The complement system is a network of soluble and cell surface proteins primarily involved in innate immune responses. Complement signaling is essential for pathogen defence and homeostasis, but an activation-regulation imbalance can lead to tissue damage. This phenomenon has been implicated in kidney diseases such as atypical Haemolytic Uraemic Syndrome (aHUS), frequently associated with dysfunction of the complement regulator Factor H (FH). Physiologically, complement interacts with the coagulation, fibrinolysis, renin-angiotensin and kallikrein-kinin systems (KKS). The KKS is a proinflammatory and procoagulant cascade comprised of the protease prekallikrein, the coagulation factors XI (FXI) and XII (FXII), and the cofactor/substrate high-molecular-weight kininogen. KKS can be activated conformationally or proteolytically. KKS activation in vitro triggers a number of biochemical interactions between FXI, FXII, FH and other complement proteins that result in direct or secondary complement activation. These functional links point to an overall complement pro-activating role for the KKS that has implications for coagulation and immunity, but whose physiological consequences in vivo remain largely unexplored. This review aims to summarize the main physiopathological events of KKS activation in the context of complement-mediated kidney disease, with particular emphasis in aHUS.
El sistema del Complemento es un entramado bioquímico de proteínas solubles y de membrana implicadas en la respuesta inmune innata. El Complemento es esencial para la homeostasis y la defensa frente a patógenos, pero un desequilibrio entre sus procesos de activación y regulación puede causar daño tisular. Un ejemplo relevante es el Síndrome Hemolítico-Urémico atípico (SHUa), que a menudo se asocia con una función anómala del regulador del Complemento Factor H (FH). El Complemento interacciona con los sistemas de la Coagulación, Fibrinólisis, Renina-angiotensina y Calicreína-Cinina (SCC). El SCC es una cascada proinflamatoria y procoagulante que se puede activar conformacionalmente o mediante proteólisis, y que incluye a la proteasa precalicreína, a los factores de coagulación FXI y FXII, y al sustrato/cofactor cininógeno de alto peso molecular. “In vitro”, el SCC desencadena una serie de interacciones entre FXI, FXII, FH y otras proteínas que provocan la activación directa o indirecta del Complemento. Esta capacidad del SCC para activar el Complemento afectaría a la coagulación y a la respuesta inmunológica del organismo, pero nunca se han explorado sus posibles consecuencias “in vivo”. Esta revisión sintetiza las principales características fisiopatológicas del SCC en el contexto del daño renal mediado por el Complemento, en particular en el SHUa.
Atypical Haemolytic-Uraemic Syndrome (aHUS) is a rare disease (OMIM#235400) that causes hmatological and renal involvement, with a pathogenesis that originates in the microvascular endothelium.1 The anatomical lesion that defines aHUS is thrombotic microangiopathy, with thrombi in the smallest renal vessels, although routine clinical coagulation studies are normal.2
