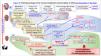
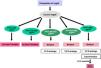
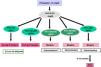
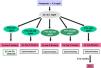
Bone-mineral metabolism recommendations
More infoAs in 2011, when the Spanish Society of Nephrology (SEN) published the Spanish adaptation to the Kidney Disease: Improving Global Outcomes (KDIGO) universal Guideline on Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD), this document contains an update and an adaptation of the 2017 KDIGO guidelines to our setting. In this field, as in many other areas of nephrology, it has been impossible to irrefutably answer many questions, which remain pending. However, there is no doubt that the close relationship between the CKD–MBD/cardiovascular disease/morbidity and mortality complex and new randomised clinical trials in some areas and the development of new drugs have yielded significant advances in this field and created the need for this update. We would therefore highlight the slight divergences that we propose in the ideal objectives for biochemical abnormalities in the CKD–MBD complex compared to the KDIGO suggestions (for example, in relation to parathyroid hormone or phosphate), the role of native vitamin D and analogues in the control of secondary hyperparathyroidism and the contribution of new phosphate binders and calcimimetics. Attention should also be drawn to the adoption of important new developments in the diagnosis of bone abnormalities in patients with kidney disease and to the need to be more proactive in treating them. In any event, the current speed at which innovations are taking place, while perhaps slower than we might like, globally drives the need for more frequent updates (for example, through Nefrología al día).
Al igual a como ocurrió en el año 2011, cuando la Sociedad Española de Nefrología (SEN) publicó la adaptación española a las guías universales Kidney Disease Initiative Global Outcomes (KDIGO) sobre alteraciones del metabolismo óseo-mineral en la enfermedad renal crónica (CKD–MBD), este documento contiene una actualización y adaptación a nuestro medio de las guías KDIGO del 2017. En este campo, al igual que en muchos otros nefrológicos, no se ha podido contestar irrefutablemente muchas cuestiones pendientes aún. Sin embargo, no hay duda acerca de la estrecha relación entre el complejo CKD–MBD/patología cardiovascular/morbimortalidad, nuevos ensayos clínicos aleatorizados en algunas áreas o la aparición de nuevos fármacos han proporcionado notables avances en este campo y crearon la necesidad de dicha actualización. Así, destacamos las discretas divergencias que ofrecemos en los objetivos ideales de las alteraciones bioquímicas del complejo CKD–MBD respecto a las sugerencias de las KDIGO (en relación, por ejemplo, con la hormona paratiroidea o fosfato), el papel de la vitamina D nativa y análogos en el control del hiperparatiroidismo secundario, así como la contribución de nuevos captores de fosfato y calcimiméticos. Asimismo, es de destacar la adopción de importantes novedades en el diagnóstico de las alteraciones óseas del paciente renal y la necesidad de tomar actitudes más proactivas en su tratamiento. En cualquier caso, la velocidad a la que acaecen novedades actualmente, aunque menor de la que sería deseable, sí impulsan globalmente la necesidad de actualizaciones con menor demora (por ejemplo, a través de Nefrología al día).
The term “Renal Osteodystrophy” was traditionally used to denote abnormalities of bone-mineral metabolism in patients with chronic renal disease (CKD). The non-profit KDIGO (Kidney Disease Improving Global Outcomes) organization suggested new definitions and a more integrated classification system to replace the traditional term of Renal Osteodystrophy.1–5
- -
Renal Osteodystrophy (ROD): this term is now applied to alterations in bone morphology and architecture intrinsic to CKD (Figs. 1 and 2). Diagnosis is confirmed by bone biopsy.
- -
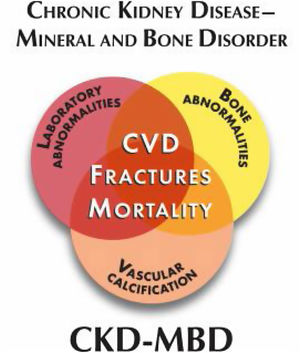
Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD): this term covers all biochemical and skeletal alterations together with the extra-skeletal calcifications occuring as a result of mineral metabolism disorders in CKD patients, integrated as a systemic entity (Fig. 3) that is associated with increased mortality. Patients may present as one or a combination of the following manifestations:
- •
Abnormalities in calcium (Ca), phosphorus (P), parathyroid hormone (PTH) and vitamin D.
- •
Disorders in skeletal remodelling, mineralisation, volume, bone growth or fragility.
- •
Cardiovascular and/or other soft tissue calcifications.
- •
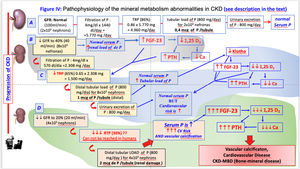
Metabolic disorders of CKD–MBD are the result of a gradual loss of renal function. Kidneys play an essential role in mineral homeostasis, as it is the source of some regulator molecules (klotho and calcitriol) while kidneys also regulate phosphate balance in the organism. It is also key in maintaiining a normal calcium concentration. These facts justify that derangements in calcium and phosphate homeostasis and consequent regulatory hormonal changes start early in the course of CKD.
In patients with moderate reductions in creatinine clearance (less than 70 mL/min) a load of phosphate causes a transitory increase in phosphataemia together with a reduction in calcaemia.6 However the elevation in serum phosphate concentration usually goes undetected until CKD stages 4 and 5, when the reduction of glomerular filtration overcomes the compensatory phosphaturic effect of fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH) which are unable to raise the fractional excretion of phosphorus (FEP) above 50%. This is why phosphataemia does not reveal the phosphaturic effort of the kidney, and it is not a very sensitive marker of the total body load of phosphate since circulating phosphate represents only approximately 1% of the total content of phosphate in the organism. Alternatively, the increase in circulating levels of FGF23, or its phosphaturic effect (measured as the increase in the fractional excretion of phosphate in urine or maximum phosphate transport relative to the glomerular filtration rate, or simply the phosphaturia divided by glomerular filtration) may be good indicators of early phosphate retention before the detection of hyperphosphatemia.7
FGF23 is produced mainly in bones (mature osteoblasts-osteocytes) and the serum concentration increases in early stages of CKD. The production of FGF23 increases in response to the intestinal absorption of phosphate relative to the filtration capacity of the kidney. FGF23 production also depends on the interaction of local factors which modulate bone remodelling and mineralization, as well as systemic factors associated with mineral homeostasis. The major stimuli for FGF23 production are phosphate retention, active vitamin D and serum PTH levels. FGF 23 controls phosphate homeostasis both by its phosphaturic effect which prevents the accumulation of phosphate, and by inhibiting the synthesis of calcitriol (1,25(OH)2D3) which reduces intestinal absorption of phosphate.
