Atypical hemolytic uremic syndrome (aHUS) is a rare, life-threatening systemic inflammatory disease that presents classically with microangiopathic hemolytic anemia, thrombocytopenia and acute kidney injury.1 Extra renal manifestations are observed in 20% of patients.2
A 42-year-old woman with unremarkable past medical history presented in our hospital reporting a 6-day history of headache, nausea and vomiting. Physical examination showed hypertension (220/120mmHg), cutaneous pallor and moderate lower limbs edema. Laboratory results revealed anemia (hemoglobin 8.4g/dl), thrombocytopenia (76,000/μl), severe azotemia (urea 16.9mmol/l, creatinine 448.8μmol/l), schistocytosis, a negative Coombs test, low blood haptoglobin (<0.07g/L) and high lactate dehydrogenase levels (1234U/l). Renal ultrasonography was normal.
Blood pressure was hardly controlled with oral medication. A diagnosis of acute thrombotic microangiopathy (ATM) was made and daily plasma exchange (PEX) was started.
Investigations for secondary causes of ATM (pregnancy, auto-immune disease, malignancy, drug-induced), infection-induced HUS and thrombotic thrombocytopenic purpura were normal. A presumptive diagnosis of aHUS was made and the administrative process of Eculizumab acquisition was initiated.
On the 15th day of admission (D15), hemodialysis was started due to progressive renal failure. All attempts to stop PEX resulted in increased hemolytic activity, forcing to maintain 3 sessions a week.
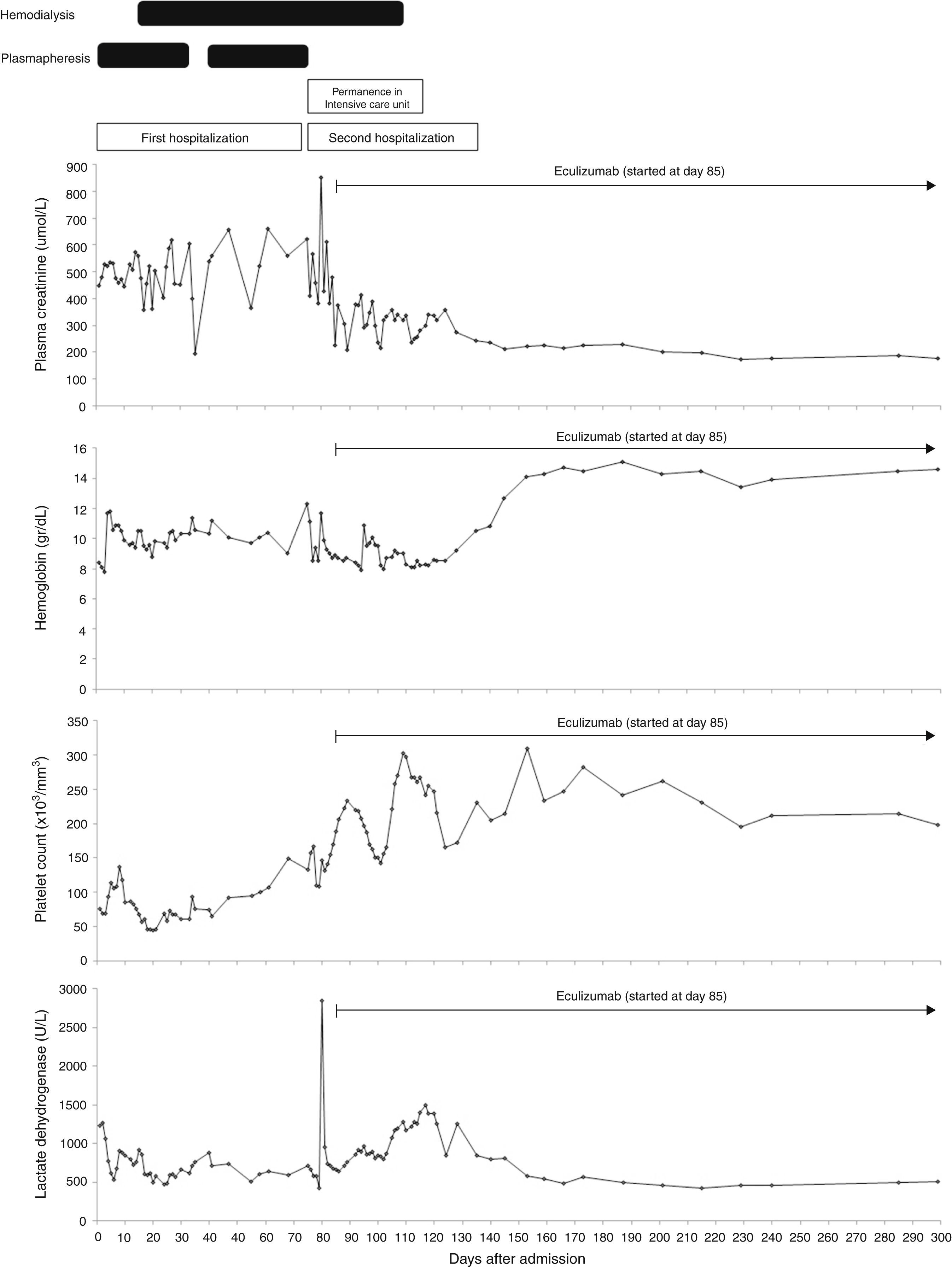
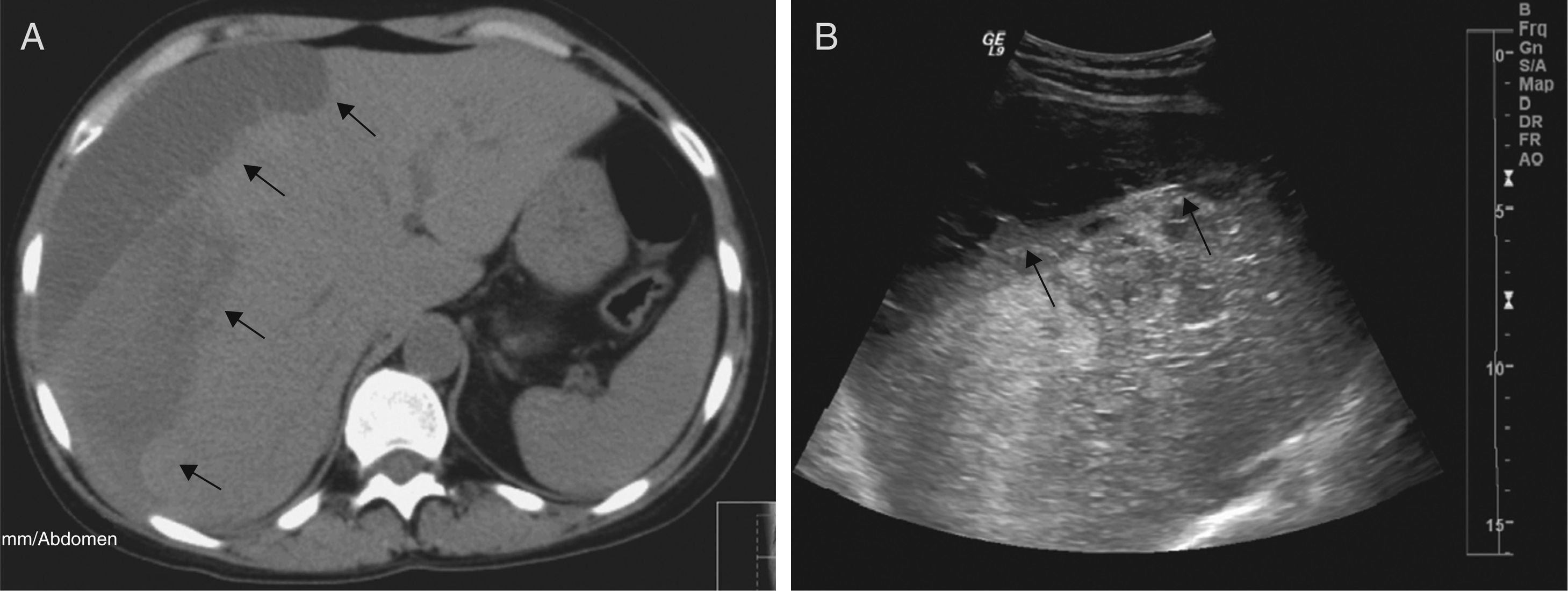
On the D72, after performing 44 PEX sessions, we were still waiting for Eculizumab acquisition. Attending to clinical and analytical stability (Fig. 1), the patient was discharged home to continue hemodialysis and PEX three times a week as an outpatient. Four days after discharge, she was admitted in the emergency room with a 12-hour history of severe right upper quadrant pain and vomiting without history of trauma. Laboratory results revealed stabilized hemoglobin (11.1g/dl) and both normal platelet count (157,000/mm3) and coagulation tests. Abdominal ultrasonography and Computed Tomography scan showed a large subcapsular liver hematoma (SLH) (Fig. 2).
