It is well known that climate change greatly affects human health, even though there are few studies on renal outcomes. Heat waves have been found to increase cardiovascular and respiratory morbidity and mortality, as well as the risk of acute renal failure and hospitalisation due to renal diseases, with related mortality. Recurrent dehydration in people regularly exposed to high temperatures seems to be resulting in an unrecognised cause of proteinuric chronic kidney disease, the underlying pathophysiological mechanism of which is becoming better understood. However, beyond heat waves and extreme temperatures, there is a seasonal variation in glomerular filtration rate that may contribute to the onset of renal failure and electrolyte disorders during extremely hot periods. Although there are few references in the literature, serum sodium disorders seem to increase. The most vulnerable population to heat-related disease are the elderly, children, chronic patients, bedridden people, disabled people, people living alone or with little social contact, and socioeconomically disadvantaged people.
Sabemos que el cambio climático afecta de forma considerable a la salud, si bien son muy pocos los estudios que recogen sus consecuencias a nivel renal. Se ha visto como las olas de calor aumentan la morbimortalidad cardiovascular y respiratoria, pero también el riesgo de fracaso renal agudo, así como el índice de ingresos de causa nefrológica, con la mortalidad que ello implica. Las situaciones de deshidratación repetidas en población expuesta de forma habitual a altas temperaturas parecen estar generando una nueva entidad dentro de la enfermedad renal crónica proteinúrica, cuyo mecanismo fisiopatológico se va dilucidando. Pero más allá de olas de calor y temperaturas extremas, se ha comprobado que existe una variación estacional del filtrado glomerular que pudiera facilitar el desarrollo de fracaso renal y alteraciones electrolíticas en periodos extremadamente cálidos. Entre las alteraciones del medio interno, parecen aumentar fundamentalmente las disnatremias, aunque es poca la evidencia bibliográfica al respecto. Los grupos de riesgo para presentar enfermedades asociadas al calor son ancianos, niños, enfermos crónicos, personas encamadas, discapacitados, sujetos que viven solos o con escaso contacto social y las poblaciones más desfavorecidas a nivel socioeconómico.
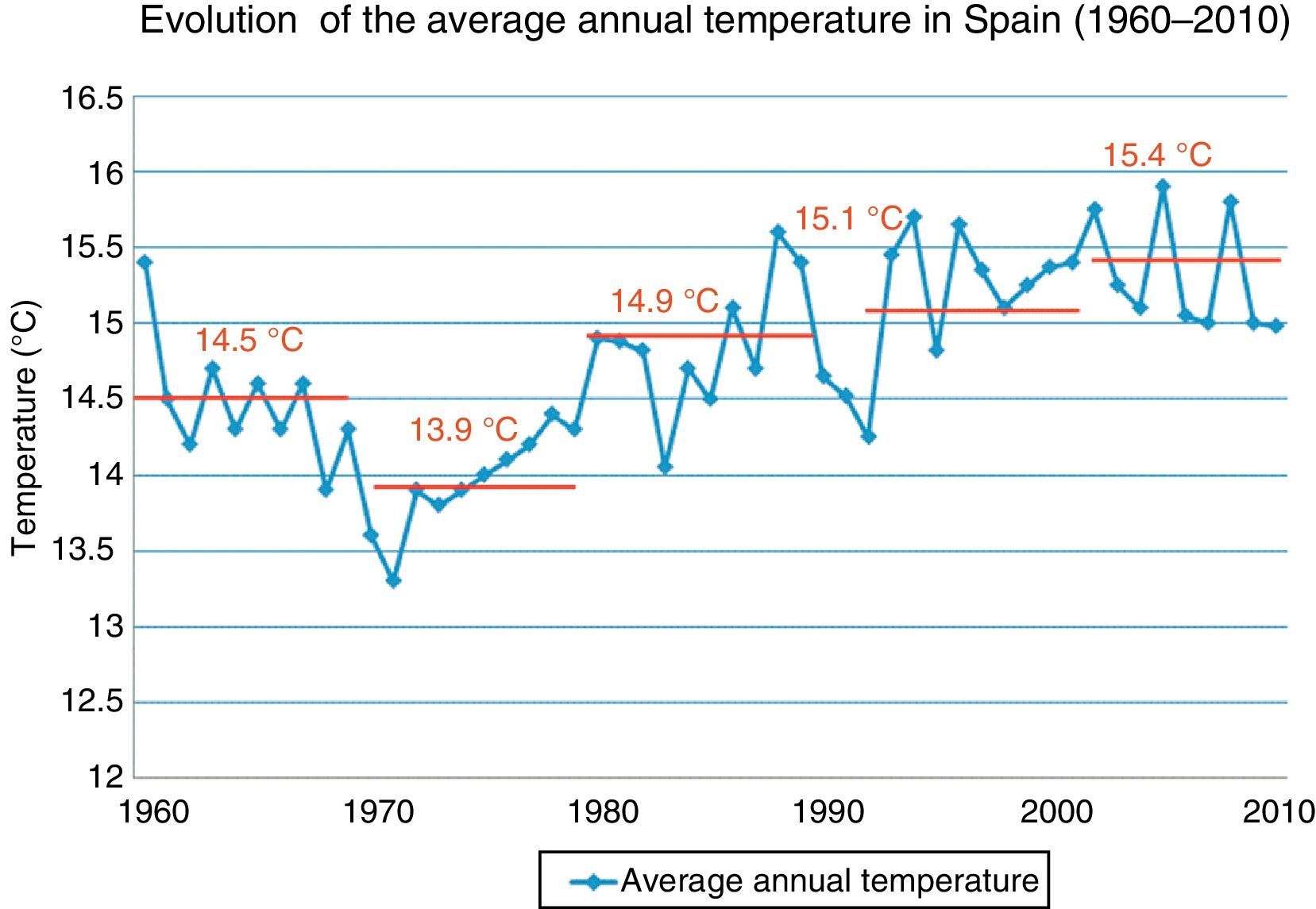
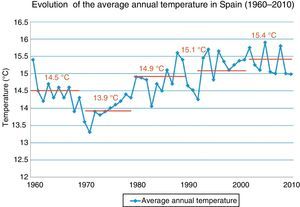
The global variation in the Earth's climate is called climate change, which occurs at various timescales and in any meteorological parameter (temperature, rainfall, cloudiness, etc.), and it is a consequence of our mode of energy production and consumption. Carbon dioxide is the main factor in this phenomenon; its atmospheric concentration has almost doubled from the pre-industrial era to the present. The Earth's temperature has increased by about 0.7°C in the twentieth century, and the rate of increase has been greater during the past 50 years (Fig. 1).1 In fact, 11 of the past 12 years have been the hottest recorded since 1850. Due to its geographical location, Spain is very vulnerable to climate change; in fact, it is expected that in the last third of the century, summer temperatures will be 5–7°C higher. The potential impact of climate change is therefore enormous, with a lack of drinkable water, difficulties in producing food and rising mortality rates due to floods, droughts and heat waves, etc. In short, this is not only an environmental phenomenon, but also involves profound economic and social health consequences.2
