Our aim was to assess the usefulness of glomerular filtration rate (GFR) and urinary albumin excretion (UAE) to predict the risk of mortality in patients with type 2 diabetes mellitus.
Material and methodsThis is a prospective cohort study in patients with type 2 diabetes mellitus. Clinical end-point was mortality rate. GFR was measured in ml/min/1.73m2 and stratified in 3 categories (≥60; 45–59; <45); UAE was measured in mg/24h and was also stratified in 3 categories (<30; 30–300; >300). Mortality rates were reported per 1000 patient-years. Cox regression models were used to predict mortality risk associated with combined GFR and UAE. The predictive power was estimated with C-Harrell statistic.
ResultsA total of 453 patients (39.3% males), aged 64.9 (SD 9.3) years were included; mean diabetes duration was 10.4 (SD 7.5) years. Median follow-up was 13 years. Total mortality rate was 39.5/1000. The progressive increase in mortality in the successive categories of GFR and UAE was statistically significant (P<.001). In a multivariable analysis, UAE (HR30-300=1.02 and HR>300=2.83; χ2=11.6; P=.003) and GFR (HR45-59=1.34 and HR<45=1.84; χ2=6.4; P=.041) were independent predictors for mortality, with no significant interaction. Simultaneous inclusion of GFR and UAE improved the predictive power of models (C-Harrell 0.741 vs. 0.726; P=.045).
ConclusionsGFR and UAE are independent predictors for mortality in type 2 diabetic patients and do not show a statistically significant interaction.
Nuestro objetivo fue evaluar la influencia de la tasa de filtración glomerular (TFG) y de la excreción de albúmina urinaria (EAU) sobre el riesgo de mortalidad en pacientes con diabetes mellitus tipo 2 (DM2).
Material y métodosEstudio de cohortes prospectivo con inclusión de pacientes con DM2. El punto final clínico fue mortalidad total. La TFG se midió en ml/min/1,73 m2 con estratificación en 3 categorías (≥60; 45-59; <45) y la EAU en mg/24h con estratificación también en 3 categorías (<30; 30-300; >300).
Se evaluaron las tasas de mortalidad por cada 1.000 pacientes/año y, mediante regresión de Cox, el riesgo de mortalidad asociado con las categorías de TFG y EAU. El poder predictivo se midió con el estadístico C de Harrell.
ResultadosSe incluyó a 453 pacientes (39,3% varones, edad 64,9 [DE 9,3] años y evolución de DM2 10,4 [DE 7,5] años). Durante una mediana de 13 años de seguimiento, la tasa de mortalidad total fue de 39,5/1.000, con incremento progresivo ante descenso de la TFG y aumento de la EAU (p<0,001). En análisis multivariante la EAU (HR30-300=1,02 y HR>300=2,83; chi2=11,6; p=0,003) y la TFG (HR45-59=1,34 y HR<45=1,84; chi2=6,4; p=0,041) fueron predictores independientes de mortalidad sin interacción significativa. La inclusión de TFG y EAU mejoró la capacidad predictiva de los modelos (C de Harrell 0,741 vs. 0,726; p=0,045).
ConclusionesLa TFG y la EAU son predictores independientes de mortalidad en pacientes con DM2, sin interacción significativa.
The presence of diabetes mellitus (DM) increases patients’ cardiovascular risk and it is considered to be equivalent to coronary heart disease risk.1 However, not all patients with DM have the same cardiovascular risk.2 It is widely known that the presence of chronic kidney disease (CKD) increases the risk of overall mortality and cardiovascular mortality (CVM) in the general population. This increased risk is associated to both increased urinary albumin excretion (UAE) and reduced glomerular filtration rate (GFR). These two variables provide independent information.3
Patients with diabetic nephropathy have CVM rates well above the very high risk4 threshold that has been defined by the European Society of Cardiology (ESC) guidelines.5 Due to their prognostic significance, monitoring of both GFR and UAE is recommended in follow-up of patients with DM.6,7 Furthermore, it is recognised that in DM patients with CKD the approach to reduce the increased cardiovascular risk, should be multidisciplinary.