Clinical course: laboratory data and treatment. Blood transfusions were done when hemoglobin got around 8g/dl. Plasma exchange consisted of one volume exchange with fresh frozen plasma. It was started on the second day of admission and maintained until there was no evidence of hemolytic activity. Attempts at weaning plasma exchange were unsuccessful due to increased hemolytic activity, forcing us to maintain 3 sessions a week until the diagnosis of the subcapsular liver hematoma. We performed a total of 44 plasma exchange sessions. Hemodialysis was commenced on the 15th day of admission to manage her renal failure. Eculizumab was administered at a dose of 900mg per week for 4 weeks, started on the 85th day after admission, followed by a dosage of 1200mg 1 week later and then a maintenance dose of 1200mg every 2 weeks. During the first days of eculizumab usage, we could not use hemoglobin neither lactate dehydrogenase to monitor hematologic response attending to the presence of the subcapsular liver hematoma that would distort their interpretation. Hemodialysis was stopped 26 days after the first infusion of eculizumab. Eculizumab is still being continued on the maintenance schedule of 1200mg every 2 weeks. Reference range values: lactate dehydrogenase – 313–618U/l; platelet count – 150–400×103/mm3; hemoglobin – 12–16g/dl; plasma creatinine – 46–92μmol/l.
She was transferred to the Intensive Care Unit (ICU). Attending to hemodynamic stability, a conservative approach was attempted. PEX was suspended to prevent increased hepatic bleeding related with heparin systemic anticoagulation. Ultrasonography follow-up examinations revealed almost unchanged size of hematoma.
Eculizumab was started on the 10th day of admission in the ICU (ICU D10) at a dose of 900mg per week for 4 weeks, followed by subsequent doses of 1200mg every 2 weeks since the 5th week. Thereupon, platelet count increased and hemodialysis was suspended on the ICU D35. She was transferred to the Nephrology ward on the ICU D42 and was discharged home three weeks later. The final genetic workup was available only after discharge and detected a complement factor H haplotype H3 (−332T, c.184G, c.1204T, c.2016G, and c.2808T).
Currently, 8 months after eculizumab initiation, hematologic remission persists, renal function remains stable (figure 1) and last ultrasonography control showed a significant decrease in the size of hematoma – 3.94cm×1.3cm. Alternate-week eculizumab infusions are still being continued.
Discussion: Complement system dysregulation in aHUS induces thickening and inflammation of vascular wall, widening of subendothelial space and microvascular thrombosis.1 Renal microvasculature is predominantly affected but extrarenal ischemic events, mainly involving central nervous system, were also reported.2 Few hemorrhagic events were also reported in patients with infection-induced HUS and in secondary causes of ATM.3–5 Only three hemorrhagic events, affecting pulmonary vasculature, were previously reported in aHUS patients.6–8
SLH is a rare event that is mainly related with pregnancy-induced hypertension (preeclampsia and HELLP syndrome) and primary or metastatic liver tumors.9 Since HELLP syndrome is an ATM like aHUS,1 we speculate that SLH pathogenesis might be similar in both diseases. In HELLP syndrome, SLH is thought to be a consequence of hepatic blood flow obstruction due to fibrin deposits within hepatic sinusoids, resulting in periportal necrosis and intrahepatic hemorrhage.10 Likewise, we believe vascular rupture in aHUS might result from hepatic blood flow obstruction secondary to complement-induced microvascular thrombosis. Other possible contributors for bleeding diathesis in our patient were uremic platelet dysfunction, difficult-to-control hypertension and heparinization during dialysis and PEX. It is important to note that at the time of SLH diagnosis platelet count and coagulation tests were normal.
There is currently no documentation on the role of eculizumab to rescue aHUS-related hemorrhagic events since none of the previously reported cases used it. However, we believe that the blockade of vascular injury achieved by eculizumab might have had some beneficial effect.
Current treatment of aHUS relies on early onset of eculizumab based solely on clinical and laboratorial data.1 The decision to start eculizumab in our patient was made one week after presentation but the high cost of Eculizumab limited its immediate availability and forced us to rely on extensive and ineffective PEX while waiting for the immunoglobulin. Conversely, Eculizumab initiation allowed immediate hematologic complete remission and induced remarkable renal recovery with discontinuation of dialysis after 3 months of replacement therapy.
In summary, our case highlights the first report of a SLH complicating an aHUS and reinforces the importance of an earlier intervention with Eculizumab to improve clinical outcomes and to prevent the onset of severe complications.
Conflict of interestThe authors declare that they have no conflicts of interest related to the contents of this article.