Numerous studies have shown that abrupt changes in temperature, including both cold snaps or heat waves, have a direct effect on the number of hospital admissions, morbidity and mortality.3–10 In the United States, 650 people die each year due to heat waves; this is the most lethal weather event and one which has been occurring more and more frequently. These periods of extreme warm temperatures can cause life-threatening situations, such as hyperthermia and heat stroke.
It is easy to imagine how sweating combined with an inadequate water intake or an excess intake of water can cause electrolyte imbalances during periods of high temperatures and variable humidity may cause electrolyte imbalances that are independent predictors of mortality.11–13 Further, compensatory physiological mechanisms, such as circulatory adaptation and thermoregulation, may compromise kidney function. Several studies have demonstrated the relationship between high ambient temperature and an increase in the number of hospital admissions due to kidney failure.5,14–17 During the heat wave that struck Europe in August 2003, more than 70,000 people died across the continent (6500 in Spain); France was the country most affected, with 14,729 deaths,18 most of whom were elderly people dehydrated with kidney failure.19–21
The groups that are at a risk of heat-related illness include children, the elderly, the chronically ill (heart, respiratory and kidney disorders and diabetics), those who are confined to bed, disabled people, those living alone or who have little social interaction,14 as well as the most socio-economically depressed and disadvantaged populations. According to different studies, although a priori the youth are a low-risk group, more time doing outdoor activities for work or recreation, increases their susceptibility heat related disease.18,22–27
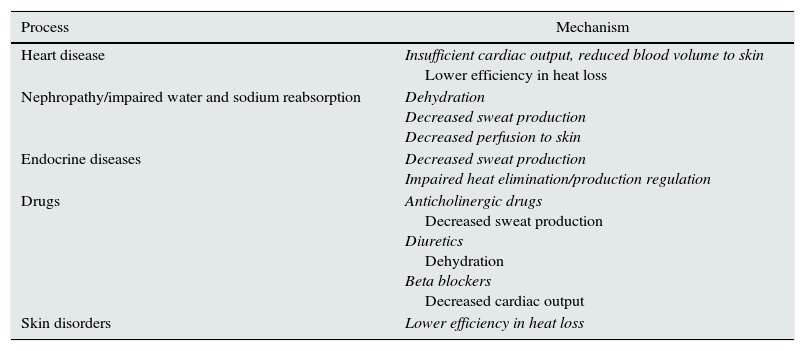
Heat-related illnessHeat-related illness is a condition that may occur either in mild forms (sunburn, heat rash, oedema, syncope, cramps) or in serious and potentially life-threatening forms, such as heat exhaustion and heat stroke, which occur when the physiological mechanisms of thermoregulation fail (Table 1). The skin is the main mechanism for thermoregulation through the loss of heat by conduction (transmission of heat from one object to another by direct contact), radiation (emission of infrared radiation), evaporation and convection (transfer of heat by the movement of a substance through the passage and contact with another of higher temperature). To work more effectively, the sweat glands excrete a saline solution that evaporates quickly, which improves heat transfer. In addition, peripheral vasodilation occurs, which involves increased cardiac output to achieve a greater flow to the skin, thereby reducing blood temperature before returning to the central circulation.28
Processes that alter thermoregulation mechanisms.
