The experience of a tertiary hospital and four hemodialysis centers attached to it during the COVID-19 epidemic is described. The organization of care that has been carried out and the clinical course of the 16 cases of COVID-19 in hemodialysis patients are summarized. The joint application of measures, including patient screening, the early investigation of possible cases, the isolation of confirmed, investigational or contact cases, as well as the use of individual protection measures, has enabled the epidemic to be controlled. The clinical course of these 16 patients is compared with the series published by the Wuhan University Hospital and with the data from the Covid-19 infection registry of the Spanish Society of Nephrology. In our experience, and unlike what was reported by the Wuhan Center, COVID-19 disease in hemodialysis patients is severe in a significant percentage of cases, and high lethality is mostly caused by the infection itself. Measures to contain the epidemic are effective.
Se describe la experiencia de un hospital terciario y cuatro centros concertados de hemodiálisis adscritos al mismo durante la epidemia de COVID-19. Se resume la organización asistencial que se ha llevado a cabo y el curso clínico de los 16 casos de COVID-19 en pacientes en hemodiálisis. La aplicación conjunta de medidas que incluyen el cribado de pacientes, la investigación precoz de casos posibles, el aislamiento de los casos confirmados, en investigación o en contactos, así como la utilización de medidas de protección individuales, ha permitido controlar la epidemia. Se compara el curso clínico de estos 16 pacientes con la serie publicada por el Hospital Universitario de Wuhan y con los datos del registro de infecciones Covid-19 de la Sociedad Española de Nefrología. En nuestra experiencia y, a diferencia de lo comunicado por el centro de Wuhan, la enfermedad COVID-19 en los pacientes en hemodiálisis es grave en un porcentaje importante de los casos y la letalidad, elevada, es mayormente causada por la propia infección. Las medidas de contención de la epidemia son eficaces.
The SARS-CoV-2 coronavirus pandemic, which originated in the Wuhan region (China) at the end of 2019, has spread across the globe at a shocking speed, although with different repercussions in different parts of the world.1,2
The first published case series1,3,4 and data from the Centre for Disease Prevention and Control in China5 suggested the disease followed a benign course in most infected people, with mild symptoms in more than 80% of cases, 15% of cases requiring hospital care, but not being serious, and 5% of serious cases. Advanced age, cardiovascular disease, diabetes and hypertension were identified as factors associated with greater severity and a mortality rate as high as 49%.5
In Europe, the epidemic initially expanded most rapidly in Italy,6 followed by Spain, where 5753 patients had been registered by 15 March, when the state of alarm was decreed.7 The epidemic has affected the whole of Spain, but unevenly, with a very high incidence in the regions of Madrid, Catalonia, Castile-La Mancha and Castile-Leon.8,9
Initial reports from northern Italy already showed that the epidemic was following a "less benign" course than in China, with a higher percentage of admissions to intensive care units (ICU).10 In Spain, data from the National Statistics Centre confirm that impression, with 5% of admissions to ICU and a crude mortality rate of 11.9%.9
There is still very little information on the impact of COVID-19 in patients with chronic kidney disease on haemodialysis. A study carried out at the Wuhan University Hospital11 did not show that development of SARS-CoV-2 infection in this group of patients carried a significant risk of serious or fatal disease.
In Italy, the Brescia Renal COVID Task Force presented an organisational model for the care of patients with kidney disease during the epidemic, based on the active surveillance of patients and isolation of suspected and confirmed cases of COVID-19. At the time of publication, they had recorded 21 cases of haemodialysis patients with COVID-19.12
In Spain, as of 9 May 2020, the Sociedad Española de Nefrología (SEN) [Spanish Society of Nephrology] COVID-19 registry has recorded 937 cases on centre-based haemodialysis, with a mortality rate of 27.2%.13
This study describes the experience of SARS-CoV-2 infection in 478 haemodialysis patients from two healthcare areas distributed in one tertiary hospital and four associated centres. The tertiary hospital is the only referral centre for patients on dialysis, covering a population of over 600,000 people.14 During the study period, from 13 March to 27 April 2020, 16 cases of COVID-19 were confirmed in the hospital, coinciding with the expansion phase of the epidemic in Spain. This paper describes the organisational and epidemiological aspects and the clinical outcomes of this experience.
