We have read with great interest the review article authored by Sharaf El Din and colleagues, summarizing the reno-protective effects of sodium-glucose co-transporter-2 (SGLT-2) inhibitors, based on clinical evidence retrieved from the hallmark cardiovascular and renal outcome trials, also providing some interesting mechanistic insights.1 SGLT-2 inhibitors confer cardiovascular protection for patients with heart failure (HF) regardless of diabetes mellitus (DM) or chronic kidney disease (CKD) status at baseline.2 However, it has not been extensively studied whether SGLT-2 inhibitors exert reno-protective effect in patients with heart failure (HF), either with reduced or preserved left ventricular ejection fraction (HFrEF and HFpEF, respectively). Declining of renal function is of utmost importance for patients with HF, thus, development of optimal treatment strategies to prevent or delay this progression to kidney failure is required.3,4 During the last 2 years we have welcomed the results of 3 hallmark trials, 2 in HFrEF and 1 in HFpEF populations, assessing the efficacy and safety of SGLT-2 inhibitors in subjects with HF, with or without DM.5–7 Reno-protection with SGLT-2 inhibitors has been established, as demonstrated in relevant meta-analyses.8,9 Therefore, since it has not been addressed previously, we sought to evaluate whether SGLT-2 inhibitors confer reno-protection in patients with HF, pooling data from the 3 dedicated trials.5–7
Two independent reviewers (D.P. and C.P.) extracted data of interest from the eligible reports. We evaluated the following surrogate outcomes: the composite renal outcome, as defined across the 3 included trials, and the ≥40% decrease in estimated glomerular filtration rate (eGFR) from baseline. Definition of composite renal endpoint is as follows: time to first occurrence of: (1) chronic dialysis; (2) renal transplantation; (3) sustained reduction of ≥40% in estimated GFR; or (4) sustained estimated GFR.
Differences were calculated with the use of risk ratio (RR) with 95% confidence interval (CI) for dichotomous variables, after implementation of the Mantel–Haenszel (M–H) random effects formula. Statistical heterogeneity among studies was assessed by using I2 statistics. All analyses were performed at the 0.05 significance level.
Two independent reviewers (D.P. and C.P.) assessed the quality of the included RCTs, by using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome. Discrepancies between reviewers were solved by discussion, consensus or arbitration by a third senior reviewer (M.D.).
We pooled data from 3 trials in a total of 14,462 enrolled subjects with HFrEF or HFpEF, assigned either to SGLT-2 inhibitor treatment or placebo. Overall risk of bias was evaluated as low across all trials.
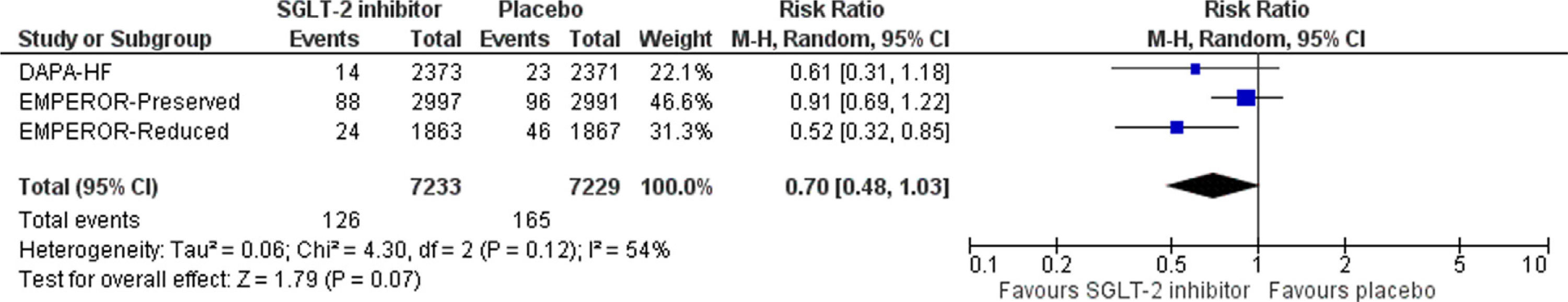
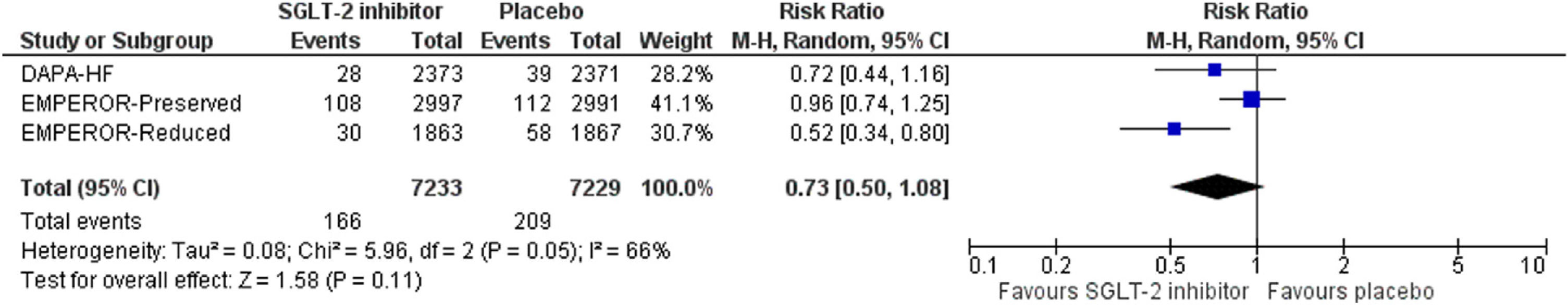
SGLT-2 inhibitor treatment resulted in a non-significant decrease in the risk for the primary composite outcome by 27% (RR=0.73, 95% CI; 0.50–1.08, I2=66%, p=0.11), as shown in Fig. 1. In addition, SGLT-2 inhibitor treatment led to a non-significant decrease in the risk for ≥40% decrease in eGFR from baseline, compared to placebo (RR=0.70, 95% CI; 0.48–1.03, I2=54%, p=0.07), as depicted in Fig. 2.
In conclusion, SGLT-2 inhibitors provide significant cardiovascular benefits in patients with HFrEF or HFpEF, however, they do not exert significant reno-protection. Of course, preservation of renal function with this drug class is of utmost importance for subjects with an established diagnosis of HF, contributing to further risk reduction for “hard‿ cardiovascular endpoints.
FundingNone.
Conflict of interestNone declared.
None.