| Process | Mechanism |
|---|---|
| Heart disease | Insufficient cardiac output, reduced blood volume to skin Lower efficiency in heat loss |
| Nephropathy/impaired water and sodium reabsorption | Dehydration Decreased sweat production Decreased perfusion to skin |
| Endocrine diseases | Decreased sweat production Impaired heat elimination/production regulation |
| Drugs | Anticholinergic drugs Decreased sweat production Diuretics Dehydration Beta blockers Decreased cardiac output |
| Skin disorders | Lower efficiency in heat loss |
Adapted from Sankoff.28
Heat exhaustion and heat stroke are the most severe forms of this condition. In heat exhaustion, core body temperature never rises above 40°C and one's mental status remains unchanged. The clinical symptoms vary: tachypnoea, tachycardia, nausea, vomiting, sweating, myalgia, skin redness, headache, etc.29,30 In heat stroke, core body temperature rises to 41.5–42°C and causes cell damage, a clinical condition that is due to the systemic inflammatory response syndrome that is triggered and the disseminated intravascular coagulation caused by the activation of a coagulation cascade. The liver and the central nervous system are primarily affected and, vertigo, confusion, ataxia and coma may occur. In addition, the decrease in renal and splanchnic blood flow produced to achieve a greater blood supply to the skin impairs glomerular filtration and the already reduced liver function; intestinal and myocardial ischaemia may also appear.31 In these cases the prognosis depends on factors such as comorbidity, existence of organ damage (seizures, kidney failure, coagulopathy, elevated transaminases) and response to treatment with improvement of mental status when temperature falls below 40°C.
High temperatures and kidney functionImplications on acute kidney injurySeveral studies have demonstrated a causal relationship between heat exhaustion/heat stroke and kidney failure.16,17,19,32 These studies show that different mechanisms, such as dehydration or rhabdomyolysis, may participate in the development of acute kidney injury (AKI). The elderly population is the most vulnerable, due to a low tolerance to high temperature, impaired sensation of thirst, decreased glomerular filtration rate and reduction in the tubular reabsorption of water and sodium during dehydration.19,21 Moreover, elderly patients are often taking multiple drugs, some of which can increase the risk of heat-related illness due to their inhibitory effect on thermoregulation: neuroleptics, anxiolytics, antidepressants, anticholinergics, barbiturates, antihypertensives (beta blockers, diuretics) and antihistamines.17,19,21
In 2008, Hansen et al.7 published a retrospective study in which they looked at exposure to high temperatures and their relationship to renal morbidity over a 12-year period in a population of 1.15 million inhabitants. A heat wave was defined as temperatures above 35°C for more than 3 days. At the end of the period, there were a total of 31 episodes of heat wave of an average duration of 3.8 days, showing a 10% increase in hospital admissions due to impaired kidney function. Significant differences were found in the population aged 15–64 years in terms of the relative risk (RR) of admission due to kidney pathology (1.13) and the RR of admission for AKI (1.78), with the RR of admission due to kidney disorders being also significant in women over the age of 85 (1.22). There were no differences in the incidence of acute dialysis. Similar results were obtained in related studies during the heat waves in Chicago (1995) and California (2006), among others.4,14,22,23 These studies revealed similar findings except on to the susceptibility of diabetic patients to present kidney disorders, which Hansen et al. were not able to show. A study carried out during the 2006 heat wave in France found a higher number of visits to the A&E and hospitalisations due to kidney disorders: mainly (AKI), chronic kidney disease (CKD) and kidney stones, with a high incidence of AKI and kidney stones in people under the age of 75.9
At the end of 2014, Gronlund et al.3 published data collected during 1992–2006 from 114 US cities on the impact of moderate heat (temperature greater than or equal to the 90th percentile), extreme heat (temperature greater than or equal to the 99th percentile) and a heat wave (temperature greater than or equal to the 95th percentile for at least 2 consecutive days) in people older than 65. They found an increase in the number of admissions due to kidney disorders of 4.3%, 14.2% and 23.2%, respectively.
