COVID-19 has proven to be particularly aggressive in patients with chronic kidney disease (CKD). The lower immune response rate and the greater susceptibility to progress to severe forms of the disease have contributed to this phenomenon, which has persisted in the post-vaccination era of the pandemic. Paradoxically, CKD has been excluded from most clinical trials of the main therapeutic tools developed against SARS-CoV-2. However, experience in the use of these drugs has been accumulating in different stages of CKD, supporting their use with guarantees of efficacy and safety.
The objective of this review is to gather all treatment indications for COVID-19 in the different phases of the disease, tailored to CKD in its various stages, including renal replacement therapy.
La COVID-19 ha demostrado ser especialmente agresiva con los pacientes con enfermedad renal crónica (ERC). La menor tasa de respuesta inmunológica y la mayor facilidad para la progresión a formas graves de enfermedad ha propiciado este hecho que se ha mantenido en la era post-vacunal de la pandemia. Paradójicamente, la ERC ha sido excluida de la mayoría de los ensayos clínicos de las principales herramientas terapéuticas desarrolladas frente a SARS-CoV-2. Sin embargo, se ha ido reuniendo experiencia de uso de estos fármacos en distintos estadios de la ERC que avala su uso con garantías de eficacia y seguridad.
El objetivo de esta revisión es reunir todas las indicaciones de tratamiento frente a la COVID-19 en los distintos estadios de la enfermedad adaptadas a la ERC en sus distintas fases incluyendo el tratamiento sustitutivo renal.
The severity of COVID-19 associated with chronic kidney disease (CKD) has been especially evident in two groups of patients: patients undergoing renal replacement therapy (RRT) via dialysis and renal transplant and patients with CKD at any stage who are also undergoing immunosuppressive treatment. In the case of patients with CKD and RRT, the COVID-19 registry of the Spanish Society of Nephrology (SEN) showed a high rate of infection with increased hospital admission (up to 85%) and a mortality rate that reached 25% in the pre-vaccination era.1 These findings have been replicated in samples from other countries, such as France and Great Britain, which confirm that the increased risk of progression to severe disease and death from COVID-19 associated with CKD has remained clearly higher than that of the general population in the post-vaccination era.2,3
Patients with glomerular disease and COVID-19 have been shown to have worse prognosis than the general population, showing increased mortality and acute renal failure independently of the use of immunosuppressive therapy at the time of SARS-CoV-2 infection.4
Despite low response rates to vaccination, both severity and mortality associated with SARS-CoV-2 appear to have improved substantially in successive waves of infection.5 Nevertheless, CKD and immunosuppression remain a risk factor for progression to severe forms of disease (Tables 1 and 2).
Risk groups for ranking the use of anti-SARS-CoV-2 drugs.a,b
| People with severe immunosuppression | Adequate immune response to vaccination is not expected |
| Nonvaccinated persons | Over 80 years of age or over 65 years of age with risk factors for progression to severe forms (Table 2) |
| Vaccinated persons (more than six months after the last dose) | Over 65 years of age and at least one risk factor for progression to severe forms of the disease |
SARS-CoV-2 (Severe Acute Respiratory Coronavirus 2).
Risk factors for progression to severe forms of the disease.a
| - Chronic kidney disease (GFR < 45 ml/min). |
| - Chronic liver disease (Child-Pugh class B or C) |
| - Chronic neurological disease (multiple sclerosis, ALS, myasthenia gravis, or Huntington's disease). |
| - Cardiovascular disease (AMI, stroke, TIA, HF, angina pectoris on nitroglycerin prescription, coronary revascularization grafts, percutaneous coronary intervention, carotid endarterectomy and aortic bypass). |
| - Chronic lung disease (high risk COPD with FEV1 < 50% or 2 or more exacerbations year or one hospital admission, asthma with requiring daily treatment), diabetes mellitus with target organ involvement |
| - Obesity (BMI ≥ 35) |
| - Low body weight (BMI ≤ 18.5) |
AMI, acute myocardial infarction; ALS, amyotrophic lateral sclerosis; COPD, chronic obstructive pulmonary disease; BMI, body mass index; LVEF, left ventricular ejection fraction; HF, heart failure; TIA, transient ischemic attack; HF, heart failure.
Since the first cases of COVID-19 up to now, multiple strategies have been developed to treat the disease. Unfortunately, CKD patients has been excluded from the main clinical trials to evaluate these therapies; furthermore, CKD patients with estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 are absent from most of the data sheets of monoclonal and antiviral antibodies active against SARS-CoV-2. However, in this period, experiences of their use in patients with advanced CKD and/or renal transplantation have been reported that support the use of these drugs in our patients.
