Introducción: La natremia en los pacientes en hemodiálisis (HD) se considera constante, contrariamente a lo observado en la clínica diaria. Su relación con parámetros clínicos, de diálisis y con la distribución del agua corporal (AC) no está aclarada. Objetivos: Estudiar: 1) la variabilidad intrasujeto de la natremia, 2) la relación entre natremia y parámetros clínicos y dialíticos y 3) la relación entre natremia y distribución del AC por bioimpedancia. Material y métodos: Estudio observacional retrospectivo de 98 pacientes en HD crónica. Se recogieron características clínicas, de HD, natremia, glucemia y medidas de bioimpedancia. Resultados: Sesenta y tres varones y 35 mujeres de 69,6 (21-91) años con seguimiento de 23,2 (10) meses. Variabilidad: 1802 determinaciones de sodio: natremia media 138 (3,2) y corregida para glucemia: 139,1 (3,6) mEq/l, p < 0,0001. El coeficiente de variación (CV) intrasujeto fue 2 (0,8) % (rango: 1-5,6 %) y correlacionó negativamente con la natremia (r = –0,63, p < 0,0001). Parámetros clínicos: en diabéticos la natremia corregida era inferior a en no-diabéticos 138 (2,4) frente a 139 (2) mEq/l, p < 0,003, con CV de 2,3 (0,9) frente a 1,9 (0,7) % (p < 0,01) y desviación estándar de 3,2 (1,2) frente a 2,5 (0,9) mEq/l (p < 0,04). No encontramos diferencias según sexo, edad, tiempo en diálisis, cardiopatía, hepatopatía, fármacos, función renal residual ni mortalidad. Parámetros de HD: relación positiva entre natremia y conductividad del líquido de diálisis y negativa con ganancia de peso interdiálisis (GID). Bioimpedancia: no relación entre distribución AC y natremia. Conclusiones: La natremia varía en cada paciente y se relaciona positivamente con la conductividad y negativamente con la GID. En diabéticos la natremia es más baja y el CV es mayor. No existe relación entre natremia y la distribución del AC.
Background: Natraemia in haemodialysis (HD) patients is considered constant contrary to daily clinical observations. Its relationship with clinical parameters, dialysis parameters and body water (BW) distribution is not clear. Objectives: The aims of this study were to know 1) the intraindividual variability of natraemia, 2) the relationship between natraemia and clinical and dialysis parameters and 3) the relationship between natraemia and BW distribution by bioimpedance. Material and Method: Observational retrospective study on 98 chronic HD patients. Clinical, HD and natraemia, glucose and bioimpedance data were collected. Results: We included 63 males and 35 females of 69.6 (21-91) years of age, with a follow-up of 23.2 (10) months. Variability: 1802 sodium measurements: mean natraemia 138 (3.2) mEq/l and corrected for glucose: 139.1 (3.6) mEq/l, p<.0001. Intraindividual coefficient of variation (CV) was 2% (0.8) (range 1-5.6%) and it correlated negatively with natraemia (r=-0.63, p<.0001). Clinical parameters: corrected natraemia was lower in diabetics than in non-diabetics 138 (2.4) compared with 139 (2) mEq/l, p<.003, CV 2.3 (0.9) compared with 1.9 (0.7)% (p<.01) and SD 3.2 (1.2) compared with 2.5 (0.9) mEq/l (p<.04). No differences according to gender, age, HD time, cardiac or liver disease, medication use, residual renal function or mortality were found. HD parameters: a positive relationship was found between natraemia and total dialysate conductivity and it was negative with interdialysis weight gain (IDG). - Bioimpedance: no relationship was found between natraemia and BW distribution. Conclusions: Natraemia varies in each patient and is related positively with conductivity and negatively with IDG. In diabetics natraemia is lower and CV is higher. There is no relationship between natraemia and BW distribution.