Outside heat waves, Fletcher et al.8 studied the correlation of summer temperatures with admissions due to kidney disorders in New York State during the months of July and August between 1991 and 2004. They found a 9% increase in the odds ratio for hospitalisations due to AKI for every 2.78°C increase in the mean ambient temperature, showing greater susceptibility those aged 24–44 years. An increased odds ratio for admissions due to renal stones and urinary tract infections, probably associated with dehydration, was also observed. In contrast to the above findings, a UK study conducted in Greater London during the summers of 1994–2000 found differences only in the number of visits to the A&E and admissions due to kidney conditions in children under 5, and this may be related to the lowest maximum temperatures in this region during the summer (18.2°C on average; 26.7°C, 95th percentile)5 (Table 2).
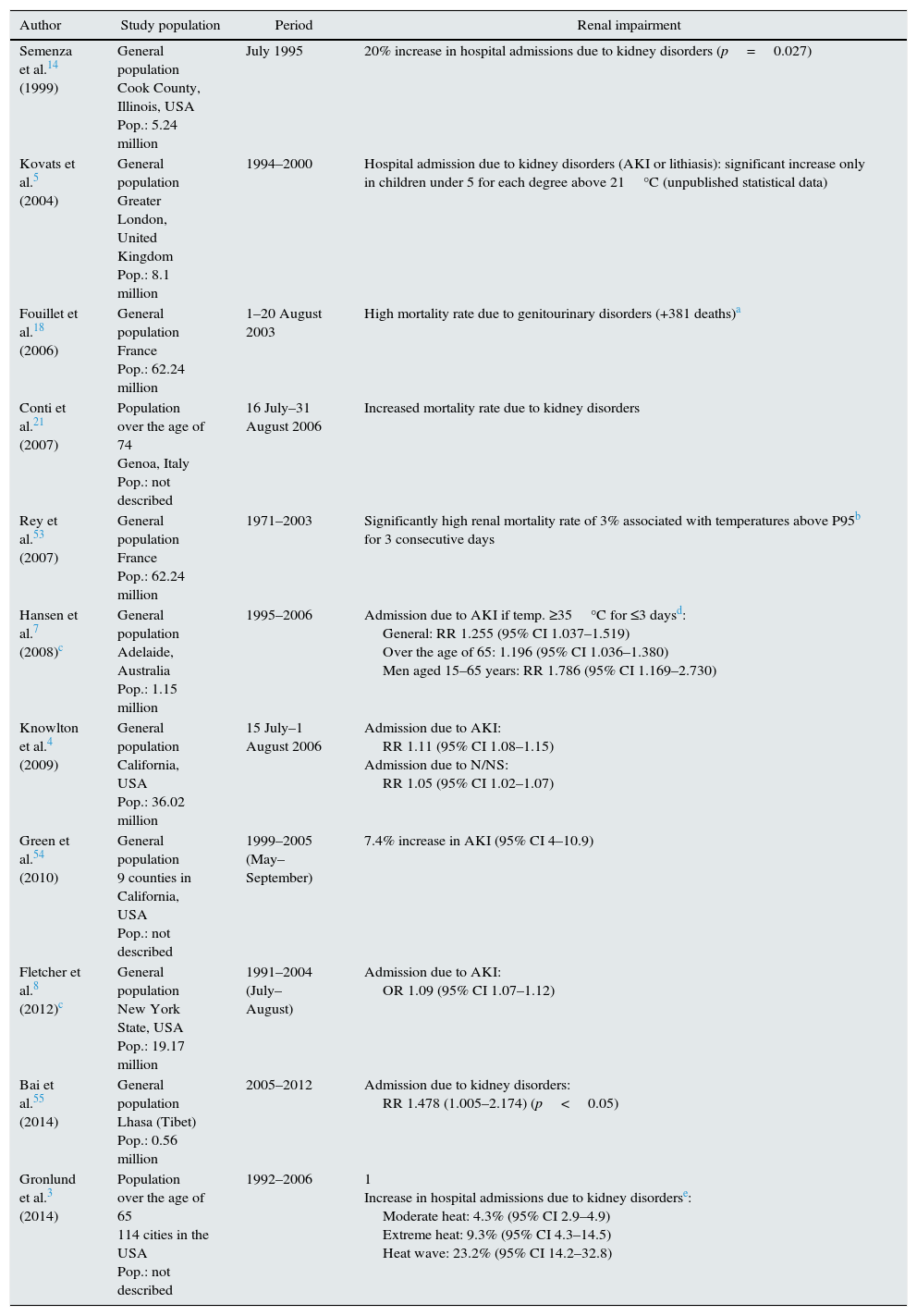
Epidemiological studies of the impact of high temperatures and heat waves.