Material and methodsWe conducted a descriptive study of SARS-CoV-2 in the haemodialysis units, departments 6 and 7 of the Regional Ministry of Health and Public Healthcare for the Generalitat Valenciana [Autonomous Government of Valencia]. We describe the organisational aspects developed to provide care in suspected or confirmed cases of COVID-19 and the clinical outcomes of diagnosed cases. The period of study was from 13 March to 27 April 2020.
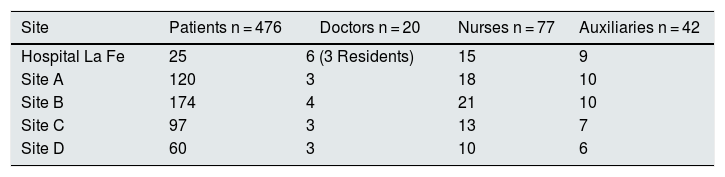
Study populationThe study includes data from 16 COVID-19 cases out of a total of 478 haemodialysis patients and four cases from among 138 healthcare professionals. Of the patients, 451 belong to the outpatient chronic haemodialysis programmes of four associated centres and 25 to the hospital programme. Two other cases of COVID-19 who were on haemodialysis and admitted to the hospital during this period were included in the study (Table 1).
Diagnostic considerationsDuring the study period, all patients with symptoms compatible with COVID-19 infection (pyrexia, respiratory or abdominal symptoms) were referred from the haemodialysis centres to the hospital for assessment.
The confirmed-case diagnosis was made by reverse transcription polymerase chain reaction (PCR) for the detection of SARS-CoV-2 in nasopharyngeal swab. All patients or staff with symptoms compatible with COVID-19 were tested.
Virology studies were not performed on asymptomatic individuals, except at associated centre A, where they had an accumulation of several cases within a few days; this situation was reported to Public Health to allow the PCR testing of patients who had contact with the initial case (25 of 120) and of all the centre’s staff.
Organisational aspectsBoth the centres and the hospital had their own contingency plans and action protocols for the prevention of infection and the management of confirmed or suspected cases of SARS-CoV-2 infection. The hospital’s nephrology department was notified of these procedures.
Measures included the following points: screening patients on arrival by taking temperature; questioning about key symptoms or contact with probable, confirmed or suspected cases; separation of possible cases; use of masks by patients during transport and in the waiting room, changing room and treatment room; attendance using own transport or reduction to two people in the same transport vehicle; use of personal protective equipment (PPE) adapted to the situation of the patients to be treated; isolation in different rooms, with independent circuits and with special precautions in suspected or confirmed cases; separation of contact cohorts; telephone notification of the centre prior to attendance in case of symptoms; transfer to hospital of all patients with pyrexia or compatible symptoms. The measures listed above were implemented progressively from 9 to 21 March.
Permanent communication channels were set up between the dialysis centres and the hospital’s nephrology department to coordinate the flow of patients, determine whether or not there had been any contact with confirmed or suspected cases, anticipate their arrival at the hospital and notify the centre as early as possible of the test results.
Admissions were made to wards prepared for COVID-19, managed, as far as possible, by the Internal Medicine and Respiratory Medicine departments. The patients were seen by the specialists at least once a day. The medical treatment for COVID-19 was as indicated by the department running the ward. All other medical treatment was agreed with the patient’s nephrologist.
A process was also established to coordinate the hospital discharge of these patients, whether with positive PCR or negative PCR, and to schedule their follow-up by the home hospitalisation unit and by the associated haemodialysis centres, where safe conditions were created for them to continue their treatment sessions. It was agreed to keep the patient in isolation in a separate room with contact precautions for at least 14 days after the first negative PCR test. No plan was established for performing repeat PCR or serology after a negative PCR test.