In CKD patients it is also detected an early, discrete but significant fall in calcitriol which is caused by :
- -
A reduction in the bioavailability of substrate [Calcidiol (25(OH)D3)] necessary for the renal synthesis of calcitriol. Calcidiol is filtrated by the glomerulus and it is transported by proximal tubular cells with the intervention of megalin (an endocytic receptor located on the apical membrane). Within the tubular cells, calcidiol is transformed into calcitriol by the action of 1-alpha hydroxylase. The fall in glomerular filtration reduces the access of calcidiol to the tubular cell, and this is aggravated if it coexists with a nutritional vitamin D deficiency (which is very common even in the general population) due to lack of exposure to sunlight and/or an unsuitable diet. In more advanced stages of CKD the reduction in megalin (which is upregulated by calcitriol itself) also contributes to the reduced availability of calcidiol.
- -
The high level of FGF23 inhibits the activity of 1 alpha-hydroxylase (or CYP27B1) which causes a reduction in the renal synthesis of calcitriol. At the same time FGF23 promotes the catabolism of circulating calcitriol by increasing the activity of 24-hydroxylase (or CYP24A1).
- -
The synthesis of calcitriol is also reduced by the loss of renal mass with leads to a lower availability of 1-alpha-hydroxylase.
The reduction of calcitriol production is partially corrected by the stimulation of 1 alpha-hydroxylase induced by PTH.
In CKD stages 2 and 3, the decrease in circulating levels of calcitriol contributes to a reduction in the intestinal absorption of calcium. Nevertheless, calcitriol is also synthesized in other cells of the organism, where it has autocrine and pleiotropic paracrine functions. These cells are able to incorporate circulating calcidiol into cytoplasm and produce calcitriol for their own consumption. The autocrine activation of the vitamin D receptors (VDR), which is fundamental for the survival of the renal cell itself as well as other cells of the cardiovascular system, depends on specific tissue factors and it is not regulated by PTH and FGF23.
In addition, a reduction of calcidiol, which also occurs in CKD, may compromise this auto-paracrine activation. The decrease in calcidiol in CKD is caused by: a) reduction in sun exposure and a Vit D deficient diet, uraemia, biliary and pancreatic dysfunctions, among others; b) an FGF23-induced increase in 24-hydroxylase activity which also catabolizes 25(OH)D); and (c) greater difficulty in accessing the inside of these cells due to the downregulation of receptors such as megalin or others, which are induced by calcitriol itself. Additionally, a study shows that the capacity of the liver to produce 25(OH)D3 is reduced in uraemic rats, and that parathyroidectomy improves liver calcidiol production.8
Phosphate retention, together with calcitriol deficiency and its associated hypocalcaemia, leads to the development of secondary hyperparathyroidism (SHPT). The increase in PTH levels is usually observed with glomerular filtration rates below 60 mL/min/1.73 m2. Additionally, there are molecules produced by the diseased renal tissue itself that may also contribute to the reduction, lack of affinity or downregulation of receptors such as megalin, or other co-regulating factors.
Klotho is a molecule which functions as a co-receptor of FGFR19 and in addition, independently of FGF23, klotho has phosphaturic activity.10 The expression of Klotho declines with the reduction of renal function, and it may be the responsible for the resistance to the phosphaturic effect of FGF23 and the lack of this independent action of Klotho on phosphaturia. It is possible that the excessive tubular load of P causes a fall in the renal expression of Klotho.9,11
In the parathyroid glands, three classic and well-known receptors are present (the Vitamin D receptor, the Calcium sensing-receptor, and the Fibroblastic growth factor receptor 1 (FGF-R1) / Klotho) which modulate the synthesis and secretion of PTH, as well as the parathyroid cell proliferation. These receptors are potential target for treatment of SHPT and CKD–MBD.
- 1.
Vitamin D receptor.
- -
The inhibitory action of calcitriol (active vitamin D) on parathyroid cells is mediated by its specific cytosolic receptor (VDR), which translocates to the nucleus to act on DNA vitamin D response elements (VDRE).
- -
In CKD, the number of parathyroid cell VDR is reduced; the uraemic state itself may reduce the stability of the VDR mARN, resulting in a decrease in the synthesis of VDR. Moreover, “uraemic toxins” reduce the translocation of the VDR–vitamin D complex to the nucleus and its binding to the VDRE is impaired.13
- -
The decrease of parathyroid cell VDR causes a resistance to the inhibitory action of active vitamin D on PTH synthesis.
- -
In CKD, the increase in parathyroid cell proliferation (hyperplasia) is accompanied by a reduction in VDR expression. In advanced hyperparathyroidism (“nodular” hyperplasia) there is a remarkable decrease in parathyroid cell VDR expression.
- 2.
Calcium sensing-receptor (CaSR)
- -
CaSRs are located on the parathyroid cell membrane and they are able to detect small changes in serum calcium concentrations and act at a post-transcriptional level by modulating the half-life of PTH mARN. If ambient calcium decreases there is insufficient calcium binding to their specific receptors and the inhibitory effect on PTH secretion ceases.
- -
A decrease in parathyroid cell CaSR causes a resistance to the inhibitory effect of Ca on the parathyroid cell activity.
- -
In CKD, the gradual development of parathyroid hyperplasia is associated with a fall in the CaSR expression in parathyroid cells.
- -
High levels of P directly stimulate PTH synthesis and secretion.14–17 This effect may be explained by a reduction of CaSR activity18
- -
High phosphate acts on specific sites of the CaSR interfering with calcium induced activation of the receptor12
- 3.
Fibroblast growth factor receptor 1 (FGF-R1) and Klotho. The FGF-R1 receptor and the Klotho protein, its co-receptor, are expressed in the parathyroid cells allowing FGF23 to exert an inhibitory effect on parathyroid cells. However, in hyperplasic parathyroid glands from uremic rats and CKD patients the expression of FGFR1 and klotho is reduced thus in uremic hyperparathyroidism the parathyroids are resistant to the inhibitory action of FGF23.
