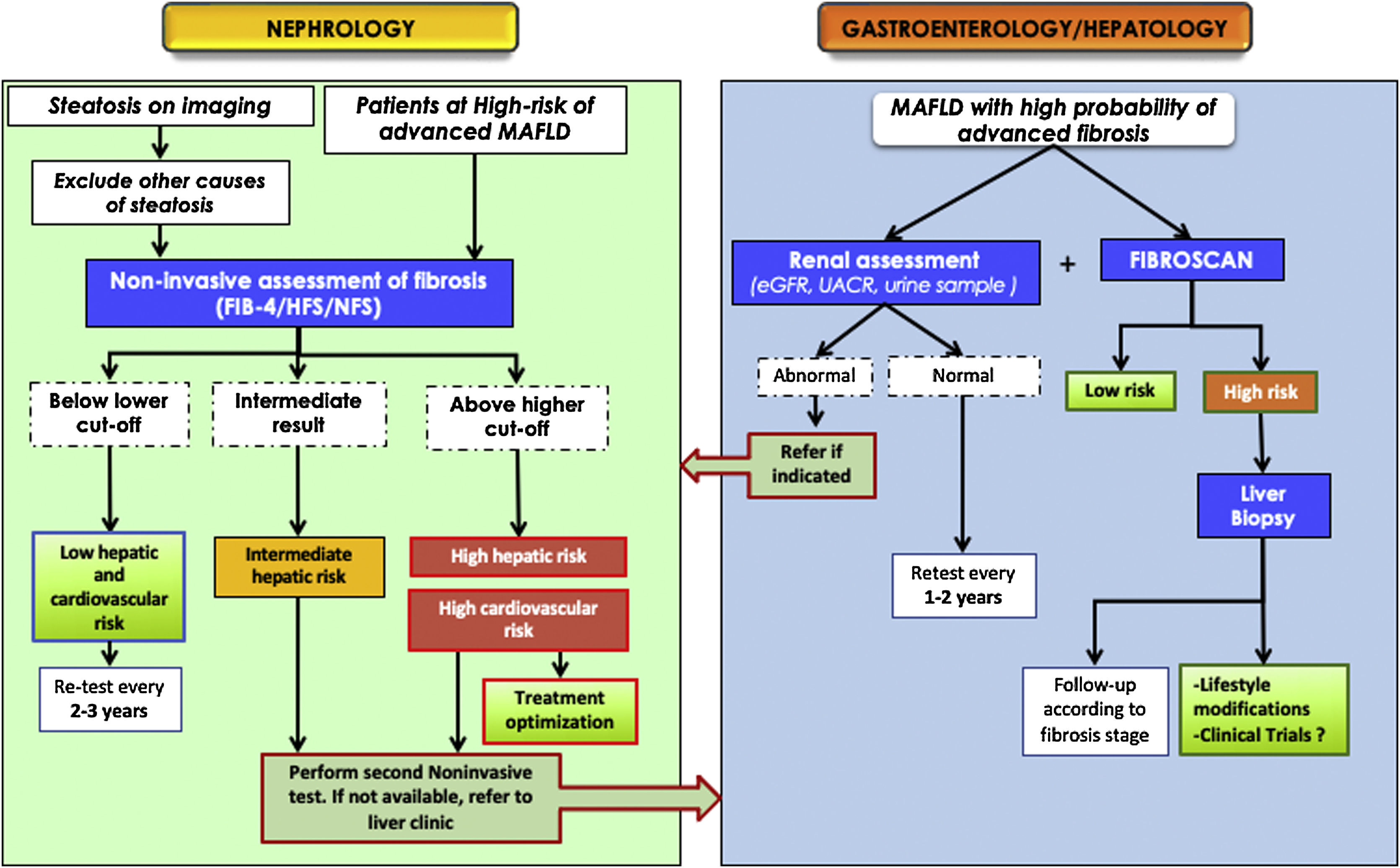
Moreover, given the great health impact of cardiovascular morbidity and mortality associated with CKD and MAFLD, it is also necessary to maximize the control of cardiovascular risk factors to improve the prognosis of these patients. Therefore it should not be surprising that the multidisciplinary approach is of particular importance. In this regard, some recommendations have been recently published to facilitate the joint management of diabetic patients with MAFLD.14,17,49 Following the scientific evidence and being consistent with the main consensus documents and clinical practice guidelines regarding the diagnosis and management of MAFLD,9,14,15 the target population at the greatest risk would be patients with T2DM, obesity or metabolic syndrome, especially with an age >50 years. A 2-step algorithm is recommended: firstly, clinicians should start by requesting a simple noninvasive study such as FIB-4, HFS or NFS to rule out or detect significant fibrosis. If the test result is indeterminate or is compatible with advanced fibrosis, another more accurate test should be performed, preferably liver elastography (FibroScan®), and if not available, proprietary tests such as ELF® or OwlLiver®. If none of these tests are available or the results are still indicative of advanced fibrosis, referral to the gastroenterologist should be indicated in order to complete the diagnostic work-up, adjust follow-up and assess therapeutic options.
Fig. 4 shows a possible flow chart in which the necessary interaction between the different health professionals caring for patients with CKD, diabetes, DN, or heart disease is evident. The methods and intervals for screening and monitoring are approximate, pending further prospective studies that will finally elucidate the best model of care.
Proposed algorithm for the joint management between nephrologists and hepatologists for metabolic -associated fatty liver disease or MAFLD.
ACR: albuminuria/creatininuria ratio; MAFLD: metabolic-associated fatty liver disease; eGFR: estimated glomerular filtration rate; HFS: hepamet fibrosis score; NFS: NAFLD fibrosis score.
*Patient at risk of advanced MAFLD (significant fibrosis): patient with obesity (especially if BMI > 35) or type 2 DM or with metabolic syndrome, especially if older than 50 years.9
MAFLD and CKD, especially diabetic nephropathy, are two very prevalent entities with important consequences for cardiovascular health. They share common and complex risk factors and pathophysiological pathways, and also MAFLD can precede CKD. Moreover, when they coexist, their deleterious effects are potentiated. The multidisciplinary management approach algorithm is a call to action to detect high-risk patients in whom to adjust follow-up and treatment and, ultimately, improve their prognosis.
FinancingThe work has no source of funding.
Conflict of interestSB declares no conflict of interest in relation to this work. FM declares no conflict of interest and JLG reports personal fees from NovoNordisk (Fees, Advisory), Boehringer (Fees, Advisory and Consulting), Eli Lilly (Fees), AstraZeneca (Fees, Advisory and Grants).