Management on haemodialysisA safety procedure was put in place in order to allow haemodialysis sessions for patients admitted with COVID-19 in the hospital’s haemodialysis unit. The unit has a general ward, with 12 stations, and two isolation wards, each with three stations. The general ward was prepared for the use of COVID-19 patients in the afternoon shift, with greater separation between patients, isolation from the rest of the areas with partitions and signage, special access for COVID-19 patients, removal of all non-essential material, use of personal protective equipment (with FFP2 mask, impermeable gown, long gloves, cap and face shield) and subsequent disinfection of all surfaces by washing and wiping down with disinfectant solutions. Staff received precise and frequent instruction on the protective measures and on the organisation of work.
For the treatment of suspected cases awaiting test results, the two isolation rooms were used, with the same measures as for patients with a confirmed diagnosis.
In order to create these treatment spaces, it was necessary to transfer seven patients from the hospital’s chronic outpatient programme to associated centres.
As a general rule, the haemodialysis schedule was three sessions lasting 240 min per week. Within the unit, an online haemodiafiltration technique was used, with a high-flux polysulfone filter (after confirming absence of intolerance), with the aim of producing a greater elimination of inflammatory mediators and seeking a target Kt greater than 45 and V online of 21−23 l. The dialysis bath was adapted to requirements according to the patient’s biochemistry values, generally using a calcium concentration of 1.5 mmol/l and potassium concentration of 2−3 mmol/l. In the ICU, conventional haemodialysis was used with a high-flux polysulfone filter.
Statistical methodThe Excel database (Microsoft Office 2019) was used to obtain the medians and their interquartile range (IQR) in the continuous variables. The discontinuous variables are shown as percentages.
ResultsEpidemiological descriptionFrom 13 March to 27 April 2020, PCR tests were performed on 101 individuals, 61 of whom were haemodialysis patients and 40, healthcare personnel.
Virology study was performed on individuals with suspected COVID-19 symptoms or contact with confirmed cases. Of the 32 patients given PCR tests due to symptoms, 15 (46.8%) were confirmed as having COVID-19 infection, and of the nine suspected staff, only one (11.1%) was confirmed. Of the 29 contacts tested in the patient group, one (3.4%) was confirmed, and in the staff group, 3 (31%).
Of the 16 confirmed cases included in the study, 14 were from associated centres. The cumulative incidence in the period for chronic outpatients was 2.94% (14/476). Of these 14 cases, 9 were from the same centre (centre A). It is thought that in 6 of these cases the index case was a patient who had travelled to a community transmission area from 6 to 8 March and then had two sessions at their centre before the onset of symptoms. In the remaining cases, it was also possible to determine the index case in two instances of transmission to patients; in centre A to a contact and in centre B to 3 contacts, some days later. Transmission from these index cases to staff members was not considered, as it was more difficult to confirm.
The two cases which did not correspond to patients from associated centres were one patient from another department and another admitted for a different reason, who started chronic haemodialysis while in hospital and developed a fever 12 days after discharge.
All cases were diagnosed between 13 March and 8 April. Since then, no new cases have been detected. Of the 16 confirmed cases, 14 were confirmed from the Accident and Emergency department, one from Public Health (a positive contact case, whose symptoms began on the day of the test result) and two during admission. The ruled-out cases were all ruled out from Accident and Emergency, and the contacts, from Public Health.