The effect of molecules and hormones on the parathyroid receptors:
- -
A decrease in extracellular calcium is detected by the parathyroid cell CaSR resulting in postranscriptional stimulation stimulation of PTH synthesis and secretion.
- -
Phosphate retention stimulates PTH synthesis and secretion as well as parathyroid cell proliferation. This is produced by adirect effect on the parathyroid cells through CaSR and also by indirect mechanisms such as: a) inhibition of the production of active vitamin D, b) reduction in the calcaemic response to PTH (skeletal resistance to the action of PTH). It is important to keep in mind that high phosphate, by inducing parathyroid hyperplasia, causes a reduction in CaRS and VDR expression that subsequently favours PTH synthesis, secretion and further parathyroid cell prolifertion.
- -
Active vitamin D (calcitriol) binds to the VDR and acts at a transcriptional level suppressing PTH synthesis. A deficit of calcitriol reduces this inhibitory effect on parathyroid cells.
- -
Calcitriol deficiency produces a downregulation of parathyroid VDR expression and an increase in calcitriol is capable to upregulate its own receptor in different tissues including the PTG; although the effects of the different vitamin D analogues are not identical.19 Similarly, a decrease in calcium concentration causes a downregulation of both VDR and CaSR expression. Inversely, an increase in calcium upregulates VDR expression and regarding its effect on CaSR, the results from different experimental studies are not totally uniform. But certainly the expression of CaSR is augmented by the action of calcimimetics.19 It also seems that calcimimetic drugs would be able to increase VDR expression in the parathyroid gland.20
- -
Calcitriol is also able to increase CaSR expression. This effect decreases in case of hypocalcaemia, and it is enhanced when calcium levels are normal or high, or when calcimimetics are being administered.20
The previously mentioned abnormalities will cause damage in target tissues. Skeleton and the cardiovascular system are the most importantly affected. Soft tissue calcifications and calciphylaxis are important complications, as they are clearly associated with increased morbimortality in CKD patients.
Valvular and vascular calcifications are not a passive process of calcium and phosphate deposition. The increase in phosphate and calcium, inflammation mediators and uraemia per se, among others, have been found to favour the active transformation of smooth muscle cells into chondro-osteogenic type cells. These cells produce collagen matrix that subsequently mineralizes leading to cardiovascular “ossification”. There are multiple systemic (such as fetuin A) and local (such as Matrix Gla Protein) inhibitors of calcification to counterbalance the accelerator effect of renal failure on vascular calcification. In fact, calcium and phosphate combine with calcification inhibitor proteins to form primary or secondary calciprotein nanoparticles which take part in these vascular calcification processes.21 Metabolic acidosis may slow the progression of calcifications22 while alkalosis (e.g., post-dialysis) may favour calcification. Cardiovascular calcifications progress rapidly in patients with CKD.
CKD is also a process of accelerated ageing that is multifactorial. As such, bone fragility is increased and it is associated with a high incidence of fractures (senile or postmenopausal osteoporosis). There is also muscle weakness and a propensity to falls. Arteriosclerotic disease itself may not be a direct consequence of CKD but it does coexist and becomes aggravated by it. Moreover, all these factors influence the damage of CKD on its target organs and therefore affects treatment and prognosis.
These harmful effects go beyond bone alterations. Hyperphosphataemia has been associated with an increase in intima-media thickness, vascular rigidity and calcification, myocardial hypertrophy and mortality.23,24 It induces inflammation, oxidative stress and endothelial dysfunction, and it favors CKD progression.23,24 Classically, PTH has been considered an uraemic toxin, and it has been associated to a number of systemic effects.5 More recently, vitamin D deficiency is also considered an important pathogenic factor in and beyond CKD; it is associated with alterations of immunoregulation, inflammatory response, regulation of cellular proliferation, insulin secretion and renin production, among others.25 Calcidiol also has a direct action on bone metabolism, and it is the local substrate for the generation of calcitriol.
In addition, the increase in FGF23 has been shown to directly induce left ventricular hypertrophy by acting through the FGFR426 and it also cause alteration of endothelium-dependent vasodilation. It is also associated with multiple systemic effects, including inflammation and calcium and sodium control,27 although the latter has been questioned.
The rise in FGF23 occurs at early stages of CKD, and it actually precedes the increase in PTH even when the serum phosphate concentration is normal. However, the earliest alteration appears to be the reduction of klotho in kidney causing resistance to the phosphaturic action of FGF23, and in its free circulating fraction. Klotho deficiency may be associated with premature ageing mechanisms, at least partially independent of FGF23.28
Bone produces molecules that are capable to act on other organs through specific receptors an modify different functions. Therefore, bone may be considered as a new endocrine organ not only due to the generation of FGF23 but also to other molecules, such as osteocalcin, or others that modify the Wnt- (sclerostin/Dkk1) and the osteoprotegerin/RANKL- signalling pathways. The abnormalities associated with mineral metabolism have been clearly shown in the population with CKD, mainly patients in dialysis. However, they have also been found in the general population. These mineral metabolism absnormalities are independent predictors of mortality, especially due to cardiovascular disease. This is based on findings from multiple epidemiological studies, meta-analyses, pre-specified secondary analyses from randomized clinical trials and post-hoc studies.24,28–36
Nevertheless, no definitive proof of causality exists because, among other reasons, the most relevant studies have been directly or indirectly affected by a lack of sufficient statistical power.37,38
Diagnostic strategiesThe objective is to define the best diagnostic methods for the study and management of mineral metabolism disorders. This information was obtained from a synthesis of the recommendations extracted from clinical guidelines, such as the K/DOQI, KDIGO, and also from the expert opinions found in the literature and the authors of the present recommendations.1,2,4,39,40
Regarding the periodicity of biochemical determinations, although they are established by the S.E.N. Quality Guide,41 we are showing below more specific recommendations.