Research conducted in the first decade of the 21st century in small cohorts of patients with aHUS, mostly of European origin, represented a major advance in the understanding of the pathogenic mechanisms, which pointed the complement system as a key factor in disease development.3 These findings coincided with the availability of a biological drug, eculizumab, which blocked complement activation and was used in the treatment of paroxysmal nocturnal hemoglobinuria. Since its approval for the treatment of aHUS,4 eculizumab has dramatically reduced the morbidity and mortality of aHUS and has stimulated the development of other drugs capable of inhibiting or modulating the complement system at different levels.5
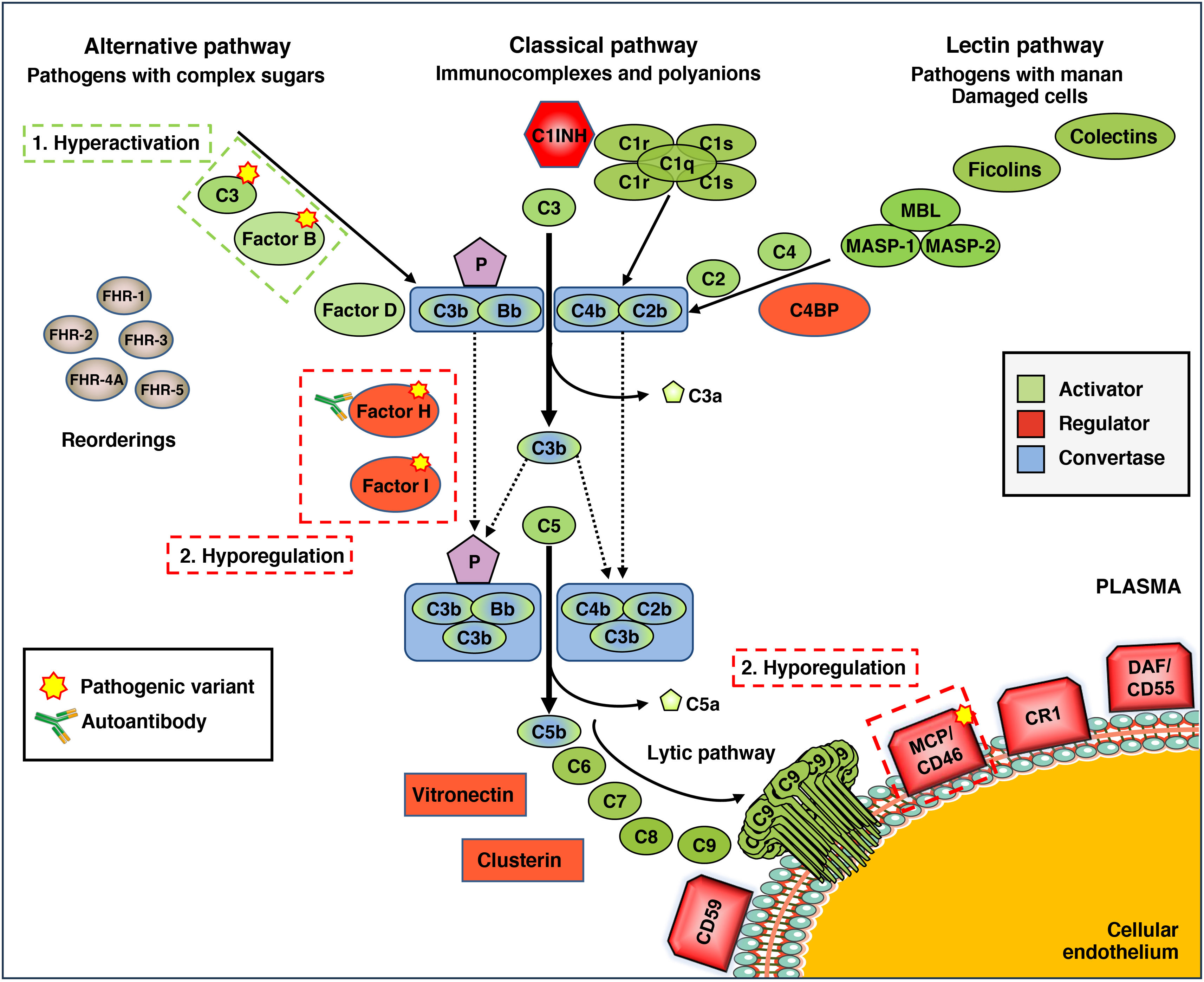
The complement system is one of the so-called "proteolytic cascades" that operate in plasma, whose best-known function is innate and immediate defence against infections. Evolutionarily older than immunoglobulins, the complement system is a biochemically complex system involving numerous plasma proteins that are activated in the presence of different pathogens or molecules (Fig. 1).6 Once activated, these proteins are unable to differentiate between the surface of pathogens and the surface of the body's own cells. For this reason, other complement proteins act as "regulators" of the system, preventing damage to autologous tissues, such as the microvascular endothelium. These characteristics mean that proper functioning of the complement system depends on a very precise balance between its activation and regulatory processes. Disrupting this balance favors the development of diseases such as aHUS or C3 glomerulopathy (C3G), among others.7
Complement defects in atypical hemolytic-uremic syndrome (aHUS). General diagram of complement function, which can be activated by three pathways: alternative, classical and lectin, depending on the pathogen or molecule involved. This activation generates enzyme complexes called C3 convertases because they activate component C3, which is the most important for complement function. The activating components, regulatory components, and C3 and C5 convertases are highlighted in different colors. The convertases of the alternative pathway are stabilized by properdin (P). Factor H-related proteins (FHRs) may act as activators and/or regulators of complement. In many patients with aHUS, the function of the alternative pathway is altered by the presence of mutations in activating components (C3 and Factor B) or regulatory components (Factor H, Factor I, membrane cofactor protein [MCP]/CD46), circulating anti-FH autoantibodies, or as a result of abnormal genetic rearrangements involving Factor H and FHRs. The overall result is hyperactivation or hyperexpression of the complement, which allows the lytic pathway to be activated and directly damage the endothelial cell. C1INH: C1 inhibitor; C4BP: C4b-binding protein; CR1: complement receptor 1; DAF: decay accelerating factor; MBL: Mannan-Binding Lectin; MASP-1: MBL-Associated Serine Protease 1; MASP-2: MBL-Associated Serine Protease 2.