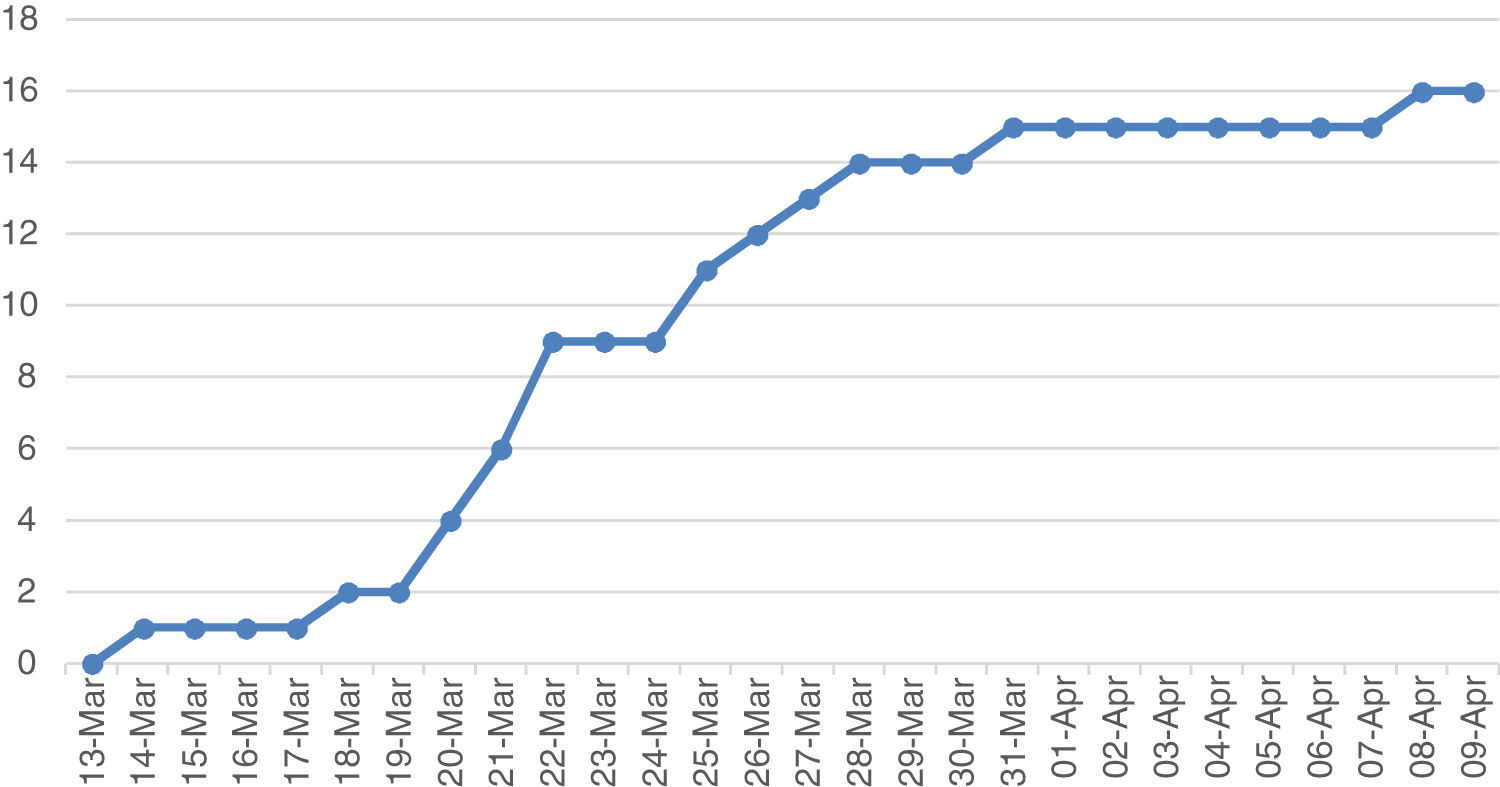
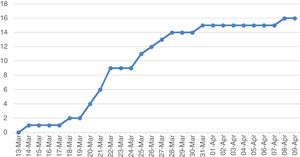
The cumulative incidence curve shows that the highest number of cases were detected in the second week after diagnosis of the first case (Fig. 1). In the third week, the curve flattened out. Only three cases were detected after 27 March.
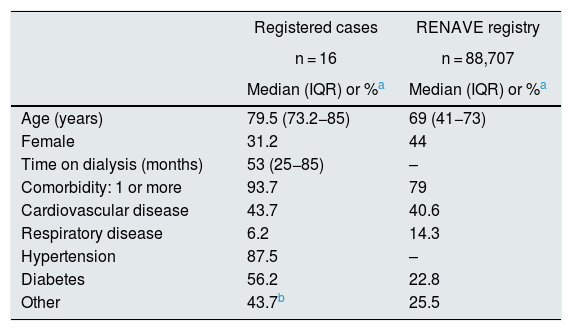
The demographic data and baseline characteristics of the patients who developed COVID-19 are shown in Table 2. For purposes of comparison, data are shown for the subgroup of hospitalised patients in the Red Nacional de Vigilancia Epidemiológica (RENAVE) [Spanish Epidemiological Surveillance Network] registry.15 We found that the patients in our study were older and more likely to have diabetes.