In the review by the Chronic Kidney Disease Prognosis Consortium,3 most of the studies included that assessed mortality risk in association with the presence of CKD were of less than 10 years’ duration and did not include populations from Spain.
The aims of our study, using long-term follow-up of a cohort of patients with type 2 DM, were:
- 1.
To describe mortality rates as a function of GFR, UAE, and the combination of the two variables.
- 2.
To ascertain that GFR and UAE offer independent information on patient mortality risk at long-term follow-up.
A prospective cohort study.
Study populationThis is described in detail in previous articles.8,9 Between 1 June 1994 and 1 June 1998, we selected 463 patients with type 2 DM being followed as endocrinology outpatients at the Hospital Comarcal de Alcañiz (Alcañiz district hospital). This hospital has a referral population of 70,000 patients.
The inclusion criteria were: diabetes diagnosed using the World Health Organisation criteria valid at the time of diagnosis,10 older than 35 years at the time of diagnosis, and absence of treatment with insulin for at least 1 year after diagnosis. Exclusion criteria were: severe somatic disease, pregnancy, uncontrolled hyperthyroidism or hypothyroidism, systemic corticoid treatment, or advanced renal failure (creatinine greater than 3mg/dL). The study design was approved by the hospital ethics committee, and all participants gave their verbal consent before being enrolled.
Baseline examinationOn enrolment, patients were interviewed, noting their age, sex, known time with DM, smoking habits (active smoker, ex-smoker, or nonsmoker), DM treatment (diet, oral hypoglycaemics, or insulin) and any manifestation of existing vascular disease (ischaemic heart disease, lower limb ischaemia, or cerebrovascular disease). Physical examination included measurement of weight and height (and calculation of body mass index, defined as weight in kg/[height in metres] squared), and systolic and diastolic blood pressure, with classification of patients as hypertensive if systolic pressure was above 140mmHg or diastolic above 90mmHg or if they were on antihypertensive treatment. A venous blood sample was taken, after an overnight fasting (10h) and before taking antidiabetic medication; biochemical measurements included: blood glucose, glycosylated haemoglobin (HbA1c), creatinine, total cholesterol, and triglycerides. A 24h urine sample was collected, and after excluding urinary infection, UAE was measured, with samples classified as normoalbuminuria (<30mg), microalbuminuria (30–300mg), or macroalbuminuria or proteinuria (>300mg). Biochemistry was measured with a Shimadzu CL 7200 auto-analyser. HbA1c was measured with a Cobas Mira Plus automatic analyser, with a normal range of 4.5–5.7%. UAE was measured using immunoturbidimetry on a Cobas Integra 700 analyser.
GFR was calculated in mL/min per 1.73m2 using the CKD-EPI formula:
If (woman and creatinine≤0.7) GFR=144*([creatinine/0.7]**−0.329)*(0.993**age).
If (woman and creatinine>0.7) GFR=144*([creatinine/0.7]**−1.209)*(0.993**age).
If (man and creatinine≤0.9) GFR=141*([creatinine/0.9]**−0.411)*(0.993**age).
If (man and creatinine>0.9) GFR=141*([creatinine/0.9]**−1.209)*(0.993**age).
GFR was categorised into 3 groups (≥60, 45–59, <45), as there were only 4 patients with a filtrate <30mL/min per 1.73m2
Cohort follow-upAll patients were prospectively followed up until their death or date of study closure on 31 August 2012. Causes of death were obtained from the hospital clinical notes or by contacting the general practitioner who signed the death certificate. Deaths were considered cardiovascular if they were sudden death, due to myocardial infarction, due to end stage heart failure, or due to cerebrovascular disease. In 4 patients (0.9%), it was not possible to determine their vital status and they were considered lost to follow-up.
Statistical methodsQuantitative variables are reported as mean and standard deviation (SD), and qualitative variables are reported as frequency distribution. Quantitative variables were compared using Student t test or the nonparametric Mann–Whitney test (time since onset of DM and triglycerides). For comparison of qualitative variables, a chi-square test was used.
The primary outcome variable was overall mortality. Patients were stratified into 9 categories according to their GFR and UAE. The category with GFR≥60 and UAE<30 was used as the reference category.