In the last two years we have read several studies that establish a link between hyponatraemia and mortality in haemodialysis (HD) patients.1-4 In another study, Sahin et al.5 reported that hyponatraemia was only a predictor of mortality in diabetic HD patients. It is important to highlight that in each of these studies the plasma sodium value chosen as the predictive value was different: Natraemia at the time of inclusion (single value) or mean natraemia (over 3 months or one year). This decision is justified on the basis of publications that state that each patient has fixed natraemia or what has come to be known as setpoint.6-10
Natraemia indicates the relationship between the quantity of sodium and water in plasma. In HD patients, the determining factors of these elements are: 1) intake and 2) removal via HD and via the kidneys if there is residual renal function (RRF). Intake is regulated by physiological and non-physiological factors (Table 1);11 losing the renal capacity to remove water and salt makes the water/salt intake relationship a determining factor of natraemia. In fact, the study by Maduell et al. that measured plasma conductivity demonstrated that a low salt diet decreases pre-dialysis plasma conductivity.12 If we consider HD in terms of the balance achieved, we may generate mechanisms that stimulate or inhibit intake.13 For example, a positive sodium balance will increase thirst, blood pressure (BP) and extracellular volume (ECV).14-16 By contrast, if dialysate conductivity decreases progressively, as demonstrated by Manlucu et al., sodium removal will increase and BP and interdialysis weight gain (IDG) will decrease, which will modify plasma conductivity.17 Thomson et al.18 also demonstrated how changes in the HD regimen may alter natraemia. With the foregoing in mind for clinical practice, due to multiple determining factors, natraemia may vary both in accordance with intake and variations in the HD regimen, and as such, it is necessary to distinguish between them, particularly if we wish to correct hyponatraemia.
Moreover, natraemia governs volume distribution in different body spaces (intracellular volume [ICV]/ECV).19 Hypervolaemia, which has been associated with mortality,20 depends on an increased amount of sodium, not natraemia. But measuring the state of hydration is complicated and it has led to bioimpedance becoming widespread in clinical practice. However, we are unaware of any study that has related natraemia to hydration or the distribution of body volumes.
With these premises and in order to improve our clinical practice, we considered: 1) the variability of natraemia over time in each patient in relation to the measuring method; 2) the relationship between natraemia and different clinical and HD parameters, and 3) the relationship between natraemia and the distribution of body volume measured by bioimpedance.
MATERIAL AND METHOD
This is a retrospective, observational, clinical study of natraemia in 98 patients, with the only inclusion criteria being time on chronic HD of more than 3 months in the Hospital Universitario Infanta Leonor de Madrid between January 2010 and October 2012. Follow-up ranged from 4 months to the entire collection period, with a mean follow-up of 23.2 (10) months.
We collected data using the normal software (TSS®, Fresenius) on:
- Patients’ demographic and clinical characteristics: sex, age, time on HD, RRF measured as mean urea/creatinine clearance (>3ml/min), diabetes mellitus (yes/no), heart disease (ejection fraction <40%), cirrhosis with episodes of fluid retention (yes/no) and treatment with diuretics or antidepressants.
- HD sessions: technique, duration and total conductivity in dialysate. We also measured the annual mean (years 2010, 2011 and 2012) dry weight, pre-dialysis systolic and diastolic BP and IDG. We calculated IDG as a percentage of dry weight (IDG%).
- In our unit, we individually managed total dialysate conductivity. This was carried out in accordance with: BP, IDG and tolerance to the session (cramp, low blood pressure). Initial conductivity was 14mS/cm. If BP values were higher than 150/90mmHg, IDG higher than 1l/24h and haemodynamic tolerance during the session was good, we gradually reduced it by 0.1 to a minimum of 13.6Ms/cm. However, in patients with cramp and low BP, we increased conductivity by 0.1 up to a maximum of 14.2mS/cm.
- Pre-dialysis tests carried out routinely: date and time of removal of glucose and sodium.
- Bioimpedance measurements (carried out with a Body Compositor Monitor [BCM]®, Fresenius) performed before dialysis on the middle day of the week, in accordance with the normal protocol. We only used the measurements carried out in the same week as the natraemia biochemical test and we measured: pre-dialysis weight, overhydration (OH), ECV and ICV. We calculated relative overhydration (OH/ECV X 100)%, ECV/ICV, ECV percentage (ECV X 100/pre-dialysis weight) and ICV percentage (ICV x 100/pre-dialysis weight).