| Author | Study population | Period | Renal impairment |
|---|---|---|---|
| Semenza et al.14 (1999) | General population Cook County, Illinois, USA Pop.: 5.24 million | July 1995 | 20% increase in hospital admissions due to kidney disorders (p=0.027) |
| Kovats et al.5 (2004) | General population Greater London, United Kingdom Pop.: 8.1 million | 1994–2000 | Hospital admission due to kidney disorders (AKI or lithiasis): significant increase only in children under 5 for each degree above 21°C (unpublished statistical data) |
| Fouillet et al.18 (2006) | General population France Pop.: 62.24 million | 1–20 August 2003 | High mortality rate due to genitourinary disorders (+381 deaths)a |
| Conti et al.21 (2007) | Population over the age of 74 Genoa, Italy Pop.: not described | 16 July–31 August 2006 | Increased mortality rate due to kidney disorders |
| Rey et al.53 (2007) | General population France Pop.: 62.24 million | 1971–2003 | Significantly high renal mortality rate of 3% associated with temperatures above P95b for 3 consecutive days |
| Hansen et al.7 (2008)c | General population Adelaide, Australia Pop.: 1.15 million | 1995–2006 | Admission due to AKI if temp. ≥35°C for ≤3 daysd: General: RR 1.255 (95% CI 1.037–1.519) Over the age of 65: 1.196 (95% CI 1.036–1.380) Men aged 15–65 years: RR 1.786 (95% CI 1.169–2.730) |
| Knowlton et al.4 (2009) | General population California, USA Pop.: 36.02 million | 15 July–1 August 2006 | Admission due to AKI: RR 1.11 (95% CI 1.08–1.15) Admission due to N/NS: RR 1.05 (95% CI 1.02–1.07) |
| Green et al.54 (2010) | General population 9 counties in California, USA Pop.: not described | 1999–2005 (May–September) | 7.4% increase in AKI (95% CI 4–10.9) |
| Fletcher et al.8 (2012)c | General population New York State, USA Pop.: 19.17 million | 1991–2004 (July–August) | Admission due to AKI: OR 1.09 (95% CI 1.07–1.12) |
| Bai et al.55 (2014) | General population Lhasa (Tibet) Pop.: 0.56 million | 2005–2012 | Admission due to kidney disorders: RR 1.478 (1.005–2.174) (p<0.05) |
| Gronlund et al.3 (2014) | Population over the age of 65 114 cities in the USA Pop.: not described | 1992–2006 | 1 Increase in hospital admissions due to kidney disorderse: Moderate heat: 4.3% (95% CI 2.9–4.9) Extreme heat: 9.3% (95% CI 4.3–14.5) Heat wave: 23.2% (95% CI 14.2–32.8) |
AKI: acute kidney injury; 95% CI: 95% confidence interval; N: nephritis; Pop.: population in millions of people; NS: nephrotic syndrome; RR: relative risk.
The studies of high temperatures and heat waves are shown where the results involve data on renal morbidity, although there are only 2 studies in which this was the only objective.
With these data in mind, we suspect that there may be seasonal variations in glomerular filtration rate that occasionally require admission to hospital; but very few publications have studied this phenomenon and their results are somewhat debatable. Masugata et al.33 compared estimated glomerular filtration rate (eGFR) in 102 hypertensive patients with CKD (not on dialysis) and without CKD. In both groups the eGFR was decreased during the summer, with a reduction, as compared with springtime, that was more accentuated in the CKD group (−13.8±9.4 vs. −7.7±8.3%; p=0.001). A significant difference was seen in patients over the age of 73 (−12.2±9.8 vs. −7.2±6%; p=0.002) and among those treated with combinations of renin-angiotensin system (RAS) inhibitors and diuretics (−14±11 vs. −8.4±7.1%; p=0.004). In patients with CKD, systolic blood pressure was significantly lower during the summer. It might be that dehydration may cause a decrease in eGFR. This seasonal variation has also been observed in patients undergoing heart surgery. In 2014, Ranucci et al. published the results from a study of 16,023 patients undergoing heart surgery, where variation in kidney function according to ambient temperature (excluding patients on dialysis) was analysed. Patients undergoing surgical interventions between May and October had significantly higher baseline creatinine and postoperative peak creatinine levels, thes differences were heightened during July and August. However, the difference in the incidence of AKI (according to the Acute Kidney Injury Network classification) was not significant. At higher temperatures, humidity and wind velocity, the eGFR was lower, perhaps because a greater degree of dehydration. Mortality was greater in August (5.1 vs. 3.5%; p=0.018) and in AKI patients: 27.2 vs. 2.4%, with a RR of 15.1 (12.5–18.3; p<0.001).34 The authors emphasised the significant association between of AKI with ejection fraction as well as with by-pass and non-elective surgeries; no significant relationship was observed betwee AKI and the presence of diabetes, use of balloon counterpulsation or anaemia. Although these studies may not have an optimal design to support the conclusions (an important limitation is the use of eGFR as a measure of kidney function, especially in patients without CKD), they are consistent with what has been pointed out in previous sections of this review. Well designed and structured studies are needed to analyse this phenomenon and to identify the population at risk and the concomitant factors so kidney failure and hospital admissions can be prevented, with all the implications that this entails, in hot weather and climates, as well as during heat waves.