Biochemical parameters (Table 1)First of all, we recommend that laboratories inform clinicians about the methods of measurement used and that any change in the methodology should be reported. The lab should also inform about sample origin (serum or plasma) and type of handling required for a correct interpretation of the results. In CKD, the isolated value of a parameter is less important than the change or trends observed in such a parameter; this is the reason why it is so important that the methods are maintained the same and if a change it is necessary, it must be notified to allow for appropriate corrections. This recommendation is especially relevant for the measurement of PTH, calcidiol and other hormones, as well as for creatinine (methods with adequate traceability) and albumin (with important differences between methods that use blue or purple bromocresol).42–44
Periodicity of biochemistry evaluation.
| Parameter | Stages 3−4 | Stages 5−5D | Renal transplant |
|---|---|---|---|
| Ca/P | Each visit | Every 1−2 months | Each visit |
| PTH | 6−12 months (depending on the values) | Every 2−3 months | 6–12 months (depending on the values) |
| Alkaline phosphatase | With each PTH determination | With each PTH determination | With each PTH determination |
| Calcidiol | 6−12 months | 6−12 months | 6−12 months |
| Mg | 6−12 months | 6−12 months | 6−12 months |
Periodic determination of serum calcium and phosphate concentrations, together with PTH and alkaline phosphatase, is decisive for the therapeutic management of patients. Ideally, ionic calcium should be used, but there are processing and cost issues for their routine use. Total calcium should be adjusted for actual albumin (or plasma protein) levels, especially in cases of hypoalbuminemia or hypoproteinemia.
We should take into consideration that the accuracy of albumin-corrected calcium and ionized calcium is only weak, probably due to variations in albumin, pH, hemoconcentration and others, present in dialysis patients or with CKD.45,46 Correction formulas have even been developed that also take into account plasma phosphate in addition to albumin.47
It is also important to remember that extracellular calcium concentration does not always correlate with calcium balance (which can be positive or negative with normal plasma calcium). The same occurs with phosphate, so a normal plasma phosphate does not exclude the presence of systemic overload.
On the other hand, the isolated values of calcium and phosphate are not sufficient for the appropriate management of patients with CKD, since normal calcium and phosphate values are common, but at the expense of a SHPT, that could be even severe.
- -
We consider that the monitoring intervals suggested by the KDIGO are reasonable; Ca and P should be measured every 6–12 months in CKD Stage 3; every 3–6 months in CKD Stage 4; every 1–3 months in CKD Stage 5, and in CKD Stage 5D monthly/bimonthly determination seems the most appropriate.
- -
A closer monitorization may be necessary in patients under treatment with calcimimetics or with active vitamin D metabolites, especially during the period of dose adjustment.
- -
In hemodialysis patients, blood samples should be obtained pre-dialysis in the middle of the week.
- -
The calcium–phosphorus product provides information that may be useful, but only in dialysis patients and never separately assessing serum calcium and phosphorus values.
The level of circulating iPTH used to be measured with the no longer available Nichols Allegro kit (the normal range in the general population was 10–65 pg/mL). iPTH is now measured by immunoradiometry or immunochemiluminescence assays. PTH is the biochemical parameter (along with alkaline phosphatase) that best correlates with the histological lesions of SHPT, especially with osteoblastic activity. In fact, PTH better reflects actual parathyroid activity, and alkaline phosphatase the bone activity.
For this reason, the level of PTH is considered a good marker of underlying bone disease (at least the best readily available) in combination with total alkaline phosphatase or bone-specific alkaline phosphatase). Thus, considering the values of PTH and alkaline phosphatase may be enough to assess bone turnover and in most cases there is no need to perform a bone biopsy. This suggestion is especially relevant in these new guidelines in which an active treatment of the risk of fracture in patients with CKD is proposed (i.e. with antiresorptive agents). These should probably be avoided if adynamic bone disease is suspected (see below).
- -
In dialysis patients, iPTH levels >450−500 pg/mL (or equivalent) are usually specific for high remodeling bone disease, specifically osteitis fibrosa or the mixed form and, virtually exclude low remodeling disease. In one study, the best PTH value to classify as high remodeling bone disease was iPTH levels >323 pg/mL (approximately 5X the upper limit of normal).48 Obviously, the values proposed by the 2009 and 2017 KDIGO guidelines (>9X upper limit of normal) increase specificity but at the expense of accepting other risks associated with high PTH levels.3,4
- -
In dialysis patients, iPTH levels <100−120 pg/mL (or equivalent) are linked to low bone remodeling (adynamic form or, more rarely, osteomalacia) with a prediction value close to 90%. In one study, the best point of discrimination for low remodeling bone disease was iPTH levels <104 pg/mL (slightly less than 2X the upper limit of normal).48 Therefore, the intermediate levels of PTH2,4,49,50 have less specificity to predict the underlying bone pathology and do not necessarily correlate with worse or increased survival. It is important to take into consideration that PTH values should be avaluated according to the ongoing treatment devoted to control PTH.
Although it has not been established a direct correlation between PTH levels and cardiovascular injury, relatively higher or lower levels of PTH are associated with increased risk of mortality, especially cardiovascular. Older publications have described an association between elevated PTH levels and left ventricular hypertrophy.51 Thus, in dialysis patients, both levels below 150 pg/mL and above 300 pg/mL (approximately 2X–5X the upper limit of normal, targets of the KDOQI 2003 or SEN 2011 guidelines)1,50 have been associated, at a population level, with an increase in a mortality. In the COSMOS study,52 it was established that the PTH value associated with a minimal mortality in European patients was 398 pg/mL. In addition it is necessary to highlight that there is an agreement that low bone remodeling (i.e. PTH < 2X the low limit of normal) is also associated with vascular calcifications, fractures and mortality. In any case, mortality associated with elevated PTH is modulated by the level of P which is cause of the most severe hyperparathyroidism.
PTH levels should be measured every 6−12 months in CKD stages 3−4 depending on the baseline value and the degree of progression of CKD. Although treatment is not modified, it is convenient to know the rate of increase in PTH in order to implement measures not only in extreme cases but also with clear evolutionary increasing or decreasing trends. In stage 5 (including 5D) PTH should be measured every 3–6 months as recommended by the KDIGO guidelines.
A more frequent monitorization of PTH may be necessary in patients on treatment for hyperparathyroidism, especially during dose titration to analyze efficacy and side effects, as well as to evaluate the rate of change in PTH. It is important to highlight that rather than taking into consideration the individual values of calcium, phosphate or PTH, we should evaluate the combined change (trends) of these parameters rather than “isolated values”, which may be punctually discordant. It is also important to assess the changes in PTH, calcium and phosphorus in the context of the other CKD–MBD parameters (calcidiol, alkaline phosphatase, vascular calcification, etc.).