The aim of this document is to compile the indications and the experience of different treatments against SARS-CoV-2 infection in CKD patients with the objective of facilitating homogeneous access to an effective treatment in this population.
Tables 1–4 show the definitions and risk stratification considered by the Spanish Agency of Medicines and Health Products (AEMPS) to prioritize access to anti-SARS-CoV-2 drugs.
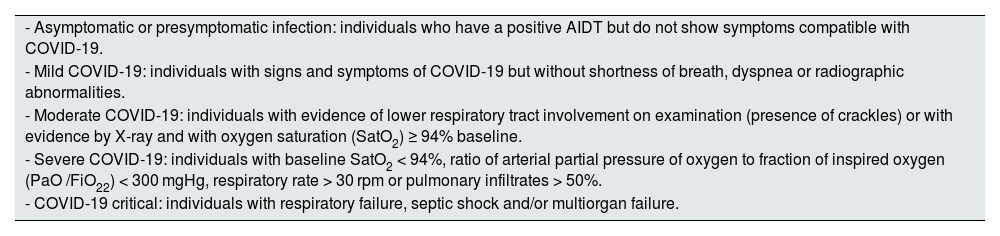
Definition of COVID-19 clinical settings.
| - Asymptomatic or presymptomatic infection: individuals who have a positive AIDT but do not show symptoms compatible with COVID-19. |
| - Mild COVID-19: individuals with signs and symptoms of COVID-19 but without shortness of breath, dyspnea or radiographic abnormalities. |
| - Moderate COVID-19: individuals with evidence of lower respiratory tract involvement on examination (presence of crackles) or with evidence by X-ray and with oxygen saturation (SatO2) ≥ 94% baseline. |
| - Severe COVID-19: individuals with baseline SatO2 < 94%, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO /FiO22) < 300 mgHg, respiratory rate > 30 rpm or pulmonary infiltrates > 50%. |
| - COVID-19 critical: individuals with respiratory failure, septic shock and/or multiorgan failure. |
AIDT: test for detection of active infection (antigen test or PCR); SatO2: oxygen saturation.
Patients with severe immunosuppression.
| - Recipients of solid organ, hematopoietic progenitor or CAR-T cell transplants in the first two years after transplantation or under immunosuppressive treatment to avoid rejection. |
| - Hemodialysis and peritoneal dialysis |
| - Combined primary immunodeficiencies and lack of vaccine response |
| - Patients with oncologic or hematologic disease with active treatment with myelotoxic chemotherapy or with neutropenia (< 500 neutrophils/µL) or lymphopenia (< 1000 lymphocytes/µl). |
| - HIV-infected patients with lymphocyte T CD4 count < 200 cells/mm.3 |
| - Patients with Cystic fibrosis. |
| - Down syndrome patients 40 years of age and older |
| - Treatment with oral corticosteroids at high doses and for a prolonged period. |
| - Treatment with certain non-biological immunomodulators in the previous three months: methotrexate, leflunomide, mercaptopurine, azathioprine, cyclosporine, mycophenolate, tacrolimus and sirolimus. |
| - Treatment with biological immunomodulators within the previous three months: inhibitors of cell proliferation B cell proliferation, lymphocyte T suppressor fusion proteins, interleukin inhibitors, anti-CD 52 monoclonal antibodies, sphingosine l-phosphate modulators, protein kinase inhibitors or JAK or BK family inhibitors |
| - Treatment with anti-CD20 monoclonal antibodies in the previous six months |
BK: Bruton's kinase; CAR-T: chimeric antigen receptor; JAK: Janus Kinase; HIV: human immunodeficiency virus.
The rapid development and commercialization of SARS-CoV-2 vaccines has clearly changed the course of the disease in countries with high vaccination rates. However, there is controversy about the response to vaccination in patients with CKD and/or RRT.6 In March 2022, the data from the prospective SENCOVAC study was published: this study assessed the immediate humoral response and safety of vaccines against SARS-CoV-2 in patients with CKD.7 This registry included patients on RRT (hemodialysis, peritoneal dialysis and renal transplantation) and also CKD of any etiology and stage who received mostly messenger RNA (mRNA) vaccines. The rate of antibody (Ac) generation against the S protein was practically 100% at 28 days post-vaccination except in the renal transplant population, where it remained below 80%. The measurement of anti-protein S antibodies was repeated at three and six months. In the majority, the drop of antibodies observed between months three and six was recovered after the third dose, except in the renal transplant population, where again, the seroconversion rate remained below 80%.8 The fourth dose of vaccine maintained a similar efficacy profile, such that patients without serological response have maintained a suboptimal response after the fourth dose.9 However, the current strategy of vaccination has demonstrated the ability to reduce the severity and mortality associated with COVID-19 in all CKD patients including renal transplant recipients, probably due to a relevant role of cell-mediated immunity acquired through vaccination.10,11
One of the main barriers to adherence to recommended vaccine regimens has been the concern about adverse effects. The Cochrane review conducted on data from 41 randomized clinical trials including more than 43,000 participants shows that the rate of side effects of the main vaccines - mRNA and ChAdOx1 (Oxford-AstraZeneca)/SII-ChAdOx1 (Serum Institute of India) - is very low and no different from that of the placebo,12 a fact that has been confirmed in the population with CKD in the SENCOVAC study.9
Given the low occurrence of serious and long-term adverse effects, there are currently no contraindications or specific exceptions in patients with CKD and therefore patients with CKD should follow the vaccination guidelines proposed for the general population, adjusted to their risk stratification (Table 4).