Demographic data and baseline characteristics of the COVID-19 patients registered in the study and of the people hospitalised from the national registry (RENAVE) as of 11 May 2020.
| Registered cases | RENAVE registry | |
|---|---|---|
| n = 16 | n = 88,707 | |
| Median (IQR) or %a | Median (IQR) or %a | |
| Age (years) | 79.5 (73.2−85) | 69 (41−73) |
| Female | 31.2 | 44 |
| Time on dialysis (months) | 53 (25−85) | – |
| Comorbidity: 1 or more | 93.7 | 79 |
| Cardiovascular disease | 43.7 | 40.6 |
| Respiratory disease | 6.2 | 14.3 |
| Hypertension | 87.5 | – |
| Diabetes | 56.2 | 22.8 |
| Other | 43.7b | 25.5 |
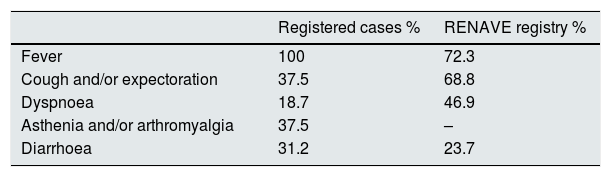
The interval between the onset of symptoms and virological diagnosis in confirmed cases among patients was generally short, due to the high index of suspicion. The PCR was positive in the first sample in all cases, except one, which was positive in the subsequent test carried out 72 h later. Compared to the data from the RENAVE registry in the general population15, a markedly low frequency of cough and dyspnoea was reported in patients on haemodialysis, despite the fact that 11 patients had radiological changes suggestive of viral pneumonia at the time of the initial assessment (Table 3).
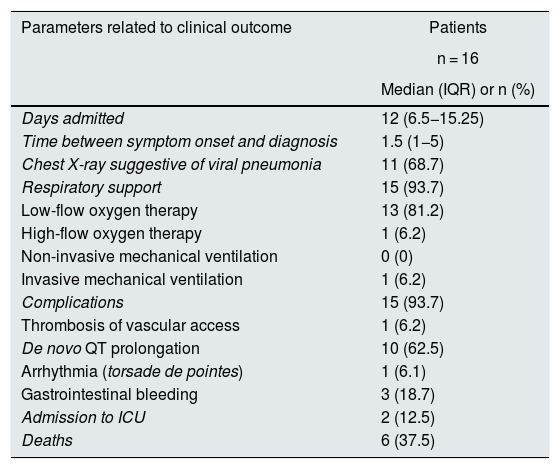
Most patients required some type of respiratory support (93%). The disease course was classified as severe or very severe in nine patients, six of whom died. Five of these six patients died as a consequence of the infection, while the death of the other patient was due to torsade de pointes multi-focal ventricular arrhythmia. Of the two patients who were admitted to ICU (the other very seriously ill patients were not candidates), one survived and the other died within a few hours from a systemic inflammatory response syndrome (Table 4).
Data relating to the clinical course of patients with COVID-19 on haemodialysis during their hospital admission.