In HUS, one of the complement activation pathways, called the alternative pathway (AP), does not function properly (Fig. 1). Many patients have pathogenic genetic variants (i.e., mutations) in activating proteins, such as C3 or Factor B (FB), or in regulatory proteins, such as Factor H (FH), Factor I (FI), or the membrane protein MCP/CD46.8 Other patients form autoantibodies against FH that have a similar effect to mutations.9 A third, less common group of alterations are abnormal genetic rearrangements affecting FH and its homologous proteins FHRs.10,11 Although functional studies are limited, it is believed that both genetic alterations and autoantibodies cause an imbalance between the activation and regulation of the complement alternative pathway, which damages the body’s own cells and tissues and promotes the development of aHUS.
The most common alterations in aHUS, and those with the worst prognosis, are those affecting FH, which is the main regulator of the alternative pathway.12 Therefore, early identification is particularly important when establishing treatments to prevent renal function deterioration or post-transplant recurrence. Despite the availability of massive sequencing techniques and other genetic analysis tools, the identification of mutations and genetic rearrangements affecting FH still requires several weeks. Tests for the presence of anti-FH autoantibodies, on the other hand, can be carried out in the first few days of onset using classic immunological techniques that detect FH and anti-FH immune complexes in plasma; in this way, plasma exchanges can be performed to rapidly eliminate autoantibodies and, eventually, initiate immunosuppressive treatment.13
Genetic studies in patients have also identified a second group of predisposing factors that may be related to the prothrombotic mechanism underlying the pathogenesis of aHUS. Some patients, particularly very young children, have pathogenic variants in the gene encoding diacylglycerol kinase epsilon (DGKE), an intracellular enzyme with antithrombotic activity.14 The alternative pathway variants identified in DGKE are usually recessive and sometimes occur in combination with alterations in complement genes, such as C3.15 Similarly, certain pathogenic variants in the gene encoding thrombomodulin (THBD) have also been associated with the development of aHUS.16 Thrombomodulin is a receptor located on the endothelial membrane and is involved in the activation of protein C and the regulation of the thrombosis process. A recent study, however, questions the actual contribution of THBD variants to aHUS.17
Furthermore, massive sequencing studies in cohorts of aHUS patients have revealed an excess of potentially pathogenic variants in the plasminogen (PLG) gene, and investigators suggest that low plasminogen or plasmin activity could reduce thrombus degradation in aHUS and act as a disease-modifying factor.18 Finally, although data are still limited, several studies in cohorts of patients with C3G have observed an excess of heterozygous mutations in DGKE and described individuals carrying variants in THBD, PLG and von Willebrand factor (VWF).18,19
Taken together, these data suggest that, even with normal plasma coagulation factors and hemostatic values, patients with aHUS may suffer from a loss of local regulation that triggers some activation of the coagulation cascade. In this context, activation of the kallikrein-kinin system (KKS) would explain the presence of microthrombi in the renal tissue observed in aHUS, as well as secondary activation of the complement system. In the following sections, we will review the functioning of the KKS and its impact on renal physiology, as well as the interactions between the KKS and the complement system that could contribute to pathologies.