Pre-exposure treatmentSeveral neutralizing antibodies against SARS-CoV-2 have been developed, but only Evusheld® (Tixagevimab 150 mg + Cilgavimab 150 mg) has been authorized for use as pre-exposure prophylaxis.
Evusheld® is a combination of two long-acting immunoglobulin G1k (IgG1k) monoclonal antibodies, cilgavimab and tixagevimab, derived from B cells from convalescent patients with SARS-CoV-2 infection. They simultaneously bind to nonoverlapping regions of the RBD receptor binding domain of the SARS-CoV-2 protein S, which prevents Receptor Binding Domain (RBD) binding to the human angiotensin 2-converting enzyme receptor, and thus virus entry into cells.13
The PROVENT clinical trial tested the efficacy and safety of Evusheld® in pre-exposure prophylaxis of symptomatic COVID-19 disease in unvaccinated adults without previous SARS-CoV-2 infection, seronegative and at increased risk of inadequate response to vaccination. Of the 5197 participants, only 10% had CKD (5% in the treated group and 5% in the placebo group). A relative risk reduction of symptomatic disease positive by reverse transcription PCR (RT-PCR) for SARS-CoV-2 of 77% (CI 95%: 46–90%, p < 0.001)) was demonstrated. The duration of the protective effect after a single dose is estimated to be at least six months.13 In terms of safety, most of the adverse effects were mild, in the form of headache, fatigue and cough.
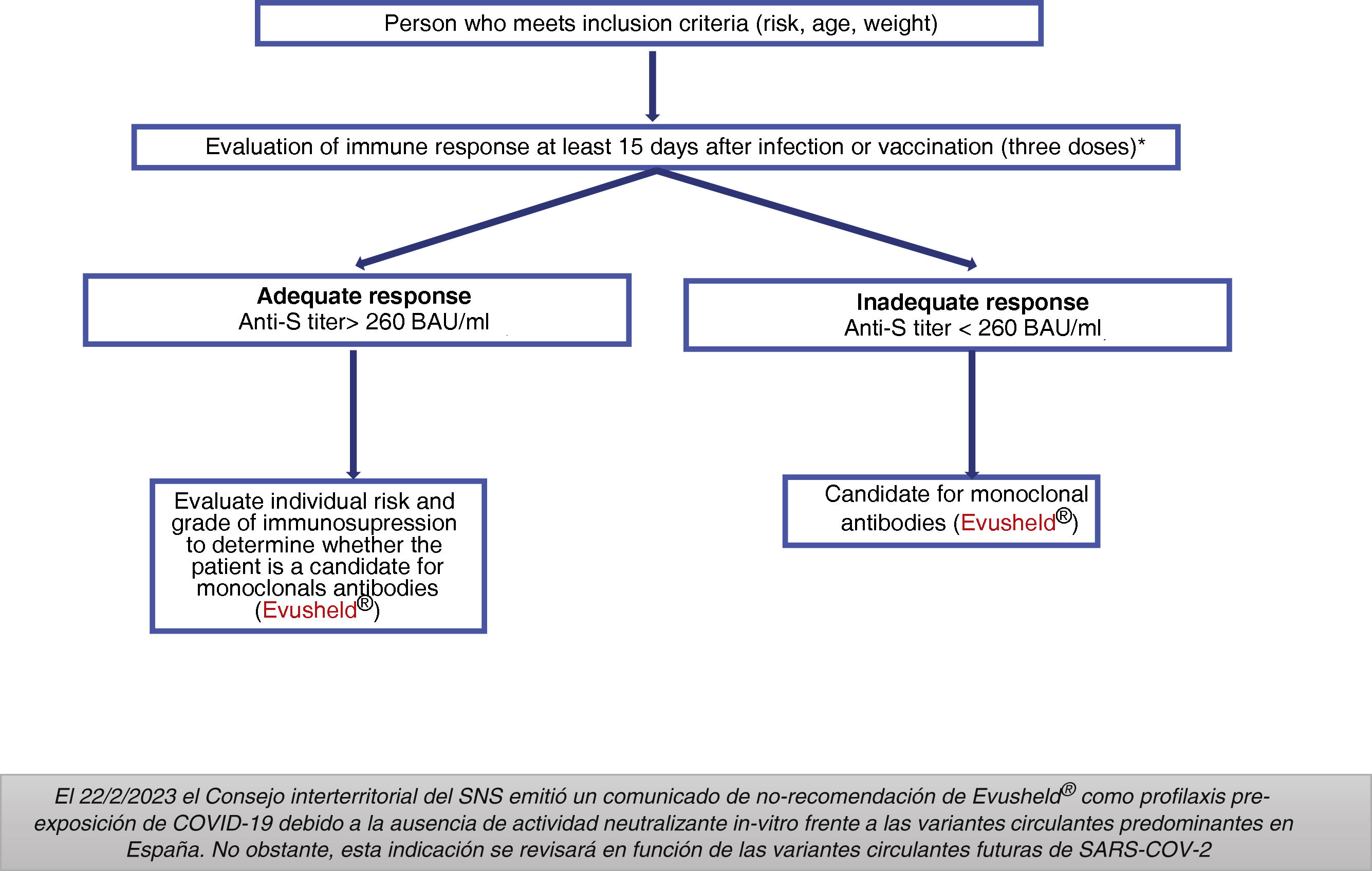
Indications: On 2/22/2023 the National Health System (SNS in Spanish) Interterritorial Council issued a statement of non-recommendation of Evusheld® as pre-exposure prophylaxis for COVID-19 due to the absence of in vitro neutralizing activity against the predominant circulating variants in Spain. However, this indication will be reviewed in the light of future circulating SARS-CoV-2 variants.