Currently, we have to face the problems derived from the lack of homogeneity of the different methods used for the measurement of iPTH. There are not good correlation coefficients between the different assays which makes it difficult to compare the laboratory results. The Spanish Society of Nephrology prepared a document aiming to clarify the interpretation of these different methods,53 including correction formulas to calculate iPTH (available in applications developed in Spain such as “Global Nephro Calculator”) with respect to the reference kit classically used (Allegro de Nichols) and from which almost all the information on PTH was originally obtained. It is important to note that these initial inter-method adjustment algorithms were established in patients with CKD 5D on hemodialysis, and therefore they should not be used in other CKD populations where the proportion of PTH fragments (cleared by the kidney) is different. Therefore, they are also not applicable to patients on peritoneal dialysis for whom different algorithms have been proposed.54
The determination of “whole” or “bio-PTH” with methods that quantify PTH 1–84 (biologically active PTH) with the interference of amino-PTH (PTH 1–84 phosphorylated at amino acid 14), as well as the calculation of the ratio between different PTH fragments,55 are not currently recommended in daily clinical practice. Likewise, the use of non-oxidized PTH measurements (iPTH — oxidized PTH) is not yet justified in daily clinical practice.55
25(OH) vitamin D (calcidiol)It is recommended to measure the levels of 25(OH) D (calcidiol) to prevent and treat if necessary, the frequent insufficiency or deficiency of this prohormone in CKD patients. The levels of 25(OH) D reflect the vitamin D stores and may vary with the diet and the degree of sun exposure. Nevertheless, the optimal level of 25(OH) D and the level at which it is considered insufficient remain controversial both in the general population and in CKD patients.
According to the results of clinical trials conducted in the general population it can we concluded that levels below 20 ng/mL (50 nmol/L) are probably suboptimal for CKD patients. In these cases it is recommended the intake of native vitamin D supplements (especially cholecalciferol or ergocalciferol) as recommended for the general population. Values between 20 and 40−50 ng/mL should probably be the target in patients with CKD, although there is still much controversy in this regard.56,57
It is unknown the relative importance of the measurement of vitamin D with the different kits available on the market, as well as the clinical value of free vitamin D measurement.
Low serum levels of 25-OH-vitamin D have been associated with higher overall and cardiovascular mortality in patients with CKD (on dialysis or not ) and in the general population58; however, improved survival with native vitamin D supplementation has not been reported.59
Alkaline phosphataseTotal alkaline phosphatase together with PTH are useful to assess bone turnover in patients without liver disease. The measurement of Bone alkaline phosphatase may have some advantages as compared with total alkaline phosphatase but these do not necessarily justify the additional cost. However, the measurement of both iPTH and bone alkaline phosphatase is prpobably the best approach correlating with bone turnover in CKD.60–62 It may be necessary to reconsider the use of alkaline phosphatase in patients with CKD, especially if it is being considered a proactive treatment for osteoporosis. The use of antiresorptive agents should probably be avoided in cases with a high suspicion of adynamic bone disease (low levels of PTH and alkaline phosphatase — i.e. bone alkaline phosphatase below the median reference limits60–63). In a multicenter study, the best cut-off point to discriminate low-turnover bone disease was bone alkaline phosphatase <33,1 U/L.48
Presently, alkaline phosphatase is associated with a positive linear increase in the risk of mortality (not as the U, J or inverted J shape observed in the relationship PTH-mortality), mainly in hemodialysis patients.64 It has also been described an independent association between of total alkaline phosphatase levels >120 IU/L and coronary calcification,65 mortality and other adverse effects.66 A high alkaline phosphatase activity reduces the serum concentration of pyrophosphate, an important inhibitor of vascular calcification. In hemodialysis there is an increase in alkaline phosphatase activity with the consequent reduction of serum pyrophosphate and an increased risk of vascular calcification.67–69 For this reason, alkaline phosphatase (and the related pyrophosphate) may be considered potential targets in the treatment of the CKD–MBD complex in the future.66,70,71
1,25-(OH)2 vitamin D (calcitriol)There is no evidence that monitorization of calcitriol is useful in the control of CKD patients. However, it can be used for research purposes or in the differential diagnosis of some cases of hypercalcaemia (sarcoidosis and other granulomatous diseases, lymphomas, etc.). In the presence of increased PTH levels, it is unknown what would be the normal or advisable values of calcitriol, but in advanced CKD calcitriol levels should be low; therefore, a “normal” level indicates pathology.
Calciuria–phosfaturiaThroughout the progression of kidney disease, it may be observed a decrease in the excretory capacity of calcium or phosphorus. It has been suggested that serial determinations of calciuria would make it possible to monitor potential calcium overload in patients with CKD. Also the previously mentioned FEP or maximum tubular reabsorption of phosphorus (TmP) (Normal FEP = 10%–20%) can be early markers of phosphorus overload.
However, in order to achieve a phosphate balance, phosphate intake should be maintained relative to the reduced glomerular filtration, requiring an increase in the FEP. The increase in FEP is accomplished by a reduction of the tubular absorption of phosphate which is accomplished by the action of an increased production of phosphatonins, mainly FGF-23 and others (FGF-7, secreted frizzled related protein 4, etc.) and PTH. These hormones modulate the expression of sodium-phosphate transporters (NaPi-IIa, NaPi-IIc, and PiT-2 type III) on the apical membrane of proximal tubule cells. However, the FEP may be increased up to a maximum limit (approximately 50%–55% in patients without proximal tubulopathies) which means that, given a certain phosphorus load, a critical reduction in glomerular filtration cannot cope with excretion, resulting in positive phosphorus balance with hyperphosphatemia.