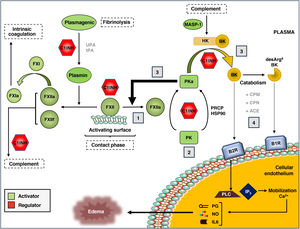
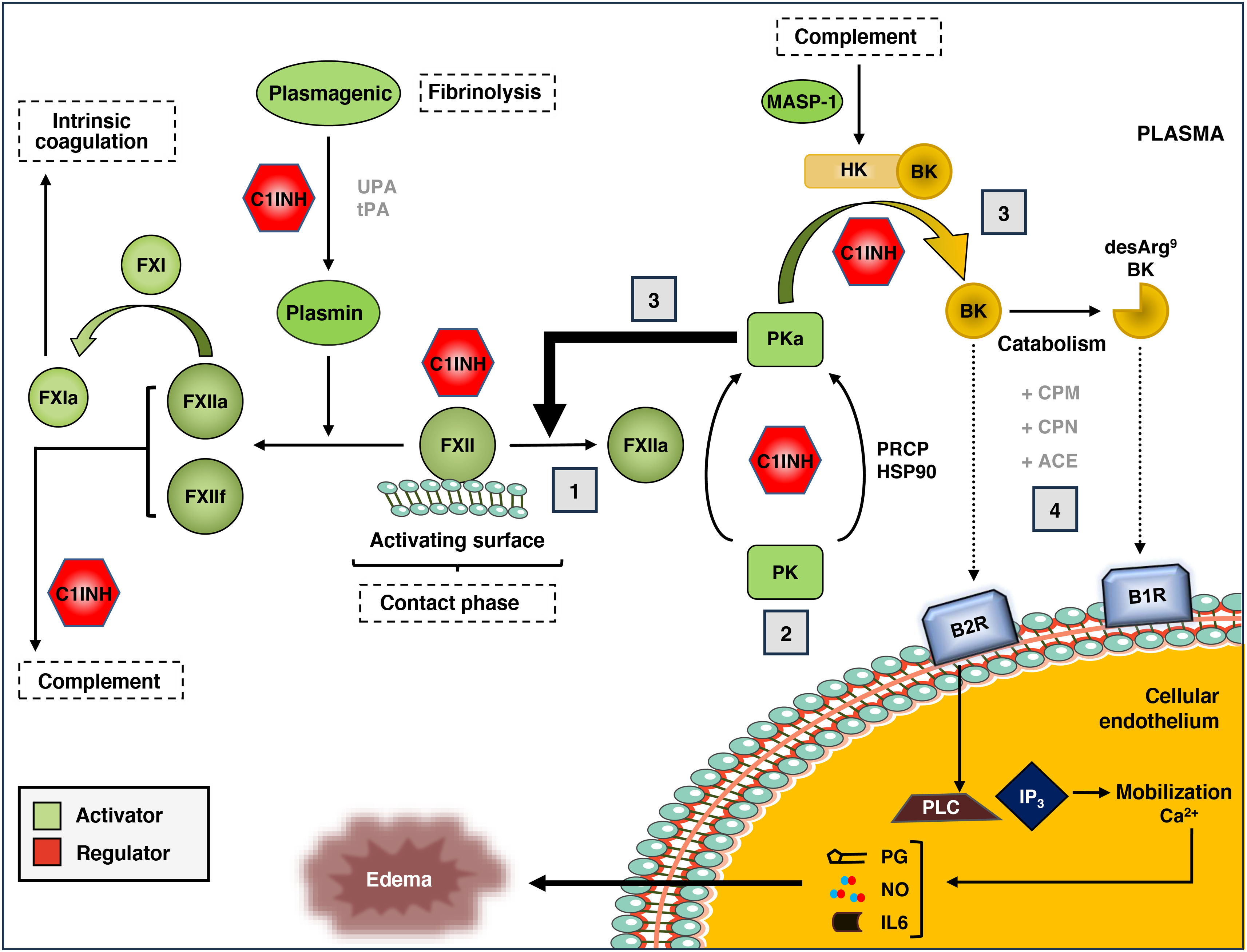
Kallikrein-kinin systemThe KKS is a proteolytic cascade in plasma associated with the activation of proinflammatory and procoagulant pathways (Fig. 2).20 It is composed of the coagulation zymogens factor XI (FXI) and factor XII (FXII), prekallikrein (PK), and the high molecular weight kininogen substrate and cofactor (HK). The KKS can be activated through the contact pathway, in which the binding of FXII to negatively charged surfaces promotes conformational changes that result in its partial proteolysis to generate the protease FXIIa.21,22 Contact activation can be initiated in response to a variety of biological molecules such as polyphosphates, aggregated or misfolded proteins, amyloid β peptide or artificial surfaces such as glass, dextran sulfate and kaolin.
The kallikrein-kinin system (KKS) of blood coagulation.
The components and main activation pathways of the KKS are shown, as well as its connection to the fibrinolysis process, intrinsic coagulation and the complement system.
(1) The contact phase of the KKS is mediated by the interaction of coagulation factor XII (FXII) with negatively charged surfaces and the subsequent generation of activated FXII (FXIIa). (2) FXIIa, in turn, activates prekallikrein (PK) to active kallikrein (PKa), although this can also be activated in a non-canonical manner through prolyl-carboxypeptidase P (PRCP) or Heat Shock Protein 90 (HSP90). (3) Once generated, PKa can activate more FXII molecules (positive feedback) or act on the high molecular weight kininogen substrate (HK), generating the kinins bradykinin (BK) and its derivative, des-Arg9-BK. (4) The binding of these kinins to their specific receptors (B1R for des-Arg9-BK, and B2R for BK) initiates an intracellular signaling cascade associated with the development of edema, with mobilization of Ca2+reserves, activation of phospholipase C (PLC) and inositol triphosphate (IP(3), and release of the secondary mediators prostaglandin (PG), nitrous oxide (NO), and interleukin 6 (IL6). All stages are controlled by the C1 inhibitor, which also regulates the complement. MASP-1: MBL-Associated Serine Protease 1.