Groups previously considered as candidates for treatment with Evusheld®Adults and adolescents aged 12 years and older with a weight > 40 kg and high degree of immunosuppression (Table 4), who do not respond appropriately to vaccination or who are unable to complete the vaccination schedule.
The AEMPS required that potential patients to have received at least three doses of the vaccine (except for severe allergic reaction or severe adverse reaction related to the administration of any of the vaccines against COVID-19) and to have an inadequate serological response at least 15 days after the last dose of the vaccine (Fig. 1).14
Dose: 150 mg of tixagevimab and 150 mg of cilgavimab, administered as two separate sequential intramuscular injections. Does not require adjustment to renal function.
Pharmacokinetic interactions. None described. It does not have hepatic metabolism or renal elimination.
Adverse effects. Reactions in the injection site and hypersensitivity (rash and urticaria).15
Treatment of SARS-CoV-2 infectionOn February 23, 2023, the following report was published on www.aemps.gob.es: "Currently, the epidemiological situation is less complex and there is availability of different treatments against COVID-19 that are already marketed and, in some cases, have even been acquired through centralized European acquisitions. Therefore, intervention and prioritization of treatments by the AEMPS is no longer necessary. It is up to the physician, within normal clinical practice, to decide the most appropriate option according to the characteristics and specificities of his or her patient, in accordance with the technical data sheet or the instructions of the various health committees at the autonomous community or hospital level".16