In addition to the high temperatures, rainfall also affects the incidence of AKI. Progressive increase in continental temperatures attracts more humid air from the ocean, thus generating more condensation that result in precipitations. In Europe these temperatures have been decreasing in the southern regions, and increasing in northern regions. As a result, there is an increase in river-flooding with negative impact mainly in underdeveloped areas with greater social health consequences. This phenomenon is maximum in Asia, as global warming produces a huge increase in the daily variability of rainfall during the Monsoon season (June–September), which also causes more floods. As a result, in Southeast Asia the incidence of AKI is now increasing by 18–24% due to new cases of malaria, leptospirosis, gastroenteritis and dysentery. This situation has effects on the mortality rate owing to a lack of access to health services and the precariousness of such services in many instances. A dramatic case is that of malaria, which is increasing in Africa, India, Thailand and New Guinea, as the disease is fatal in 45% of cases associated with AKI.35
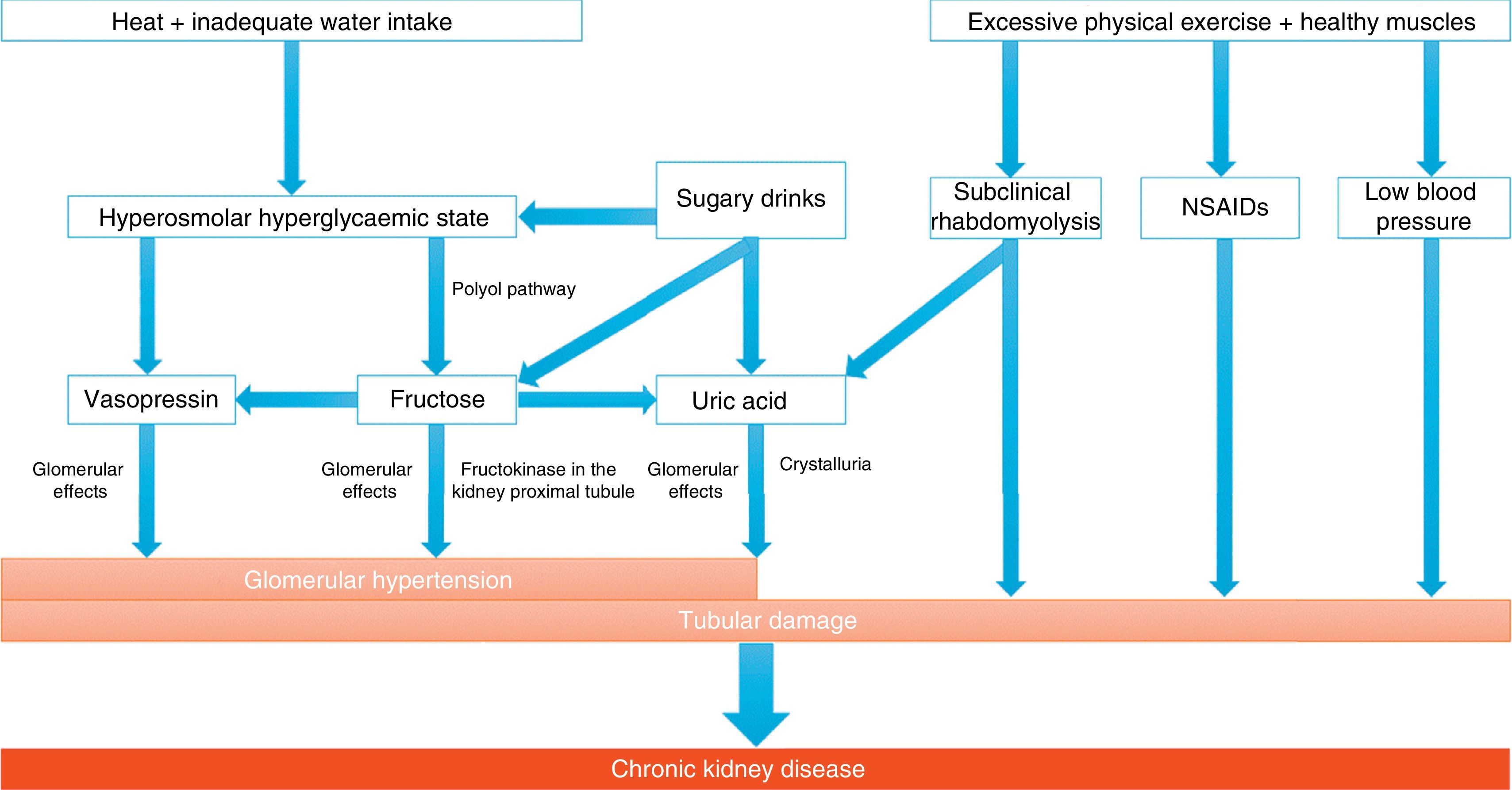
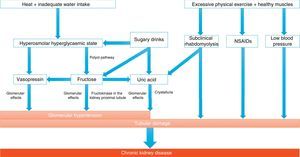
Implications for chronic kidney diseaseThe main causes of CKD are well known (diabetes, obesity, hypertension, etc.); however, there are populations with a high prevalence of kidney disease (almost epidemic levels) that are not encompassed by this series. These include areas of Central America (Guatemala, Nicaragua, El Salvador) and Asia (Sri Lanka, Thailand), and mainly people who harvest sugar cane and rice while working in high temperatures. They are patients with minimal proteinuria and the absence of an active urinary sediment, with biopsies in which tubulointerstitial fibrosis and glomerulosclerosis are detected.36 Attempts have been made to link Mesoamerican nephropathy to toxins, pesticides, silica and heavy metals, with no clear association found, though it seems that recurrent dehydration may