FXIIa can proteolyse two different substrates within the KKS: FXI and PK. The proteolysis of FXI to its active form (FXIa) initiates the intrinsic branch of the coagulation cascade, which is why it is used in diagnostic tests of coagulation capacity, such as activated partial thromboplastin time (aPTT). On the other hand, FXIIa acts on PK and generates plasma kallikrein (PKa). PKa can, in turn, activate more FXII molecules to FXIIa, in a positive feedback loop that significantly increases KKS activation, or proteolyse HK to release bradykinin (BK), which is a 9-amino acid peptide (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg).23
BK is an autacoid (local cell mediator) with potent endothelium-dependent vasodilatory activity, causing edema, redness and local sensation of heat. BK exerts its biological effects through its binding to the membrane receptor B2R, which is constitutively expressed in endothelial cells, smooth muscle, sensory neurons and epithelial cells.24 The binding of BK to the B2R receptor initiates a chain of intracellular reactions that includes the activation of phospholipase C and inositol triphosphate, the mobilization of calcium reserves, and the release of secondary mediators such as nitric oxide, prostaglandins, interleukin 6 and tissue plasminogen activator. BK-treated endothelial tissue experiences smooth muscle contraction and disruption of endothelial cadherin junctions, causing fluid extravasation and local edema.25
In vivo, BK has a short half-life (∼30 s) and transient effects on the endothelium. On the one hand, BK is rapidly catabolized in the fluid phase by the action of carboxypeptidase M (CPM) and carboxypeptidase N (CPN, kininase 1). These metalloproteases release arginine at the C-terminal position of BK and generate the octapeptide des-Arg9-BK (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe), which is subsequently cleaved by the angiotensin-converting enzyme (ACE, kininase 2) into smaller peptides with no kinin activity.26 On the other hand, persistent stimulation of the B2 receptor promotes its desensitization through phosphorylation and endocytosis mechanisms, limiting intracellular signaling and vasodilatory effects of BK to minutes or hours. The des-Arg9-BK peptide also has potent kinin-like activity through its binding to the inducible receptor B1R,27 which is expressed in endothelial cells in response to tissue damage or the presence of proinflammatory cytokines. Unlike B2R, B1R is not phosphorylated and is relatively resistant to desensitization by binding to its ligand, so it has a relatively long half-life and intracellular signaling capacity, ranging from hours to days.28
Three plasma proteins control activation of the KKS: C1 inhibitor (C-1INH), alpha-2-macroglobulin (A2M), and antithrombin III (AT).29,30 C1-INH, which is also capable of regulating the classical complement pathway, is the main plasma regulator of FXIIa, with a minor contribution from AT. In turn, C1-INH and A2M are responsible for more than 90% of PKa regulation in plasma. Together, C1-INH plays a predominant role in regulating the KKS by simultaneously inhibiting FXIIa, PKa, and FXIa.31 On the other hand, although contact-mediated activation (via FXII) is the best-studied form of KKS activation, the system can be initiated by various non-canonical pathways. These include proteolysis of FXII by plasmin,32 activation of PK by prolyl-carboxypeptidase P (PRCP),33 proteolysis of HK by complement system MBL-associated serine protease (MASP-1),34 and conformational activation of FXII by heat shock protein 90 (HSP90).35,36 However, the physiological relevance of these alternative forms of KKS activation is largely unknown.