Laboratory tests and calculations of study parameters
The normal biochemical tests were carried out with an indirect potentiometry autoanalyser (ADVIA® 2400 Clinical Chemistry System, Siemens). The coefficient of variation (CV) was 0.7 for Na of 121mEq/l and 0.8 for Na of 142mEq/l.
In a group of 59 patients, we measured natraemia using two methods: the aforementioned indirect potentiometry method and direct potentiometry from the same serum sample (Rapidlab 1265®, Siemens).
All natraemias were corrected for glucose, considering that sodium decreases 1.6mEq/l for each 100mg/dl increase in glucose above 200mg/dl.
Statistical analysis
The data in this study were presented as mean and standard deviation (SD) or median (range) in non-normal distribution values. We calculated the CV of natraemia from the mean and SD of each patient.
For comparison of the two continuous independent variables we used the Student’s t-test for non-paired samples. To compare discrete variables we used the Χ2 test and Fisher’s test when necessary (n<5). We calculated Pearson’s correlation coefficient to assess the association between quantitative variables. A P value <.05 was considered to be statistically significant.
We created two study groups in accordance with natraemia: one based on the clinical definition of hyponatraemia22 (<135mEq/l) and another based on our statistical findings by tertiles.
We used the SPSS version 15.0 (SPSS, Chicago, USA) to carry out statistical analysis and create the graphs.
RESULTS
We studied 63 males and 35 females, with a median age of 69.6 (21-91) years. The median replacement therapy time was 33 (3-322) months. Thirty-six had RRF. Forty-three were diabetic, 27 had heart disease and 6 had liver disease. We collected data from patients’ clinical records with regard to treatment with drugs that potentially induce hyponatraemia, such as diuretics (n=12) and antidepressants (n=11).
In the follow-up 13 patients had died, 11 had received a transplant and 11 had been transferred to another centre or had changed technique.
Overall natraemia tests
In the recall period we carried out a total of 1802 sodium tests. The mean value was 138 (3.2) mEq/l, with a range between 122 and 147mEq/l (mean range 10.5 [4.3] mEq/l). Mean natraemia corrected for glucose was 139.1 (3.6) mEq/l. The difference between the two natraemias was statistically significant, P<.0001. Of the total tests carried out, 215 (11.9%) were less than 135mEq/l, while 811 (45%) were greater than 140mEq/l. Their distribution is displayed in Figure 1.
To measure variability, we first analysed the mean number of natraemias obtained for each patient, which was 18.7 (4-34). The mean CV of each patient (for corrected natraemia) was 2% (0.8) (range: 1%-5.6%). There was a negative correlation between natraemia and CV: r=-0.63, P<.0001. As such, when we carried out a separation based on natraemia tertiles (Table 2), it was highlighted that lower natraemias had a significantly higher CV, SD and range.
Lastly, Table 3 displays the number of natraemias below 135 and above 140 recorded per patient.
Relationship between natraemias and clinical parameters
Diabetics had a lower (corrected) natraemia than non-diabetics, 138 (2.4) compared with 139 (2) mEq/l, P<.003, with CV of 2.3 (0.9) compared with 1.9% (0.7) (P<.01) and SD of 3.2 (1.2) compared with 2.5 (0.9) mEq/l (P<.04). There were no significant differences in range between the two groups. IDG was not statistically different.
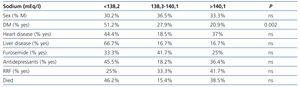
We did not observe differences in natraemias according to sex, age, time on dialysis, heart disease, liver disease, medication use or RRF (Table 4). Natraemia was not lower in those who had died during follow-up.
Relationship between natraemia and dialysis parameters
We found a positive relationship between mean natraemia in each patient and total dialysate conductivity, while the correlation between natraemia and IDG % was negative. There was no correlation between natraemia and pre-dialysis systolic and diastolic BP (Table 5). There were no differences in accordance with the dialysis technique used.
Comparison between direct and indirect potentiometry
In a prospective control that included 59 patients, we tested natraemia from the same extraction of pre-dialysis blood in both methods.