Patients with advanced CKD receiving diuretics seem to have higher serum phosphate concentrations, perhaps because diuretics interfere with the maximal capacity to excrete phosphate.72 Urinary excretion of phosphate greater than 40 mg mL/min/1.73 m2 of GFR (i.e., more than 400 mg of urinary excretion of phosphate in patients with a GFR of 10 mL/min/1.73 m2 or 800 mg in patients with a GFR of 20 mL/min/1.73 m2) have been shown to be associated to hyperphosphatemia.73 It has also been suggested that for patients with a GFR lower than 25 or 15 mL/min/1.73 m2, a phosphaturia lower than 800 mg/day or 600 mg/day respectively, would be reasonable targets.7 It is noteworthy that the total amount of P ingested does not always correlate well with daily phosphaturia; this is explained by the different bioavailability (intestinal absorption) of the different sources of P, thus intestinal absorption of P from vegetal proteins is less than that from animal proteins. The ratio phosphorus/urea nitrogen in urine (UUN) may reflect the amount of P absorbed relative to the protein intake.74 Finally, we note that proteinuria appears to independently increase phosphorus retention.75
Fibroblast Growth Factor 23 (FGF23)This phosphatonin plays an important role in the pathophysiology of mineral disorders and SHPT in CKD; in fact, the increase in FGF23 precedes the elevation in PTH. High FGF23 is associated with poor survival of CKD patients, and it is an early marker of phosphorus overload and severity of SHPT.32,34,76,77 FGF23 is mainly produced by osteocytes and new sources of FGF23 are being described, such as its production by cardiac cells. It is known that in addition to phosphate, FGF23 is also regulated by Ca, iron, sodium, among others, and that FGF23 has effects beyond the cardiovascular system (infection, inflammation…).78 However, its measurement in the clinical setting is still not recommended except for the diagnosis of some specific pathologies. There are kits commercially available for the measurement of the intact FGF-23 molecule and for the C-terminal fragments.79 There are also automated methods for measuring FGF23 that are still not widely used.
KlothoIt is a molecule of extreme interest since it acts as a co-receptor of FGF23R1, the specific receptor for FGF23. There is a circulating form of klotho with independent actions that have been closely related to healthy aging.9 There is not a widely accepted standardized method for the measurement of klotho, and there is now a significant ongoing research on klotho.
CalciproteinsThey are associated with propensity of vascular calcification and its methodology and clinical value are still a subject under debate.80
Other markers of bone remodelingCertain markers of bone remodeling, such as osteocalcin, free serum pyridolines, propeptides, and the C-terminal telopeptide of collagen, show good correlations with bone histology but do not improve the predictive power of PTH and/or alkaline phosphatase, and therefore its systematic use is not justified.60,63 The only bone derived molecules that do not accumulate in CKD are alkaline phosphatase, intact P1NP (bone formation marker) and tartrate-resistant acid phosphatase 5b (resorptive marker) because these molecules undergo hepatic clearance.60,81 The interest of their measurement could be reassessed with the appearance of the new guidelines for the treatment of osteoporosis in CKD.63
Imaging techniques. Bone and vascular radiology (Table 2)Vascular calcification and its relationship with bone pathology is clinically relevant. The radiological studies are useful as a first step to detect vascular calcifications. A simple radiology of the pelvis and hands allows the detection of vascular calcification. This images are sufficient to assess calcification using the Adragao index, (not endorsed by the KDIGO guidelines).82 Radiology of the lateral lumbar spine provides the information required to calculate the Kauppila index.83 The images obtained by radiology cannot distinguish if the calcification affects the vascular intima (secondary to atheromatosis) or the medial layer (Mönckeberg disease or atherosclerosis), the calcifications in territories of elastic arteries (such as the aorta) are more frequently associated to calcification of the intima.84 By contrast, muscular (radial and digital) or predominantly muscular (iliac and femoral) arteries seem more susceptible to medial calcification.84 Vascular calcifications are associated with mortality and hospitalizations85; the Adragao index of the hands showed not only the prognostic importance of vascular calcification but also its close correlation with the deterioration of renal function and components of the CKD–MBD complex.84 In addition, other indices of thoracic or abdominal aortic calcification have been developed.86
Periodicity of imaging techniques.
| Stages 3−4 | Stages 5−5D | Renal transplant | |
|---|---|---|---|
| X ray abdomen | Basal | Basal | Basal |
| X ray of hands | Basal (Advisable) | Basal (Advisable) | Basal (Advisable) |
| X ray lateral dorso-lumber | Basal and every 2–3 years | Basal and every 2–3 years | Basal and every 2–3 years |
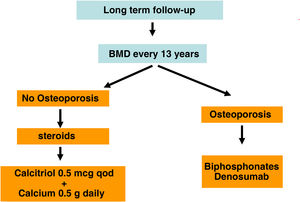
| BMD risk : >65 years. Esteroids, previous fracture | 1−2 years if on antiresorptive treatment | 1−2 years if on antiresorptive treatment | 1−2 years if on antiresorptive treatment |
It is considered that the finding of vascular calcifications may influence subsequent therapeutic choices. It is possible to accelerate or slowing down the progression of vascular calcifications with the use different drugs aimed at controlling the CKD–MBD complex.87,88 In any case, it is a controversial subject89; but certainly, there is a high degree of evidence suggesting that patients with vascular or valve calcifications should be considered as patients with the highest cardiovascular risk.3,4
Both X-rays and echocardiography can be used to detect the presence or absence of vascular or valvular calcifications and are reasonable and inexpensive alternatives to the computed tomography3,4; however they have a low negative predictive power, negative findings do not guarantee the absence of calcification.
With respect to bone, it is known that subperiosteal resorption of the radial side of the phalanges is the earliest and most specific sign of osteitis fibrosa. Other classic lesions of osteitis fibrosa are acroosteolysis, “salt and pepper” skull, “rugby jersey” vertebrae. In addition, Looser’s lines are characteristic of osteomalacia. All of them are usually late manifestations of the underlying bone disease. The aforementioned radiological extension of the lumbar spine (Kauppila index) to the thoracic spine is indicated in symptomatic patients or those with a high risk of fracture for the detection of vertebral fractures (so-called vertebral morphometric fractures, which are frequently asymptomatic).