Evidence for the involvement of the kallikrein-kinin system in renal pathophysiologyThe proteolytic components of the KKS are mainly synthesized in the liver, and there is no evidence of their expression in renal tissues under normal conditions. Despite this, several lines of evidence suggest that kinin-mediated signaling plays a complex role in the context of renal biology. First, B1R and B2R receptors are highly expressed in both the kidney and bladder,37 indicating their involvement in renal function. Furthermore, in vivo studies in canine models have shown that BK infusion produces diuresis and natriuresis without altering the glomerular filtration rate. This phenomenon, which is accompanied by the activation of protein kinase C and the release of intracellular Ca2+, is mediated by the B2R receptor and can be reproduced experimentally by administering ACE inhibitors (enalapril). These data suggest that BK participates in water reabsorption by the proximal cells of the renal tubule.38,39
In the progression to end-stage renal disease (ESRD), BK-B2R interaction reduces tubulointerstitial fibrosis by inhibiting PAI-1 expression in tubular epithelial cells and consequently increasing the degradation of extracellular matrix components mediated by plasmin and metalloproteases.40 In addition, family studies of patients with ESRD have shown a significant increase in the minor allele of a polymorphism in exon 2 of B2R (c.C181T),41 which has been associated with a younger age at onset.42 The effects of the BK-B2R interaction contrast with those observed in the des-Arg9-BK-B1R interaction. In mouse models of focal and segmental glomerulosclerosis (FSGS), both pharmacological overexpression of B1R and administration of des-Arg9-BK are associated with disease progression, manifested by increased albuminuria and urine creatinine/total protein ratio, and by loss of podocyte function. Similarly, treatment with des-Arg9-[Leu8]-BK, a B1R antagonist, confers protection against the podocytopathy that characterizes FSGS.43
The KKS is also involved in the development of kidney damage in patients with diabetic nephropathy, in whom high plasma PK levels are associated with a higher degree of albuminuria.44 In mouse models, BK has been shown to modify the gene expression pattern of podocytes to shift to an anti-apoptotic and pro-inflammatory phenotype, and B2R deficiency attenuates glomerular and tubular damage.45,46 Finally, a very recent study shows that the KKS is activated or altered in the plasma of patients with chronic kidney disease (CKD), regardless of whether or not they are undergoing dialysis treatment.47
Under physiological conditions, the KKS is in equilibrium with the renin-angiotensin-aldosterone system (RAAS), as both have antagonistic effects on blood pressure and electrolyte balance in the body. In a situation of hypotension and hyponatremia, the juxtaglomerular cells of the kidney secrete the hormone renin, which hydrolyzes plasma angiotensinogen and releases its N-terminal decapeptide, angiotensin I, initiating a series of enzymatic reactions that lead to the secretion of aldosterone by the adrenal cortex, which has a powerful vasoconstrictive effect and causes the reabsorption of water and electrolytes. There is considerable molecular evidence of interaction between the KKS and the RAAS, which occurs at several levels.48,49 First, ACE, which degrades BK generated by the KKS, is also responsible for the formation of the main effector molecule of the RAAS, angiotensin II. Then, PKa can activate prerenin and proteolyse angiotensinogen, directly generating angiotensin II. Third, the B2 receptor of BK can form heterodimers with the AT1 receptor, to which some of the peptides generated from angiotensin I bind, and perhaps also with the AT2 receptor. These and other observations provide molecular justification for the impact of certain KKS alterations on the normal functioning of the RAAS, such that the loss of balance between the two systems can have pathological consequences at the renal level, which are not the subject of this review.
Physiopathological importance of the interaction between the complement and the kallikrein-kinin systemThe complement and KKS share a number of important functional characteristics. First, both systems are physiologically in a "standby" state because the proteases they contain are synthesized in the form of inactive zymogens. Activation leads to a series of proteolytic reactions that progress sequentially in a "cascade" and through which their biological functions are manifested. Furthermore, the zymogens involved in initiating the complement and KKS activation cascades have a certain capacity for conformational self-activation in response to specific stimuli, acquiring basal levels of enzymatic activity. This phenomenon is particularly evident in the proenzyme C1r of the complement classical pathway, which undergoes a conformational reorganization when the macromolecular complex C1 of which it forms part (C1q-C1r2-C1s2) binds to immune complexes or pathogen-associated molecular patterns.6 Similarly, FXII, a functional homologue of C1r in the KKS, undergoes an autocatalytic process that generates FXIIa when it interacts with anionic surfaces.50
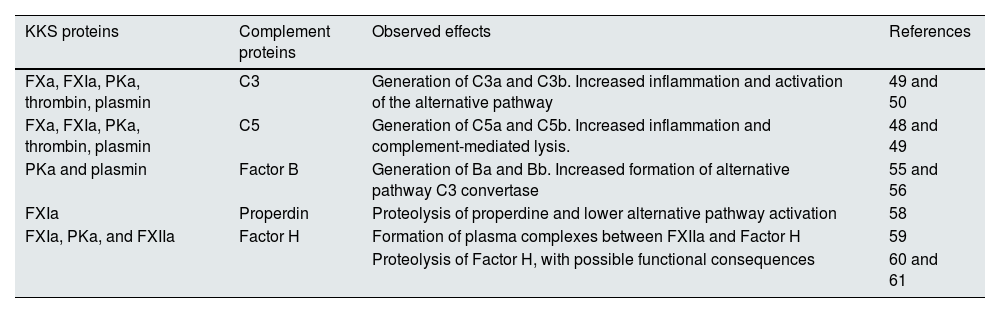
Numerous studies show that there are transactivation phenomena between the complement, the KKS, fibrinolysis and blood coagulation (Table 1). In this context, it has been observed that conditions that promote the release of thrombin and the subsequent activation of fibrinolysis in coagulation foci may facilitate the activation of some components of the complement system. Thus, in in vitro models, FIXa, FXa, FXIa, PKa, thrombin and plasmin proteolyse C3 and C5 and generate the anaphylatoxins C3a and C5a, which are capable of inducing dose-dependent chemotactic responses in a mast cell line.51–53 The activity of these KKS proteases therefore plays a crucial role in complement activation, amplifying the inflammatory response through the production of the anaphylatoxins C3a and C5a.