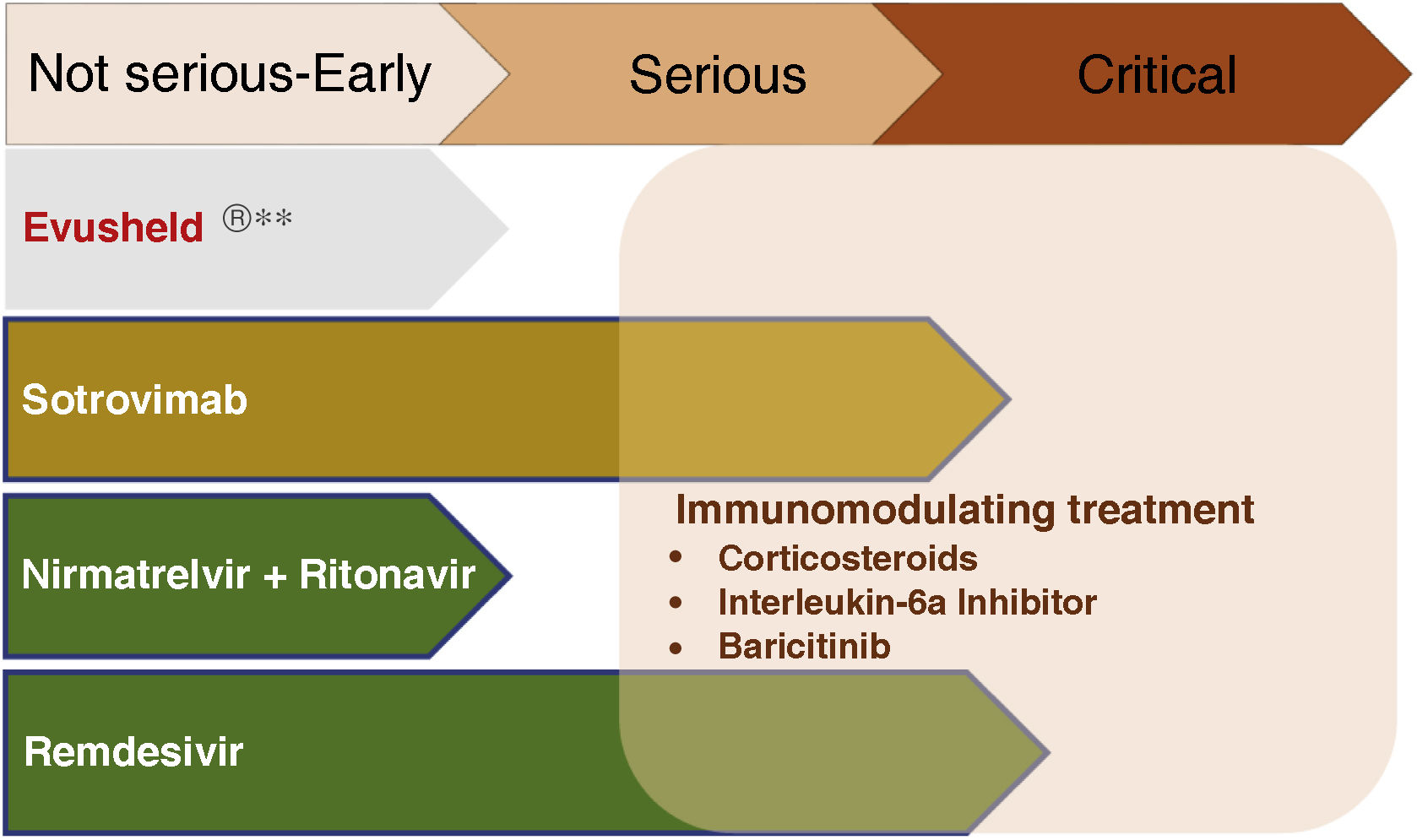
Treatment of early COVIDThe objective is to avoid progression to severe forms, hospitalization and death, both with antivirals and neutralizing antibodies. Fig. 2 summarizes the current indications in patients with CKD.
AntiviralsNirmatrelvir-ritonavir (Paxlovid)®Nirmatrelvir inhibits the major SARS-CoV-2 protease, Mpro or 3CLpro, critical for viral replication. It is combined with ritonavir, a potent cytochrome P450 (CYP)-3A4 inhibitor, which prolongs the half-life of the drug allowing dosing every 12 h.
The efficacy of this drug was tested in a randomized double-blind Phase 2–3 clinical trial versus placebo in symptomatic, unvaccinated patients with a high risk of progression to severe forms of COVID-19, demonstrating a decrease in the risk of progression to severe forms of 89% compared to the placebo group.17
The elimination of nirmatrelvir is mainly renal, and that of ritonavir is hepatic.
Indications. Adults with mild-moderate COVID-19 who are at high risk for progression to severe COVID-19, with duration of symptoms ≤ 5 days and positive antigen test.18
Dose. Within the first five days after the onset of symptoms.
- □
General dosage:
- ○
Nirmatrelvir 300 mg + ritonavir 100 mg orally twice daily.
- ○
Duration: 5 days.
- ○
- □
Dosage in renal insufficiency:
- ○
FGe ≥ 60 ml/min/1.73 m2 does not require adjustment.
- ○
GFR 30–60 ml/min/1.73 m2: nirmatrelvir 150 mg + ritonavir 100 mg orally twice daily.