Bone densitometryPresently, the Dual X-ray absorptiometry (DXA) is the standard method to determine bone mineral density (BMD) in the general population. This is because it offers accurate measurements at clinically important sites with minimal radiation. It is usually measured in the femoral neck and spine (antero-posterior and lateral projections). It provides information on changes in bone mineral content, but it does not inform what is the underlying bone disease which is especially important in the CKD patient.90
In the general population osteoporosis is defined as the presence of a BMD with a “T-score” less than –2.5 standard deviations. A significant proportion of fractures also occurs patients with osteopenia (T-score between –1 and –2.5 standard deviations). It should be remembered that for the diagnosis and treatment of osteoporosis the T-score should be determined only in the total femur, femoral neck or lumbar spine (or ultradistal radius in the case of hyperparathyroidism –see below–) because according to the WHO classification they are not equivalent to other T-scores.63,90 For this reason, it is important that the different devices must be independently validated to assess the risk of fracture. It is important to take into account this information considering the the new development of total body densitometry. In conventional bone densitometry, there is additional data such as TBS (Trabecular Bone Score) or vertebral densitometric morphometry, which may also help to determine the risk of fracture.63,90
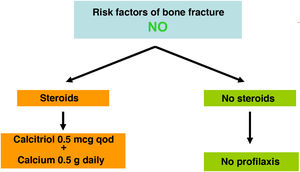
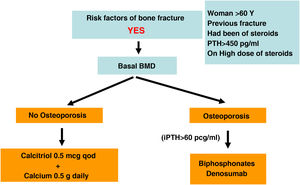
Recent studies have shown consistently a relationship between BMD and the risk of fracture in the CKD population. Also, it has been shown that antiresorptive agents increase BMD in CKD patients and that their benefits could outweigh its risks, even in the absence of bone biopsies.4,63,90,91 For this reason, current guidelines (KDIGO 2017) suggest to measure the BMD by DXA in CKD 3a-5D patients with evidence of CKD–MBD and/or with risk factors for osteoporosis (including age ≥65 years and a history of a previous fracture). This information helps to determine the risk of fracture and decide therapeutic options.4 Actually, there are various algorithms published,61,90,92 including a recent European consensus for the diagnosis and management of osteoporosis in patients with CKD stages 4–5D.63
In the general population the risk of a main osteoporotic or hip fracture within a 10 year period (or the possible recommendation of requesting BMD measurement by DXA) can be assessed using FRAX® (“Fracture Risk Assessment Tool” for its English acronym, https://www.sheffield.ac.uk/FRAX/tool.aspx?country=4) Possibly this tool could be used in patients with CKD93,94 but it may underestimate the real risk of fracture.
There is no consensus on the FRAX threshold that indicates to perform a densitometry in CKD. Initially it could be consider a 10-year risk of hip fracture >3% and/or major osteoporotic fracture (vertebral, forearm, hip or shoulder) >7.5%.90 If densitometry is performed, the FRAX should probably be recalculated incorporating the result of the densitometry to propose a more adequate treatment.
It is known that CKD increases the prevalence and incidence of fractures due to osteoporosis and it occurs at a younger age resulting on an increased mortality, even in the absence of persistent metabolic abnormalities.
In addition to the BMD measured at the femur or lumbar spine, the loss of cortical bone affecting CKD patients with hyperparathyroidism, is measured more precisely at the ultradistal radius63,95 and should correlate negatively with the PTH values.96 However, at a time point, this correlation may not be present because an isolated value of PTH does not reflect the prolonged history of changes in this hormone. The BMD cumulatively reflects changes in BMD that have occurred over time. Nevertheless the study of the distal radius could provide additional information in CKD patients, but the arm of the functioning AV fistula should be avoided.97
Histology (bone biopsy)The bone biopsy of the iliac crest with double tetracycline labeling and histomorphometry analysis is the most accurate method to identify the underlying bone lesion and it is the “gold standard” to evaluate the diagnostic and predictive value of other less invasive diagnostic techniques.
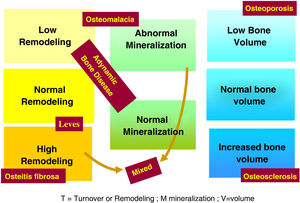
The use of histomorphometry data has allowed the classification of different forms of renal osteodystrophy (ROD). As mentioned introduction term ROD is applied exclusively to define the alterations of bone histomorphometry associated with CKD, and includes parameters of bone remodeling, rate of mineralization and bone volume (amount of bone relative to the total tissue volume). The TMV based classification is currently used for the assessment of bone histomorphometry (T = Turnover, M = Mineralization, V = Bone Volume).2–4
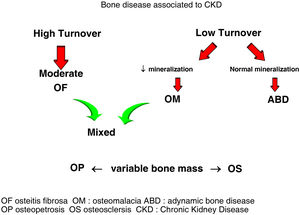
Classically, bone lesions were classified into (Fig. 1): High Remodeling (HR) and Low Remodeling (LR). The most characteristic form of HR is osteitis fibrosa (OF) and its only cause is Secondary Hyperparathyroidism (2ndHPT). The LR forms are subdivided based on the rate of mineralization. If the mineralization is normal, they are called adynamic bone disease (ABD) and if the mineralization is decreased –with the consequent increase in osteoid tissue–, they are called osteomalcia (OM). All morphological abnormalities may have variable amount of bone mass, generally decreased, so densitometry by DXA does not distinguishing between the different types of ROD.90
The KDIGO classification of Renal Osteodystrophy (ROD) (Fig. 2) takes into consideration the turnover, mineralization and bone volume.
Indication of bone biopsyThe bone biopsy is the “gold standard” for making a diagnosis and for a better understanding of the disease process. The predictive value achieved by biochemical parameters have made bone biopsy an exceptional indication, although currently its indication is being revitalized.98
The recommendations proposed by the SEN, and by KDIGO (3.50) have shown to be of limited help in decision-making. Notions previously defined as “unexplained” or “inconclusive biochemical parameters” are of little help in making the decision to biopsy; aluminum-induced bone disease has virtually disappeared with dialysis water treatment. And presently, the indication of biopsy before the use of antiresorptive agents is currently questioned.4,91
Therefore, despite its definite diagnostic value, the indication for bone biopsy must be individualized in the context of clinical cases where its diagnostic value is relevant for making therapeutic or prognostic decisions, as well as for research, with the intention to prove that there are therapeutic measures that can be applied to other patients.
To be take into consideration:
- a)
Clinical manifestations: pathological fractures and/or persistent bone pain (especially exhibited in cases of osteomalacia), radiological changes not explained by ROD or biochemical changes that are not compatible with the pattern of ROD. All these circumstances may lead to investigate another bone disease of metabolic origin.