Effects of KKS on complement.
| KKS proteins | Complement proteins | Observed effects | References |
|---|---|---|---|
| FXa, FXIa, PKa, thrombin, plasmin | C3 | Generation of C3a and C3b. Increased inflammation and activation of the alternative pathway | 49 and 50 |
| FXa, FXIa, PKa, thrombin, plasmin | C5 | Generation of C5a and C5b. Increased inflammation and complement-mediated lysis. | 48 and 49 |
| PKa and plasmin | Factor B | Generation of Ba and Bb. Increased formation of alternative pathway C3 convertase | 55 and 56 |
| FXIa | Properdin | Proteolysis of properdine and lower alternative pathway activation | 58 |
| FXIa, PKa, and FXIIa | Factor H | Formation of plasma complexes between FXIIa and Factor H | 59 |
| Proteolysis of Factor H, with possible functional consequences | 60 and 61 |
FXa: activated FX; FXIa: activated FXI; FXIIa: activated FXII; PKa: active kallikrein.
On another level, both the complement classical pathway and the KKS are regulated by the plasma protease C1-INH, whose physiological relevance is mainly evident in the treatment of patients with hereditary angioedema due to C1-INH deficiency (HAE-C1INH; OMIM#106100). HAE is a rare disease characterized by local, spontaneous, and episodic activation of the KKS, subsequent increased vascular permeability with plasma extravasation, and development of localized episodes of BK-mediated edema.54 Although BK is usually generated by the proteolytic action of PKa on HK, it has been observed that the complement lectin pathway protease MASP-1 can also hydrolyse HK and release BK34; thus, complement could contribute to the local regulation of vascular permeability and participate in the pathogenesis of HAE-C1INH.55 It has also been demonstrated, both in vitro and in vivo, that the complement proteases MASP-1 and MASP-2 are capable of activating blood coagulation through the proteolysis of prothrombin to thrombin.56,57
The first evidence supporting the activation of the alternative pathway of the complement system through the KKS dates back to the early 1980s. During this period, two independent studies demonstrated that PKa and plasmin were capable of proteolysing Factor B (FB) into its functional fragments Ba and Bb, thus mimicking the enzymatic activity of Factor D (FD) in the formation of the alternative pathway C3 convertase. However, the actual relevance of these interactions in vivo is unknown, since under physiological conditions, FD is approximately ten times more effective in molar terms than PKa.58,59 KKS may also have a modulatory effect on complement alternative pathway activation through its interaction with properdin, which is the only positive regulator of the alternative pathway. Properdin is a plasma glycoprotein capable of binding to C3 convertase and significantly increasing its half-life.60 Properdin deficiency is commonly associated with recurrent meningococcal infections and high mortality rates, probably due to reduced efficacy in alternative pathway activation. Recently, researchers have described the interaction of properdin with FXIa, which can proteolyse it.61 In addition, properdin appears to inhibit the autoactivation of FXI on negatively charged surfaces, suggesting that both proteins could establish a dynamic equilibrium with physiological implications in the context of inflammatory and thrombotic diseases.
Much more recent evidence of the interaction between the complement alternative pathway and the KKS involves the complement regulator FH. Activation of the KKS in plasma leads to the formation of complexes between FH and the protease FXIIa in which FXIIa retains its proteolytic capacity, although it is unknown whether FH activity is affected.62 On the other hand, an in vitro study has shown that PKa and FXIa can proteolyse FH in its SCR6 domain, dividing the molecule into two fragments of different sizes that remain linked by disulfide bonds and appear to regulate complement less effectively.63,64 This proteolytic process could also affect the ability of FH to bind to glycosaminoglycan/heparan sulfate molecules on the cell surfaces, altering its ability to limit complement activation on them and thus contributing to the development of aHUS. FH, for its part, directly inhibits the generation of FXIa by thrombin and FXIIa, suggesting an additional connection between the complement system and the KKS.