play a role in this condition. Kidney damage appears to be a consequence of the recurrent increase in serum osmolarity, which triggers the release of vasopressin and activates the polyol pathway. Vasopressin exerts a harmful effect because it causes a haemodynamic change and oxidative stress on mitochondria. In addition, the polyol pathway increases the production of fructose that is metabolised in the proximal tubule, re-feeding on oxidative stress and resulting in greater inflammation. Thus it ends up causing chronic inflammation and tubulointerstitial and glomerular fibrosis (Fig. 2). It is interesting to note how the frequent rehydration of these workers with sugary drinks only makes the situation worse, by adding fructose as the key substrate of the damage.37–40
Mechanisms potentially involved in the development of chronic kidney disease associated with heat.
Recurrent dehydration may cause chronic kidney disease, primarily due to the onset of a hyperosmolar hyperglycaemic state, which leads to the release of vasopressin and the generation of fructose by the activation of the polyol pathway (aldose reductase/sorbitol dehydrogenase). Vasopressin acts by increasing glomerular hydrostatic pressure, thus increasing the risk of progression of kidney disease. Endogenously produced fructose is metabolised by fructokinase in the kidney proximal tubule, not only re-feeding on the release of vasopressin and causing tubular damage together with oxidative stress, but also producing uric acid and cytokines. In this regard, rehydration with sugary drinks provides a greater amount of substrate, thus amplifying the vasopressin response and the production of uric acid. In addition to these mechanisms, there may be others involved, such as muscle damage due to strenuous physical exercise with onset of subclinical rhabdomyolysis, intake of nonsteroidal anti-inflammatory drugs (NSAIDs) and low blood pressure due to volume depletion. This would involve the activation of the RAS, which also plays an important role in kidney disease.