Patients were followed up from their inclusion in the study until leaving the study due to reaching the closing date, death, or being lost to follow-up. The rates of the different events are expressed per 1000 patient-year. Rates were compared between the different categories considered using Kaplan–Meier analysis and the log-rank test.
Subsequently, Cox-regression models were used, with calculation of hazard ratios (HR) and 95% confidence intervals (CI), to assess the risk conferred by the presence of the different categories of GFR and UAE, and their interaction. Univariate and multivariate models were used, adjusting for age, sex, smoking, presence of arterial hypertension, existing vascular disease, cholesterol and triglycerides (introduced in a logarithmic form), HbAlc, and time since onset of DM. The independent predictors of total mortality were determined using a sequential elimination procedure. The improved predictive power assumed by inclusion of GFR+UAE in the models was determined with Harrell's C statistic. Associations with a P value<.05 were considered statistically significant. The software used was SPSS version 22.0.
ResultsOf 463 patients, 453 were included; in 10 patients there was no information on GFR or UAE. Among patients included, 178 (39.3%) were men. Mean age was 64.9 years (SD 9.3 years) and the mean time since onset of DM was 10.4 years (SD 7.5 years). At the time of enrolment, 192 (42.4%) patients were on treatment with insulin and 98 (21.6%) had evidence of vascular disease.