| Parameters related to clinical outcome | Patients |
|---|---|
| n = 16 | |
| Median (IQR) or n (%) | |
| Days admitted | 12 (6.5−15.25) |
| Time between symptom onset and diagnosis | 1.5 (1−5) |
| Chest X-ray suggestive of viral pneumonia | 11 (68.7) |
| Respiratory support | 15 (93.7) |
| Low-flow oxygen therapy | 13 (81.2) |
| High-flow oxygen therapy | 1 (6.2) |
| Non-invasive mechanical ventilation | 0 (0) |
| Invasive mechanical ventilation | 1 (6.2) |
| Complications | 15 (93.7) |
| Thrombosis of vascular access | 1 (6.2) |
| De novo QT prolongation | 10 (62.5) |
| Arrhythmia (torsade de pointes) | 1 (6.1) |
| Gastrointestinal bleeding | 3 (18.7) |
| Admission to ICU | 2 (12.5) |
| Deaths | 6 (37.5) |
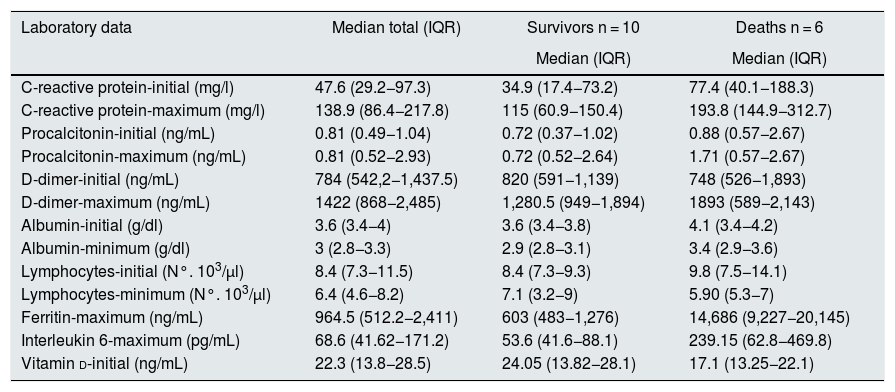
Of the laboratory parameters, the greatest differences between the patients who survived and those who died were found in C -reactive protein at baseline and, particularly, the maximum levels of ferritin and interleukin-6. These two parameters are considered discriminators for severe disease16 (Table 5).
Analytical data for the total number of patients, those living and those who died.
| Laboratory data | Median total (IQR) | Survivors n = 10 | Deaths n = 6 |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| C-reactive protein-initial (mg/l) | 47.6 (29.2−97.3) | 34.9 (17.4−73.2) | 77.4 (40.1−188.3) |
| C-reactive protein-maximum (mg/l) | 138.9 (86.4−217.8) | 115 (60.9−150.4) | 193.8 (144.9−312.7) |
| Procalcitonin-initial (ng/mL) | 0.81 (0.49−1.04) | 0.72 (0.37−1.02) | 0.88 (0.57−2.67) |
| Procalcitonin-maximum (ng/mL) | 0.81 (0.52−2.93) | 0.72 (0.52−2.64) | 1.71 (0.57−2.67) |
| D-dimer-initial (ng/mL) | 784 (542,2−1,437.5) | 820 (591−1,139) | 748 (526−1,893) |
| D-dimer-maximum (ng/mL) | 1422 (868−2,485) | 1,280.5 (949−1,894) | 1893 (589−2,143) |
| Albumin-initial (g/dl) | 3.6 (3.4−4) | 3.6 (3.4−3.8) | 4.1 (3.4−4.2) |
| Albumin-minimum (g/dl) | 3 (2.8−3.3) | 2.9 (2.8−3.1) | 3.4 (2.9−3.6) |
| Lymphocytes-initial (N°. 103/µl) | 8.4 (7.3−11.5) | 8.4 (7.3−9.3) | 9.8 (7.5−14.1) |
| Lymphocytes-minimum (N°. 103/µl) | 6.4 (4.6−8.2) | 7.1 (3.2−9) | 5.90 (5.3−7) |
| Ferritin-maximum (ng/mL) | 964.5 (512.2−2,411) | 603 (483−1,276) | 14,686 (9,227−20,145) |
| Interleukin 6-maximum (pg/mL) | 68.6 (41.62−171.2) | 53.6 (41.6−88.1) | 239.15 (62.8−469.8) |
| Vitamin d-initial (ng/mL) | 22.3 (13.8−28.5) | 24.05 (13.82−28.1) | 17.1 (13.25−22.1) |
The treatment of the patients was indicated by the specialist, according to the hospital’s own protocols, adapted to the patient’s clinical situation. The type of therapy most used was chloroquine or hydroxychloroquine (13 patients) in combination with azithromycin (12 patients). The indication for tocilizumab (2 patients), beta interferon (3 cases) and steroid boluses (4 cases) was reserved for critical patients.
The four cases of COVID-19 disease among healthcare personnel were mild and did not require admission to hospital.
DiscussionPatients on chronic outpatient haemodialysis are a high risk group for infection by SARS-CoV-2, as they have to share transport and spaces with other people in the haemodialysis units. As replication of the virus is very persistent in the upper respiratory tract, it is highly transmissible through the droplets expelled when talking and coughing or sneezing.17 Advanced age (45% of patients on renal replacement therapy are over 65)18 and the high rate of comorbidity mean that this group, a priori, has a higher risk of severe disease and fatality.