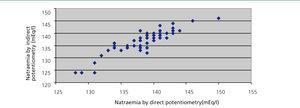
Pearson’s correlation coefficient between the two methods was 0.89 (95% confidence interval: 0.83-0.93) (Figure 2). Bland-Altman’s test showed that there were no significant differences; we were able to confirm that at least 3% of the data exceeded the two SD. We found no statistical correlation between natraemia and glucose, cholesterol or total protein concentration. Proteins ranged from 5.7 to 7.2mg/dl.
Relationship between natraemia and body volumes
We tested 136 BCM measurements in 57 patients on whom we had carried out bioimpedance and extracted the samples in the same week.
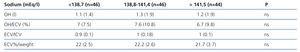
The mean values obtained were: natraemia 139.5 (3.1) mEq/l, OH: 1l (-2.8-8.1), OH/ECV %: 6.8 (–16.7-34.2), ECV 16l (3.4), ECV %/weight 22% (3), ICV 16.3l (4.1), ICV %/weight 22.4% (4.7), ECV/ICV: 1 (0.1). 16.2% of measurements displayed OH/ECV % values above 15%. We did not find any correlation between any of these parameters and natraemia at the time they were measured or when we separated them in accordance with this group’s natraemia tertiles (Table 6).
DISCUSSION
Our study has three main results. The first is that natraemia is not constant in all patients. The second is that natraemia is positively related with dialysate conductivity and negatively with IDG %. The third result is that natraemia is not related to the distribution of body water, which highlights the importance of separating the concepts of natraemia and volaemia.
Until present, three studies have stated that there is a sodium set-point or “fixed” natraemia in HD patients based on a low CV. Our data confirm that natraemia has a CV that may be considered low mathematically but clinical practice (Table 3) and the wide range reveal that the sodium/water relationship may change and may not be constant due to multiple factors. This table clearly demonstrates the situation that we find daily in clinical practice, since almost 60% of patients had some sodium measurement that was less than 135 or more than 140mEq/l. By the same token, Basile et al.8 found that despite a low CV, the intraindividual range was 6.2±2.9 and was higher than 10mEq/l in 11 subjects. In addition, Peixoto et al.6 despite claiming that this set-point exists, demonstrated that 21% of patients had a range higher than 10mEq/l. Furthermore, our results show that variability is higher in patients with natraemia of less than 138mEq/l (Table 2). It is easy to observe from the physiological point of view that although thirst is very well regulated, in daily life there are other non-physiological factors that modify liquid or salt intake and that make natraemia inconstant. Strikingly, CV is higher in diabetics, which could be attributed to the fact that in this group, thirst is stimulated in accordance with blood glucose, although IDG is not statistically significant. Although in previous studies the percentage of diabetics was high (reaching 47% in some studies6), until now, this population had not been studied separately and furthermore it has a lower (corrected) natraemia per se. This variability is important when evaluating natraemia as a mortality factor considered “constant” and determining its significance and the value to be selected for these studies and investigating whether there are differences in accordance with the studied populations.
Out of the three studies cited regarding the set-point, in two of them natraemia was measured with indirect potenciometry7,9 and in the other with direct potentiometry.8 In normal clinical practice the method used is indirect potentiometry, considered the reference for evaluating other methods.23 Our results show an excellent correlation between the two methods and all the results were obtained in the same laboratory, which complies with ISO standards. In any case, given that the method employed in most hospitals is indirect potentiometry, we believe that our results are the most relevant and most applicable to clinical practice.
In our study we did not find differences in natraemia in terms of the associated diseases or mortality, which may be due to the fact that we had a small number of patients in comparison to the other large studies previously published and because we used a mean natraemia over a long follow-up period. Only diabetic patients had lower corrected natraemias, which we attribute to the abovementioned causes.