Regarding GFR, 69.8% of patients had a GFR≥60mL/min/1.73m2, 23.4% had a GFR between 45 and 59mL/min/1.73m2, and 6.8% had a GFR<45mL/min/1.73m2. The prevalence of normo- micro- and macroalbuminuria was 70.9%, 23.1%, and 6%, respectively.
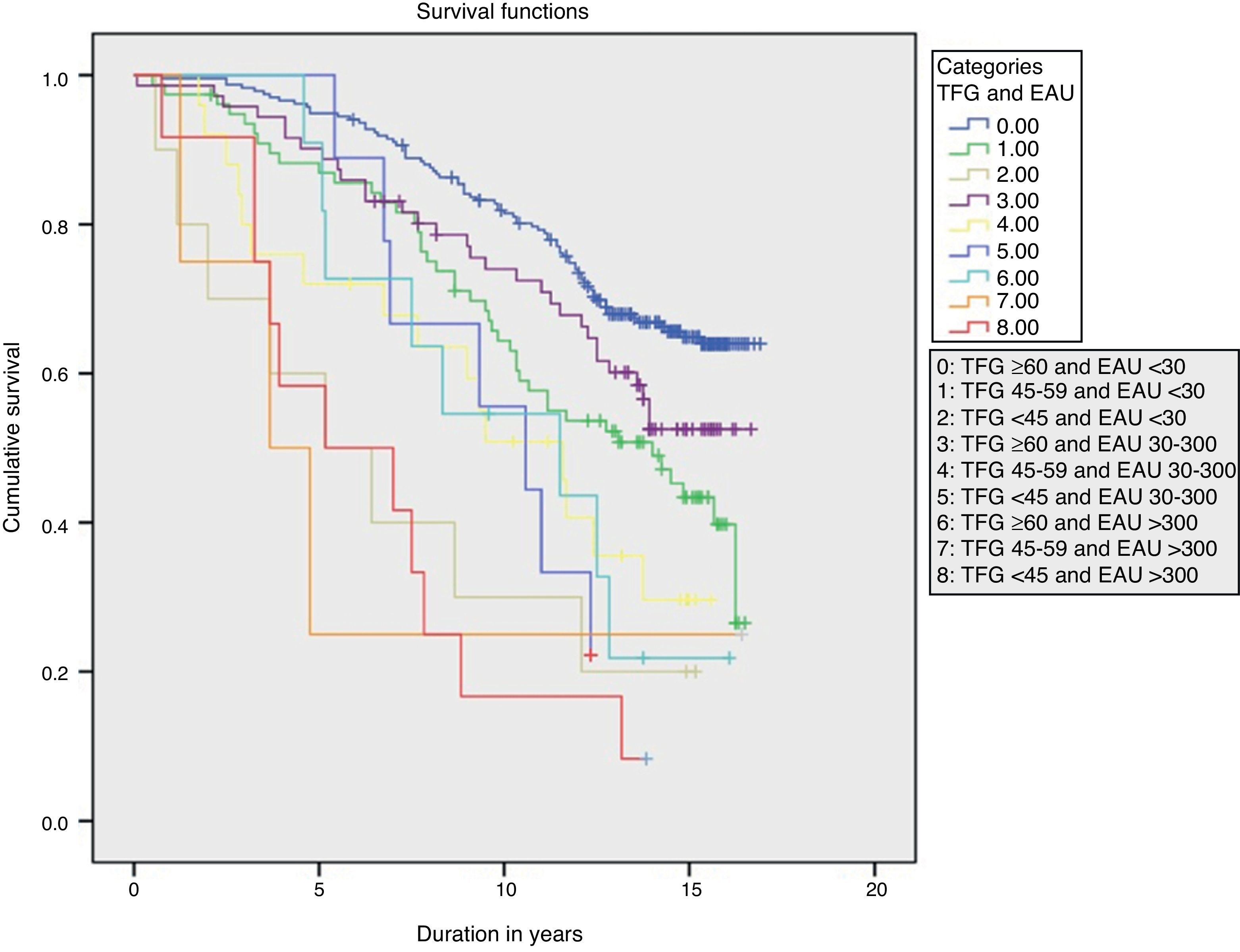
Over a median 13-year follow-up (interquartile range 6.5 years; minimum 1 month, maximum 17 years) there was a total of 207 deaths (rate 39.5/1000), of which 74 were cardiovascular deaths (rate 14.1/1000). There was a significant linear trend in the progressive increase of overall mortality rates as GFR deteriorated and UAE increased (P<.001) (Fig. 1).
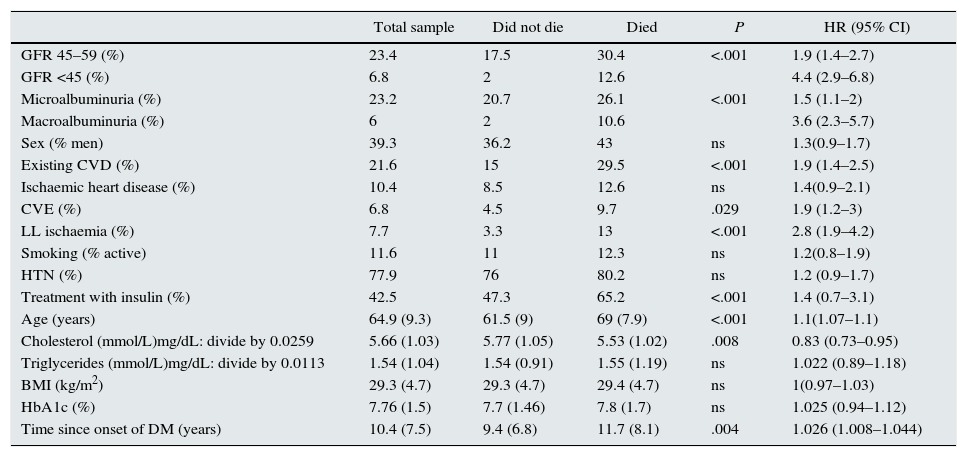
Patient characteristics according to survival or non-survival during follow-up are shown in Table 1. Compared with patients who survived, the patients who died were older and had a longer time since onset of DM, higher prevalence of initial insulin treatment, lower cholesterol levels, higher prevalence of macroangiopathy, lower GFR, and higher UAE.
Patient characteristics according to survival during follow-up. The variables are reported as mean (SD) or frequency distribution.