Adapted from Johnson et al.36
The theoretical relationship between high temperatures and disorders of plasma sodium seems clear: increased sweating and loss of hypotonic fluid combined with situations of impaired thirst sensation and/or an lack of access to water; or profuse sweating together with excessive replacement with hypotonic solutions. Both hyper- and hyponatraemia may cause an increase in mortality, especially in the presence of rapid changes in intracellular water in the central nervous system. Regarding potassium disorders, apart from hyperaldosteronism caused by volume depletion due to sweating, several experimental studies have shown an increase in the activity of Na+/K+-ATPase with an increase in ambient temperature. Thus, the higher the temperature, the greater the uptake of potassium by cells and the lower the blood potassium concentration.41
Acclimatisation is an important aspect in relation to electrolyte imbalances. It has been observed that people who have a greater capacity and rapidity for acclimatisation also have a lower sodium concentration in their sweat from the second day of exposure to high temperatures, owing to a greater sensitivity of the sweat glands to aldosterone. Therefore, inherent factors, such as the density and sensitivity of the sweat glands, as well as changes in lifestyle, are involved in one's capacity for acclimatisation.42
The evidence in the literature on electrolyte imbalances associated with climate is limited and contradictory. In early 2014, Pfortmueller et al.43 published a study on electrolyte imbalances in periods of high temperatures, retrospectively collecting the lab tests of 22,239 patients (mean age of 53) who went to an A&E from 2009–2010 in Bern, Switzerland. The incidence of hypokalaemia was 11.1%; hyperkalaemia, 4%; hyponatraemia, 8.9%; and hypernatraemia, 1.5%. It should be noted that 11% of this population took diuretics and 9.6% had CKD. There was a weak inverse correlation between daily maximum temperature and the values for sodium (R=−0.04, p<0.05) and potassium (R=−0.03, p<0.05). Considering that the temperature exceeded 25°C only for 88 days and on 16 of them it was higher than 30°C, the only significant result was the higher prevalence of hyponatraemia (11 vs. 9%; p=0.04) in the periods where the temperature was above 30°C. Hyponatraemia and hypernatraemia, an age greater than 80 years and hospital admission with ambient temperature higher than 30°C were independent predictors of mortality. In a retrospective study of a 10-year period on admissions to 12 Chinese hospitals, Zhao et al.44 concluded that sodium levels were significantly lower in August than during the rest of the year (134.2 vs. 140.1mmol/l; p<0.05) and these levels were an independent risk factor for admission. There were no differences in potassium levels. The variation in blood sodium with temperature and humidity was calculated retrospectively by Bucher et al.45 in a cohort of 13,277 patients, showing increases of 0.017mmol/l for each 1% increase in humidity above the mean (p<0.0001) and 0.033mmol/l for each degree of temperature above the mean. Although other authors support these electrolyte-related variations associated with high temperatures, it has not been demonstrated in all cases.46,47 In the specific case-series of 201 patients diagnosed with hyponatraemia associated to thiazides, there was no higher incidence in the summer; in fact, blood sodium was significantly higher in that season (118±7 vs. 114±8mmol/l; p=0.016).48
There are no studies on electrolyte imbalances in patients on dialysis during periods of heat. As seems to be the case with eGFR, some authors report some seasonal variation in electrolytes, but not all reached uniform results. Some observed higher potassium levels during the winter in relation to a higher food intake in the cold months, others showed higher potassium levels during the summer due to a higher consumption of fruit.49 There is only one reference concerning patients on peritoneal dialysis. A significant inverse relationship between temperature and plasma levels of sodium, potassium, bicarbonate, albumin, BUN, ultrafiltration volume and dry weight was found in a group of 44 prevalent anuric patients on peritoneal dialysis after a 2-year study period, with these changes being attributed to sweating caused by heat.50
Global warming; morbidity and mortalityIn addition to kidney disease, as mentioned in the previous sections, during heat waves there is an increase in hospital admissions, from 1to 10% according to different authors,3,5 due to cardiovascular and respiratory disorders. However, Turner et al.51 did not find this association in their meta-analysis, although it is true that the results were very close to reach statistical significance.
During the 1995 heat wave in Chicago, the all-cause mortality in the general population increased by 147%, whereas the number of admissions only increased by 11%. This disproportion was due to the increase in sudden deaths, mainly among people living alone or lacking social/family support.52 In other cohorts, deaths due to cardiovascular and respiratory causes increased by 5–6%, mainly in those over the age of 75.3 In Australia, from 1968–2006, the summer/winter ratio of all-cause mortality went from 71/100 to 86/100, and continues to rise as the mean temperature and the frequency of heat waves increase.6
Although it seems obvious that there is higher morbidity and mortality in relation to heat in patients with CKD, there are no data in the literature that support this. In dialysis patients, the results from the MONDO registry (87,399 patients with end-stage CKD on dialysis in different climatic zones of the world) indicate a seasonal variability in the mortality rate, which is higher in winter.10
ConclusionsThe rise in average temperatures and the increased frequency and intensity of heat waves have a direct impact on mortality rates and social health spending. There are not many studies that analyse the implications of these changes on kidney function and electrolyte disturbances, and their results are controversial and inconsistent, probably due to the lack of a suitable design and objectives. The incidence of AKI seems to increase during these periods, mainly affecting depressed populations and deprived areas with limited health resources. Also, causal models that include how temperatures affect CKD are being studied. New groups at risk are being defined, beyond the conventional ones.
We need not only public health plans based on early warning systems that enable risk situations to be identified and anticipated, but also programmes to monitor and check on susceptible populations. We should raise awareness among citizens, politicians and health professionals to promote measures to adjust to and mitigate the effects of climate change, which will in turn encourage research and technological development.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: de Lorenzo A, Liaño F. Altas temperaturas y nefrología: a propósito del cambio climático. Nefrologia. 2017;37:492–500.