Infection, after the initial replication phase, can lead to an acute systemic inflammatory reaction with serious consequences.12 This response may be less intense in patients on haemodialysis,11 perhaps reducing the influence of the risk factors on severity and mortality. This was the experience of the Wuhan University haemodialysis centre,11 where cases with pneumonia in computed tomography (16.1% of the centre’s patients) were mild. The high number of deaths (18.7%) was attributed to causes other than the infection.
Comparison of data from the SEN Spanish COVID-19 Renal Registry and the data from Wuhan revealed differences in the epidemic in Spain, with high percentages of both admissions19 (85%) and deaths (27.2%).13 The results of our series are in line with the Spanish COVID-19 Renal Registry. All our patients with symptoms required admission and the mortality rate was high (37.3%), as a direct result of infection in the vast majority of cases. These figures are similar to those reported by another Spanish tertiary hospital, with 36 admitted cases and 30% mortality in a population of 282 haemodialysis patients.20 The COVID-19 mortality rate reported in this study and our own is consistent with that of the older Spanish population with comorbidity (RENAVE, 11 May).15 In the study by Goicoechea et al.,20 no association was found between classic cardiovascular risk factors and mortality. We were unable to establish differences in mortality rates according to the presence of risk factors, as almost all the cases were older and had comorbidity. We also failed to find higher rates of lymphopenia in those patients who died, the most differentiating laboratory markers being ferritin and interleukin-6.
In our series, the cumulative incidence during the study period of confirmed symptomatic cases compared to the total number of patients in haemodialysis units was very low (2.94%), lower than that of the aforementioned study (12.7%)20 and that of another recent Spanish study, with 24.4% of symptomatic cases.21 These rates, in a similar period, are four to eight times higher than that for our study. Perhaps the lower rate of infection in the Region of Valencia at that time9 meant that the application of confinement measures in the general population and of screening and protection measures in the haemodialyses units was more effective.
To better understand how this infection has specifically affected renal patients, we shall have to wait for population data, perhaps derived from serology studies. Screening using radiological or virological methods provides a better estimate of the incidence of infection in a given period and reduces the overall rate of severe cases by detecting asymptomatic and mild cases. The study by Albalate et al.,21 carried out in one of the areas of Spain with the highest infection rate, reported a cumulative incidence of symptomatic and asymptomatic cases of 41.1%, some 40.5% of which were diagnosed by PCR screening.
Home confinement was the most important contagion prevention measure applied at a general level during the initial stage of the epidemic in Spain.7 However, confinement has to be broken for individuals who require in-centre haemodialysis several times a week. The outbreak of SARS-CoV-2 infection in one of the units in our study is illustrative of what can happen at a general level in haemodialysis units where, in Spain, over 30,000 people are treated.18 For that reason, great efforts have been made in terms of drawing up recommendations for the prevention of contagion and management of patients with COVID-19 in haemodialysis units.12,21–27 In general, they emphasise the need to apply various types of action simultaneously and at an early stage: 1) screen patients, for early detection of possible cases; 2) isolate confirmed or suspected cases; and 3) reduce the risk of virus transmission with individual protection measures. This approach seems effective, as both in the Wuhan study11 and the one we present here, after full implementation, no new outbreaks occurred and individual cases were drastically reduced.
For the screening, it is necessary to have cheap, sensitive, rapid diagnostic tests. Many units, including all of those in our study, are not able to perform virology tests for the detection and separation of asymptomatic and pre-symptomatic cases. Moreover, in an epidemic which is extended over time, unless the health authority has sufficient means, it could be very difficult to carry out effective virological screening, with repetition of the PCR testing at regular intervals, possibly every 7–10 days. Over time, once we have a greater understanding of its significance, serology may allow separation of cohorts in units, according to the risk against the virus.28 At present, the method of choice for screening, at least for us, continues to be taking the patient’s temperature on arrival at the centre, in addition to a brief survey on any symptoms of recent onset. The proportion of infected people who are asymptomatic or pre-symptomatic is unknown and varies widely according to populations, with figures as disparate as 12% in the general population29 to 56% in nursing homes30 and 25.4% among haemodialysis patients.21 These rates can vary according to the incidence rate in each subpopulation.