Despite the extensive evidence of interaction between the KKS and the complement system, and between the KKS and other physiological systems, virtually all drugs developed for the treatment of KKS target hereditary angioedema. In recent years, however, interest in this field has grown, and several molecules are currently in clinical or preclinical development.65 In terms of kidney disease, a clinical trial on the use of C1INH in the treatment of acute antibody-mediated rejection (Takeda, Shire66,67) was discontinued at 36 months due to no significant improvement over placebo, although a long-term ad hoc analysis showed a lower incidence of graft-associated glomerulopathy. Another study did observe a beneficial effect of C1INH, reducing ischemia-perfusion damage after transplantation and delaying graft function.68 A recombinant tissue kallikrein-1 (DM199) is also being tested for the treatment of preeclampsia and in diabetic patients with CKD, with the aim of slowing the progression of kidney disease and reducing proteinuria (DiaMedica Therapeutics).69,70
In summary, the complement interacts with the KKS at different levels that are not yet fully understood, but cross-activation between the two systems could result in exacerbation of the inflammatory and thrombotic processes associated with some kidney diseases. Although current evidence is very limited, it is possible that in the future, pharmacological modulation of the KKS will open up new therapeutic possibilities that will reduce recurrence and improve long-term outcomes in patients.
FundingThis work was funded by the Carlos III Health Institute (ISCIII) and the European Regional Development Fund (project PI22/00211 to PS-C) and by the Autonomous Community of Madrid (Complement III-CM; P2022/BMD-7278).
F.C. has a contract co-funded by the Senefro Foundation (http://www.senefro.org/; Research Grants 2022), the Spanish Association of Lipodystrophies (AELIP), and IdiPAZ (Luis Álvarez Grant 2024).
The authors have no conflicts of interest to declare.
We would like to thank Dr. Margarita López Trascasa and Rosario García Sánchez, from the IdiPAZ group "Complement Alterations in Human Pathology," for their critical reading of the manuscript.


![Complement defects in atypical hemolytic-uremic syndrome (aHUS). General diagram of complement function, which can be activated by three pathways: alternative, classical and lectin, depending on the pathogen or molecule involved. This activation generates enzyme complexes called C3 convertases because they activate component C3, which is the most important for complement function. The activating components, regulatory components, and C3 and C5 convertases are highlighted in different colors. The convertases of the alternative pathway are stabilized by properdin (P). Factor H-related proteins (FHRs) may act as activators and/or regulators of complement. In many patients with aHUS, the function of the alternative pathway is altered by the presence of mutations in activating components (C3 and Factor B) or regulatory components (Factor H, Factor I, membrane cofactor protein [MCP]/CD46), circulating anti-FH autoantibodies, or as a result of abnormal genetic rearrangements involving Factor H and FHRs. The overall result is hyperactivation or hyperexpression of the complement, which allows the lytic pathway to be activated and directly damage the endothelial cell. C1INH: C1 inhibitor; C4BP: C4b-binding protein; CR1: complement receptor 1; DAF: decay accelerating factor; MBL: Mannan-Binding Lectin; MASP-1: MBL-Associated Serine Protease 1; MASP-2: MBL-Associated Serine Protease 2. Complement defects in atypical hemolytic-uremic syndrome (aHUS). General diagram of complement function, which can be activated by three pathways: alternative, classical and lectin, depending on the pathogen or molecule involved. This activation generates enzyme complexes called C3 convertases because they activate component C3, which is the most important for complement function. The activating components, regulatory components, and C3 and C5 convertases are highlighted in different colors. The convertases of the alternative pathway are stabilized by properdin (P). Factor H-related proteins (FHRs) may act as activators and/or regulators of complement. In many patients with aHUS, the function of the alternative pathway is altered by the presence of mutations in activating components (C3 and Factor B) or regulatory components (Factor H, Factor I, membrane cofactor protein [MCP]/CD46), circulating anti-FH autoantibodies, or as a result of abnormal genetic rearrangements involving Factor H and FHRs. The overall result is hyperactivation or hyperexpression of the complement, which allows the lytic pathway to be activated and directly damage the endothelial cell. C1INH: C1 inhibitor; C4BP: C4b-binding protein; CR1: complement receptor 1; DAF: decay accelerating factor; MBL: Mannan-Binding Lectin; MASP-1: MBL-Associated Serine Protease 1; MASP-2: MBL-Associated Serine Protease 2.](https://static.elsevier.es/multimedia/20132514/0000004500000008/v1_202510300825/S201325142500118X/v1_202510300825/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)