- ○
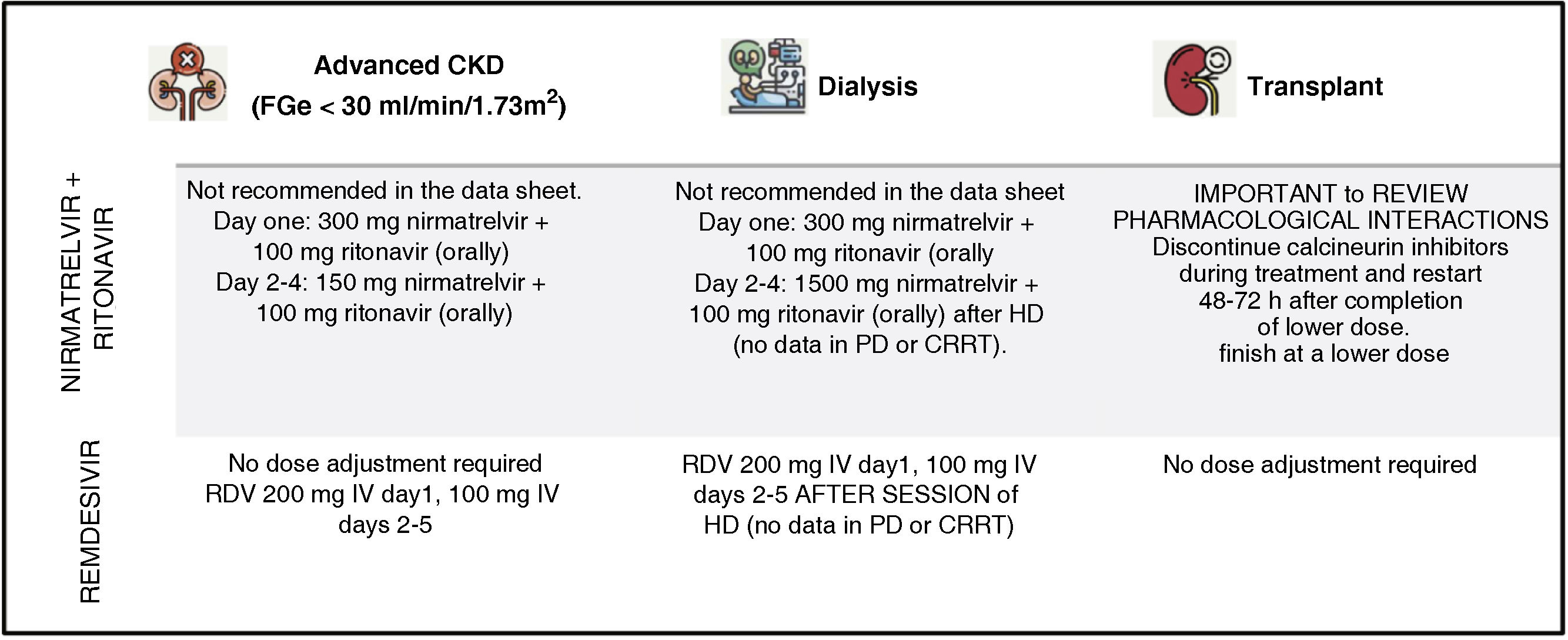
FGe <30 ml/min/1.73 m2: in the data sheet it is listed as not indicated. However, the use of 300 mg nirmatrelvir +100 mg ritonavir on day one followed by 150 mg nirmatrelvir +100 mg ritonavir once daily for 4 days has been described to have good efficacy (Mpro inhibition) a satisfactory safety profile.18–20
- ○
Dialysis patients: same dose as for eGFR < 30 ml/min/1.73 m2. No data in peritoneal dialysis and no data in continuous replacement techniques.
- ○
Renal transplantation: due to interaction with hepatic clearance (see below), calcineurin inhibitors should be discontinued during treatment with nirmatrelvir/ritonavir and restarted 48−72 h after completion of treatment at a lower dose than previously and monitoring levels.18
- ○
- □
Not recommended in severe hepatic insufficiency.
Pharmacological interactions. They are common, so it is recommended to carefully review the list of these interactions. For this purpose, you can access some of the digital tools developed, such as https://www.covid19-druginteractions.org/.21 Among the most common are direct acting anticoagulants and warfarin, statins, alpha-adrenergic antagonists, trazodone and hydromorphone, calcium channel antagonists, anticonvulsants and calcineurin inhibitors.22
Adverse effects:
- □
Nervous system disorders: dysgeusia, headache (frequent).
- □
Gastrointestinal disorders: vomiting, nausea (frequent), abdominal pain (rare).
- □
General disorders: general malaise (rare).
- □
Elevation of hepatic transaminases, clinical hepatitis and jaundice (very rare).
- □
Risk of development of HIV-1 resistance. (Ritonavir monotherapy treatment of a patient with uncontrolled or undiagnosed HIV-1 infection may develop resistance to HIV protease inhibitors).