In relation to the dialysis parameters, we found an association between natraemia and total conductivity in the dialysate, which had not been found in other studies. These results must be interpreted while bearing in mind that it is a retrospective study of a unit in which we individualised dialysate conductivity on the basis of its potential effect on BP and IDG, as was explained in the section “Material and Method”. For example, on the basis of the criteria of the year 2012: 6% of patients had a conductivity of 13.6%, 18.7% had 13.7, 31.8% had 13.8, 24.8% had 13.9, 16.9% had 14 and 1.5% had 14.1mS/sec conductivity. In the major studies cited,3,4 when they tried to explain the role that sodium selection in dialysate plays in mortality, we found disparate results that were difficult to interpret. They did find that sodium concentration in dialysate is related to IDG and BP, but not to natraemia. However, in these studies, the criteria for choosing the dialysate are unknown. The effects of changing sodium concentration in dialysate on natraemia found in the literature are variable. While this relationship is clear in the study by Manculu et al.17, in which decreasing dialysate conductivity decreases initial plasma conductivity, the results are different in the study by Paula et al.24. If the differences are due to the latter being based on sodium that is calculated by machine, this is not explained. Many current monitors calculate sodium plasma concentration on the basis of ionic dialysance. Although plasma natraemia and calculated natraemia are different,25 their values are very useful in clinical practice. The point is that when we act on dialysate conductivity to intervene in BP and IDG we modify the sodium balance by diffusion and as a result, we influence natraemia. We do not know the consequences of this action. Since our study is retrospective, we cannot know if lower natraemia is the cause or the consequence of choosing a dialysate with lower conductivity in accordance with clinical parameters. On viewing the existing literature and our data, when the dialysis regimen is modified (changes in conductivity17 or frequency18) natraemia may vary. As such, if decreasing sodium conductivity to control BP or IDG has the result of decreasing natraemia, it would be necessary to conduct new prospective studies to determine if the intended benefit might be offset by decreased natraemia.
Natraemia was also negatively related to IDG %. This could be explained by these patients having more sodium gain than water, highlighting the water/sodium relationship, or else because in those who had higher gains we tried to decrease sodium concentration in dialysate to limit the positive balance and thirst. As with the previous point, since this was a retrospective study we were unable to distinguish between cause and effect.
Lastly, we would like to comment on the bioimpedance results. Contrary to what we theoretically thought we would find, patients with hyponatraemia did not display a relative increase in ICV or differences in distribution between ECV and ICV or OH. This finding highlights that, like with the population with normal renal function, we could find hyponatraemia with hyper/hypo/normovolaemia and this highlights the complexity of managing the balances volaemia (=sodium) and natraemia (=water) with HD. Until now, no publication had been written in this regard, which means that our study is contributing very new information and is opening a field of research.
As limitations to our results, we have already mentioned that the retrospective nature is a confusing factor when it comes to interpreting the relationship between natraemia and dialysate conductivity and IDG % but it does not affect the variability results for natraemia in a normal clinical practice in which the situation can change. The small number of patients could explain why we did not find a difference in mortality or other epidemiological factors. Lastly, must say that we know bioimpedance is not a direct measure of different volumes but it is a very useful tool and one that is employed extensively in dialysis units to assess hydration. As such, although it is not the gold standard method for measuring body water, its use has been validated and on the basis of daily practice, we can say that its results on the absence of a relationship between hydration and natraemia are clear.
In conclusion, natraemia in HD patients has a low CV, but it does not display a constant value, especially in patients with a tendency for hyponatraemia and it is necessary to correct it for blood glucose. This CV is higher in diabetics, in whom natraemia is lower. The lack of association between natraemia and volume highlights the need to independently assess the sodium and salt balances that each patient requires, insisting once again on the importance of individualised HD.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Factors involved in water and salt intake
Table 2. Differences in the intraindividual coefficient of variation and standard deviation in accordance with natraemia tertiles
Table 3. Distribution of patients in accordance with the natraemias found
Table 4. Percentage of patients grouped by tertiles according to clinical characteristics
Table 5. Mean natraemia correlation for each patient with conductivity used in the dialysate over one year and weight gain during this year
Table 6. Bioimpedance results in accordance with the natraemia tertiles
Figure 1. Distribution of all natraemias measured (n=1802).
Figure 2. Relationship between direct and indirect potentiometry