The isolation of patients with COVID-19 is essential to avoid the flow and inadvertent proximity of people susceptible to infection and not adequately protected.10
Lastly, personal protection measures include hand and environment hygiene, social distancing and the use of appropriate PPE for the situation. Standard, contact and droplet precautions are indicated for direct care of COVID-19 patients. These measures involve the use of PPE in treatment rooms, including, if possible, an FFP3 or FFP2 respirator/N95 mask, eye protection, gloves, waterproof protection and long sleeves.27 For the care of patients who are not suspected or sick, the appropriate level of protection could depend on the ease of performing screening and the quality thereof. The generalised use of a surgical mask has proven its utility in curbing contagion at the population level31 and is therefore one of the key measures to be applied in haemodialysis wards and their settings.
In the Wuhan study,11 a high level of protection was ensured by using a waterproof gown, face mask, cap, and N95 mask to care for all patients in the haemodialysis unit. These measures have not been fully applied in many other countries, including ours, owing to the shortage of equipment, which has made it necessary to reserve high-protection masks and waterproof gowns for practices with the generation of aerosols in COVID-19 patients.32,33 In an environment where only clinical screening is possible, intensification and maintenance of protective measures are likely to be essential to prevent outbreaks of COVID-19 within haemodialysis units.
An additional concern for haemodialysis units is deciding when to withdraw isolation and advanced protection measures in patients who have suffered from COVID-19, once their PCR test comes back negative. In our case, this was indicated 2−3 weeks after a negative result, provided the patient’s situation did not suggest otherwise. In most cases, no further PCR or serology tests have been performed, in accordance with hospital procedures. However, cases of apparent recurrence, with a second positive PCR test, have been reported several weeks after the results in which one or more tests were negative.34 Therefore, to avoid risks to other patients, we should consider whether to extend the quarantine period in the centre and attempt to repeat the tests over a sufficient period (PCR, serology)
With a goal of "zero cases" for individuals on haemodialysis, as in many other alarm situations, we might say that we should have been quicker to publish official documents, with the same rules for all, and to apply protocols for prevention and action in haemodialysis units. We should not forget that the declaration of this situation as a Public Health Emergency of International Importance by the World Health Organisation was made on 31 January 2020.35 Globalisation makes speed important in decision-making and the application of appropriate measures to protect the population in international emergency situations. In haemodialysis units, we therefore need to have up-to-date contingency plans and procedures which enable us to react in time and in an appropriate way to different types of situations (natural disasters, terrorist attacks, etc.). Now may be the time to rethink issues such as the architecture and the appropriate size of centres. We may also need to look at the relationship between the associated centres and the health authorities, and establish the necessary conditions for the provision of services in exceptional situations, guaranteeing support from the public system when additional means, be they human, technical or material, are required.
By way of conclusion, we would point out the following: 1) Haemodialysis centres are possible sources for outbreaks of SARS-CoV-2; 2) Haemodialysis patients can develop severe and fatal disease, at rates at least similar to that of the general population of the same age and with the same comorbidities; 3) Preventive measures are effective in preventing contagion and are essential when screening cannot be performed by virological testing; 4) It is necessary to have procedures and contingency plans in place which enable early action in emergency situations, in a coordinated manner between reference hospitals and their associated centres; 5) The currently available technical document27 could be modified to include recommendations on patient screening and the withdrawal of special precautions in patients after COVID-19 infection and, to that end, facilitate the performing of specific tests.
Ethical responsibilitiesInformed consent was obtained from all study subjects. The individuals’ right to privacy was respected at all times.
FundingThis study has received no specific funding from public, private or non-profit organisations.
Conflicts of interestThe authors have no conflicts of interest.
Please cite this article as: Sánchez-Pérez P, González-Calero P, Poma-Saavedra FH, Orero-Calvé E, Devesa-Such R, Soldevila-Orient A, et al. Resultados de un modelo de organización asistencial para Covid-19 en hemodiálisis en un hospital terciario y sus centros concertados. Nefrologia. 2020;40:453–460.