Remdesivir (Veklury®) is a nucleoside analog that inhibits RNA-dependent polymerase with in vitro efficacy in inhibiting the replication of Middle East respiratory syndrome (MERS-CoV), SARS-CoV-1 and SARS-CoV-2 in respiratory epithelial cells. In experimental animal models, it has been shown to be effective in pre-exposure (12 h) and early post-exposure use. The efficacy of remdesivir was initially tested in the phase 3, randomized, double-blind, placebo-compared Adaptive Covid-19 Treatment Trial ACTT-1 clinical trial in hospitalized adult patients with COVID-19, demonstrating a decrease in hospitalization time of 10 days in the remdesivir group vs 15 days in the placebo group (relative risk [RR]: 1.29; CI 95%: 1.12–1.49; p < 0.001) overall, and of mortality in the group of patients with low-flow oxygen requirement (RR: 0.30; CI 95%: 0.14–0.64).23 Subsequently, the phase 3 PINE-TREE clinical trial24 has conclusively demonstrated a decrease in mortality associated with the use of the drug within 7 days of the onset of symptoms in patients with risk factors for progression. In addition, the adaptive clinical trial conducted by the World Health Organization, Solidarity phase 3, has demonstrated a reduction in progression and mortality in patients hospitalized for COVID-19 with the need for oxygen therapy, except for those on mechanical ventilation.25 The REDPINE study evaluated the efficacy and safety of remdesivir in patients with GFR < 30 ml/min/1.73 m2 (both CKD and acute renal failure with sustained deterioration of renal function), including patients on dialysis with a good safety profile.26
Indications27:
- □
Mild-moderate COVID-19 presenting any criteria for high risk of progression and ≤ seven days of symptoms and confirmed infection.
- □
COVID-19 severe.
- □
It is not indicated in critical COVID-19 with need for mechanical ventilation, although if the patient is on treatment with the drug prior to ventilation, it should be maintained.
Dose:
- □
Mild-moderate COVID-19 presenting any criteria for high risk of progression and ≤ seven days of symptoms or positive antigen test. Day one: 200 mg intravenous infusion. Day two and the following day: 100 mg/24 h intravenous infusion. Duration three days.
- □
Severe COVID-19: same dose as above, duration 5–10 days (even longer in the case of immunosuppressed patient and persistence of symptomatic viral replication).
Dose in renal insufficiency. Does not require adjustment, including dialysis patients. We recommend administering doses after dialysis session.16,18 There are no data on dose adjustment in peritoneal dialysis or continuous techniques.
Interactions. With chloroquine phosphate or hydroxychloroquine sulfate (antagonize in vitro antiviral activity). Remdesivir is a CYP 3A4 inhibitor, but due to its rapid elimination after intravenous administration, it is unlikely to have a significant effect on exposure to drugs that are substrates of this enzyme. However, increased levels of calcineurin inhibitors have been reported after the third day of treatment.28
Adverse effects. The most frequent adverse events are elevation of transaminases and prothrombin times, followed by nausea and vomiting, headache, skin rash (maculopapular rash). Sulfobutileter-beta-cyclodextrin, the excipient of Veklury®, is eliminated by the kidneys and can accumulate in patients with severe renal disease and also be associated with nephrotoxicity. However, in different studies evaluating the toxicity of remdesivir in patients with severe renal impairment (GFR < 30 ml/min/1.73 m2) no worsening of GFR was observed in treatments of five days duration or less.
Monoclonal antibodiesBoth hyperimmune serum and the use of monoclonal antibodies have been evaluated as a strategy to prevent the development of COVID-19 or at least its severe forms after exposure to SARS-CoV-2. In the case of monoclonal antibodies against SARS-CoV-2, these are derived from a clone of B cells directed against the S protein and can act by two mechanisms:
- □
Neutralization of the pathogen.
- □
Effector function, including antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity and opsonization through the crystallizable fragment (Fc region).29
It is worth reviewing the neutralizing capacity of the different monoclonal antibodies in relation to the currently circulating variants to assess the indication for treatment29. In the case of detection of a decreased neutralizing capacity, prioritize treatment with antivirals (nirmatrelvir or remdesivir) and/or evaluate the combined use with any of them.
SotrovimabThis is a monoclonal antibody obtained from a pool of human neutralizing monoclonal antibodies from an individual infected with SARS-CoV in 2003, which potently inhibited both human and zoonotic SARS-CoV isolates. Its anchor site on the S protein is distant from the RBM domain (common anchor site of neutralizing Ac), being less affected by variability in that site, mitigating viral escape.
The COMET-ICE Phase 3, is a randomized, double-blind, placebo-controlled clinical trial, which included patients with a diagnosis of mild or moderate COVID-19 with less than or equal to five days from the onset of symptoms and risk factors for progression to severe forms. This study showed a decrease in mortality and hospitalization for all-cause in patients treated with sotrovimab with a RR reduction of 79%; CI (50%–91%); p < 0.001.17
There is evidence for the use of sotrovimab in CKD: an observational cohort study conducted in the United Kingdom, which included 2367 patients in RRT (70% transplant patients and 30% on dialysis), observed that treatment with sotrovimab reduced the risk of hospitalization and/or death due to COVID-19 compared to treatment with molnupiravir (hazard ratio: 0.35; CI [0.17−0.71]).18 Furthermore, in the meta-analysis that analyzed the clinical outcomes of the use of sotrovimab in patients with RRT, including renal transplantation, sotrovimab significantly reduced the risk of hospitalization, ICU admission and mortality.19
Indications. Within five days of the onset of COVID-19 symptoms.