| Total sample | Did not die | Died | P | HR (95% CI) | |
|---|---|---|---|---|---|
| GFR 45–59 (%) | 23.4 | 17.5 | 30.4 | <.001 | 1.9 (1.4–2.7) |
| GFR <45 (%) | 6.8 | 2 | 12.6 | 4.4 (2.9–6.8) | |
| Microalbuminuria (%) | 23.2 | 20.7 | 26.1 | <.001 | 1.5 (1.1–2) |
| Macroalbuminuria (%) | 6 | 2 | 10.6 | 3.6 (2.3–5.7) | |
| Sex (% men) | 39.3 | 36.2 | 43 | ns | 1.3(0.9–1.7) |
| Existing CVD (%) | 21.6 | 15 | 29.5 | <.001 | 1.9 (1.4–2.5) |
| Ischaemic heart disease (%) | 10.4 | 8.5 | 12.6 | ns | 1.4(0.9–2.1) |
| CVE (%) | 6.8 | 4.5 | 9.7 | .029 | 1.9 (1.2–3) |
| LL ischaemia (%) | 7.7 | 3.3 | 13 | <.001 | 2.8 (1.9–4.2) |
| Smoking (% active) | 11.6 | 11 | 12.3 | ns | 1.2(0.8–1.9) |
| HTN (%) | 77.9 | 76 | 80.2 | ns | 1.2 (0.9–1.7) |
| Treatment with insulin (%) | 42.5 | 47.3 | 65.2 | <.001 | 1.4 (0.7–3.1) |
| Age (years) | 64.9 (9.3) | 61.5 (9) | 69 (7.9) | <.001 | 1.1(1.07–1.1) |
| Cholesterol (mmol/L)mg/dL: divide by 0.0259 | 5.66 (1.03) | 5.77 (1.05) | 5.53 (1.02) | .008 | 0.83 (0.73–0.95) |
| Triglycerides (mmol/L)mg/dL: divide by 0.0113 | 1.54 (1.04) | 1.54 (0.91) | 1.55 (1.19) | ns | 1.022 (0.89–1.18) |
| BMI (kg/m2) | 29.3 (4.7) | 29.3 (4.7) | 29.4 (4.7) | ns | 1(0.97–1.03) |
| HbA1c (%) | 7.76 (1.5) | 7.7 (1.46) | 7.8 (1.7) | ns | 1.025 (0.94–1.12) |
| Time since onset of DM (years) | 10.4 (7.5) | 9.4 (6.8) | 11.7 (8.1) | .004 | 1.026 (1.008–1.044) |
BMI, body mass index; CVD, cardiovascular disease; CVE, cerebrovascular event; CI, confidence interval; GFR, glomerular filtration rate in mL/min/1.73m2; HR, hazard ratio; HTN, hypertension; LL, lower limbs; UAE, urinary albumin excretion.
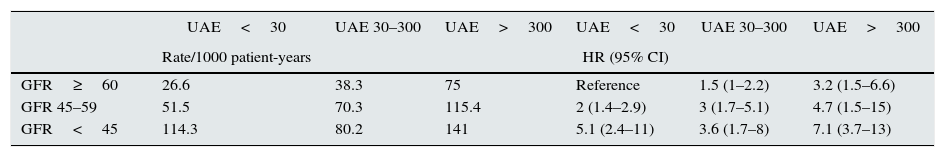
Table 2 shows the rates and HR for mortality according to the GFR and UAE levels. The interaction between GFR and UAE was not significant. In multivariate analysis, the variables that were independently associated with mortality risk, in order of predictive power, were age (HR=1.092, χ2=77.1, P<.0001), UAE (HR30–300=1.02 and HR>300=2.3, χ2=11.6, P=.003), presence of existing vascular disease (HR=1.7, χ2=9.9, P=.002), active smoking (HR=1.84, χ2=6.9, P=.032), GFR (HR45–59=1.34 and HR<45=1.84, χ2=6.4, P=.041), and initial cholesterol levels (HR=0.843, χ2=4.9, P=.026).
Mortality rates per 1000 patient-years and mortality risk according to the combined GFR and UAE categories.
| UAE<30 | UAE 30–300 | UAE>300 | UAE<30 | UAE 30–300 | UAE>300 | |
|---|---|---|---|---|---|---|
| Rate/1000 patient-years | HR (95% CI) | |||||
| GFR≥60 | 26.6 | 38.3 | 75 | Reference | 1.5 (1–2.2) | 3.2 (1.5–6.6) |
| GFR 45–59 | 51.5 | 70.3 | 115.4 | 2 (1.4–2.9) | 3 (1.7–5.1) | 4.7 (1.5–15) |
| GFR<45 | 114.3 | 80.2 | 141 | 5.1 (2.4–11) | 3.6 (1.7–8) | 7.1 (3.7–13) |
CI, confidence interval; GFR, glomerular filtration rate in mL/min/1.73m2; HR, hazard ratio; UAE, 24h urinary albumin excretion.