- □
Indicated for the treatment of COVID-19 in adults and adolescents (12 years or older and weighing more than 40 kg) who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19.
- □
There is also experience about the use of this compound in immunocompromised moderate-severe COVID-19 patients with negative serology or low level of protection for SARS-CoV-2.
Posology:
- □
Single dose of diluted perfusion of 500 mg intravenously. Surveillance is recommended in the first hour.
- □
Renal insufficiency. No dose adjustment is required in patients with renal insufficiency.
Interactions. No interaction studies have been performed. Sotrovimab is not eliminated through the kidneys nor it is metabolized by CYP enzymes.
Adverse effects. Diarrhea (2%), hypersensitivity reactions (2%), perfusion-related reactions (1%), anaphylaxis (0.05%).20
Treatment of severe COVID-19The development of therapeutic tools that reduces the proliferation of SARS-CoV-2 has decreased the frequency of progression to severe disease. However, there are still concepts that should be emphasized in the management of patients with severe forms.
The use of immunomodulatory drugs should be restricted to the inflammatory phase, avoiding their use in the early stages of the disease, where they may favor viral replication.
The following treatments, alone or in combination,21 have demonstrated efficacy in controlling the inflammatory phase of COVID-19:
- □
Corticosteroids.
- □
Interleukin-6 receptor antagonists (tocilizumab).
- □
Baricitinib.
The use of antivirals in this phase is limited to remdesivir, and only in severe non-critically ill patients. In mechanically ventilated patients, remdesivir did not modify the evolution of the disease compared to non-treatment.22 If the patient was receiving remdesivir before the establishment of mechanical ventilation, treatment will be continued until it is completed.
The use of monoclonal antibodies in this phase has been shown to decrease mortality in seronegative patients, as demonstrated by the RECOVERY study,23 with decreased mortality in hospitalized seronegative patients requiring oxygen. This trial used casirivimab-indevimab, a combination of monoclonal antibodies that are not effective against current variants and are therefore not used. Based on these results, monoclonal antibodies that maintain activity against the currently circulating variants are currently used in this indication.
In renal transplant recipients, it is recommended to suspend the anti-metabolite and maintain the calcineurin inhibitor and/or mammalian Target Of Rapamycin (mTOR) inhibitors in both the viral and inflammatory phases, given their potential immunomodulatory effect in this phase of the disease.24
Fig. 3 summarizes the use of drugs in the different phases of COVID-19.
Pharmacological treatment in the different phases of COVID-19.
**Not currently recommended by the Interterritorial Council of the National Health System based on the absence of neutralizing effect on strains circulating in Spain. Subject to modification depending on the evolution of SARS-CoV-2 variants.
CKD is a risk factor for the development of severe forms of COVID-19. Immunization obtained by successive vaccination schedules has been shown to reduce the risk of severe forms of COVID-19 in patients with CKD, including those patients with lower initial serological response rates. There are different pharmacological tools for the control of mild or moderate early disease that can be used effectively and safely in advanced CKD. In the case of transplanted patients or patients with immunosuppressive treatment, adjustment of the treatment can be useful to mitigate progression to severe forms.
Key concepts- □
Chronic kidney disease (CKD) is a risk factor for suboptimal serological response to SARS-CoV-2 vaccine strategies and for the development of complications from COVID-19.
- □
Clinical trials of the main drugs against SARS-CoV-2 have excluded patients with advanced CKD. However, during this period, cohort and real-life experience studies have been published in different groups of CKD patients.
- □
Vaccination has been shown to be effective in preventing the development of severe forms of infection with a good safety profile, and successive booster doses have improved the serological response rate even in patients with the worst response, such as renal transplant recipients. Therefore, vaccination is indicated in the entire spectrum of renal disease, including those on dialysis and with a kidney transplant.
- □
There are several treatments at the early stage of the disease to prevent progression to severe forms in patients at high risk of progression, which can be used in patients with CKD. Both the use of monoclonal antibodies and the use of antivirals in the early stages of COVID-19 can be used with guarantees even in patients with renal replacement therapy.
- □
The use of drugs aimed at controlling the inflammatory phase is also indicated in CKD. In the case of renal transplantation, suspension of the anti-metabolite while maintaining treatment with calcineurin inhibitors and/or (mTOR) inhibitors may be useful for the control of both viral and inflammatory phases of COVID-19.
The authors declare that they have no conflicts of interest.