Harrell's C statistic was 0.726 in the baseline mortality prediction model, composed of age, sex, smoking status, presence of arterial hypertension and existing vascular disease, cholesterol and triglycerides, HbAlc, and time since onset of DM. Inclusion of GFR and UAE improved the predictive power for mortality of the model (Harrell's C statistic 0.741, P=.045 vs. previous model).
DiscussionThis prospective follow-up over a median of 13 years in a cohort of 453 patients with type 2 diabetes has allowed us to prove the prognostic significance of the simultaneous inclusion of GFR and UAE in predicting mortality risk.
The issue of diabetes as a risk of coronary heart disease has been widely debated.1,11,12 In one benchmark epidemiological study13 carried out by the Emerging Risk Factors Collaboration (ERFC), with more than 820,900 participants, the presence of DM approximately doubled the mortality risk, with total mortality rates of 29/1000 in men and 23/1000 in women and CVM rates of 13/1000 in men and 11/1000 in women with DM. In our study, the mortality rate was higher (39/1000), which could be explained by the older age of our patients compared with the previous study (65 vs. 58 years). However, CVM in our study was 14/1000, similar to that described in the ERFC data.
One review4 of clinical trials with at least 1000 patients with type 2 DM in each study demonstrated that mortality rate varied between studies, ranging from 2.8 to 84.2/1000 patient-years. Therefore, considering the high prevalence of the disease,14 it is a challenge to accurately identify those patients with DM that are at the most unfavourable extreme of the vascular risk spectrum. One consistent finding4 is that the higher mortality rates are observed in trials that include patients with CKD. One study of 42,761 patients with type 2 diabetes followed over 4 years15 demonstrated a synergistic interaction between low GFR and high UAE on mortality risk. In our study, as in the previously mentioned study, both GFR and UAE were independent predictive factors for mortality risk, and furthermore, they improved the predictive power of the models; the fact that the interaction term did not reach statistical significance could be due to the relatively low number of patients included. Besides age, other factors significantly associated with mortality risk in our patients, were similar to those described in the literature: presence of existing vascular disease,1,16 low cholesterol levels (probably as a marker of weakness)17 and active smoking.18
As in the general population, in patients with DM there is a progressive increase in mortality risk as GFR decreases and UAE increases.19 Our data support this fact in a prolonged follow-up of more than 10 years. The possible pathophysiological mechanisms that explain the association between CKD and mortality include the increased prevalence of HTN and left ventricular hypertrophy, the increased activity of both the sympathetic system and renin–aldosterone system, endothelial dysfunction, low grade inflammation, and high levels of asymmetric dimethylarginine.20 It must also be kept in mind that the changes over time in both GFR21 and UAE22 offer additional prognostic information.
The greatest strength of our study was the long follow-up of patients, with only few losses, which allowed a reliable estimation of mortality rates during this period of time and reinforced the internal validity. The results are in agreement with those obtained in a previous data analysis with a shorter follow-up.23 Regarding noteworthy weaknesses, we must point out the limited number of subjects, with a low number of events in some categories, which makes the estimations less precise. Also, this study included patients with type 2 DM selected from hospital outpatient clinics, with a mean age of 65 years and a mean time since onset of DM of more than 10 years, which limits the external validity of the results. Finally, there were few patients with GFR<45, therefore the conclusions are primarily valid for stages 1–3A of CKD.
We can conclude that GFR and UAE are independent predictors, without significant interaction, of mortality risk in patients with type 2 diabetes. We consider the systematic assessment of both parameters fundamental in the follow-up of patients with type 2 diabetes.
Conflicts of interestThe authors declare no conflicts of interest
Please cite this article as: Gimeno-Orna JA, Blasco-Lamarca Y, Campos-Gutierrez B, Molinero-Herguedas E, Lou-Arnal LM, García-García B. Riesgo de mortalidad asociado a enfermedad renal crónica en pacientes con diabetes tipo 2 durante un seguimiento de 13 años. Nefrologia. 2015;35:487–492.