These may be examples:
- -
High remodeling: a) Oxalosis: severe radiological images of osteitis fibrosa, without overt increase in PTH. Genetic diagnosis should be performed before the bone biopsy, but it may not be conclusive. b) Paget’s disease, depending on its presentation.
- -
Low remodeling: a) Rickets/osteomalacia. b) Chronic hypophosphatemia without specific clinical manifestations, and with inconclusive genetic study.
- a)
Non-iatrogenic persistent hypercalcemia of uncertain etiology, with suppressed PTH and a potential systemic disease pending diagnosis.
- b)
Possible exposure to metals with a potential effect on the skeleton (aluminum, heavy metals, etc.).
- c)
Studies with relevant designs oriented to diagnosis and therapy, such as the study of the potential effect of drugs on bone damage of metabolic origin (anti-resorptive drugs, bone anabolic agents…). As mentioned before, it is reasonable to perform a bone biopsy in patients with 3a-5D CKD if knowledge of the type of ROD may impact the therapeutic decisions.4,99
However, current guidelines consider that the absence of a bone biopsy should not limit the prescription of antiresorptive treatment in a patient at high risk of fracture.4,91
Other imaging techniquesThere is not a consensus about clinical guidelines for the evaluation and follow-up of extra-osseous calcifications in CKD.
Plain X-rays: The Adragao and Kauppila indices, among others, have prognostic value, it is suggested to obtain these indices in all patients with CKD; particularly if they may help therapeutic decision-making (use of different phosphate binders, vitamin D derivatives, calcimimetics, etc.) and in patients on the kidney transplant waiting list. The limitation of these indices are the low sensitivity and the subjective nature of semi-quantitative methods.100 This is why they are not very useful for prospective follow-up.
Other methods of diagnostic imaging are used depending on their availability, the experience of the operator and the type of study to be performed.
- -
Mammography: especially useful in women, it provides a unique possibility to assess calcification of the media layer of the artery.100
- -
Echocardiogram: useful for evaluating valvular calcifications, as well as cardiac geometry and function.
- -
Carotid ultrasound: detects calcifications in atherosclerotic plaques and allows measurement of intima-media thickness in carotid vessels. It also allows the location of calcification that only affect the internal elastic layer.
- -
Carotid-femoral Pulse Wave Velocity (PWV): used to measure arterial stiffness (or loss of compliance). It is a non-invasive method, harmless to the patient, easy to perform and highly reproducible. There is a correlation between PWV and the degree of vascular calcification.
- -
Ankle-brachial index: decreased ankle-brachial index suggests the presence of peripheral arterial disease and is frequently associated with vascular calcification (as is increased pulse pressure).
- -
Angiotomography techniques: this modality, less invasive than arteriography, provides good quality images for study the morphology of the vascular tree.
- -
Helical CT or multidetector tomography: considered the “gold standard” for the study of vascular calcification as it is a widely available technique with high sensitivity.100 It is useful for the evaluation of coronary calcifications,71 being possible to evaluate the progression of the surface (Agatston index) or the calcified volume (Raggi index).101,102
- -
Electron-beam computed tomography (EBCT): the best validated technique for the detection of coronary calcifications, but it is expensive.
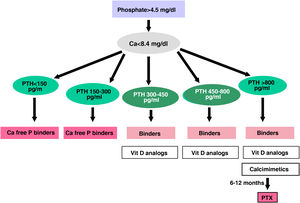
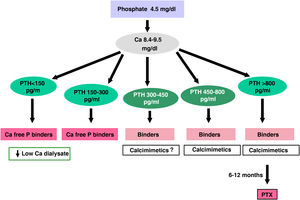
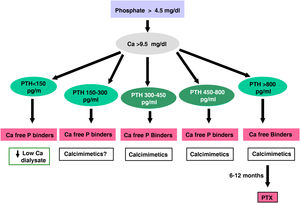
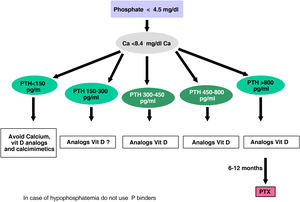
The recommended serum values, according to the K-DOQI, K-DIGO and review of the literature, are shown in the Table 3. There is no clear evidence, especially before dialysis, to support the recommendation of certain biochemical values, especially PTH.
Recommended values of biochemical parametersa.
Some authors have recommended the normalization of PTH values, others the initiation of if it is observed a some degree of increase in PTH with respect to a baseline determination if it is available (still within the normal range). Given that in CKD there is resistance (hyporesponsiveness) to the action of PTH4,103,104 it is currently considered that a moderate increase in PTH is necessary as an adaptive mechanism, to maintain a normal rate of bone remodeling; and to contribute to phosphaturia and the and stimulation of 1-α-hydroxylase.
In any case, the recommendations on the biochemical parameters are based on observational studies that, in reality, can only inform about associations. Given the important and uniform association of mortality with altered levels of calcium and phosphorus in patients with CKD, we suggest maintaining calcium and phosphorus levels in the normal laboratory range in patients with CKD 3a-5 as long as the measures to achieve this goal are reasonable.
It should not be forgotten that the therapeutic objectives of bone mineral metabolism disorders must be adapted to the clinical characteristics and global therapeutic objectives in each individual patient, without merely pursuing certain plasma concentrations. As previously mentioned, in patients with CKD 3a-5D, treatments should be based on serial determinations of calcium, phosphorus and PTH (in addition to calcidiol and alkaline phosphatase) as a whole and assessing trends.4
Regarding calcium, patients treated with calcimimetics (stage 5D) are expected to have hypocalcemia which is not only tolerable, but it could even be beneficial38,101; this is why the new KDIGO 2017 guidelines state “avoid hypercalcaemia” instead of advising normal calcium levels in all patients.4
In relation to phosphorus, the KDIGO 2017 guidelines vaguely suggest that in patients with CKD 3a-5D phosphorus levels should be lowered “towards normal”.4 We believe that phosphorus levels should be normalized whenever possible using reasonable measures.
Therapeutic alternativesDietIn CKD patients the urinary excretion of phosphate (P) is markedly reduced. Therefore the intestinal absorption of P is a main determinant of the serum P concentration. Although patients with CKD stage 5 absorb about 60% of the ingested P (as compared to 80% of the general population), the absorption of P is increased if patient is receiving vitamin D.105 Among the sources of P it is important to distinguish the organic vs inorganic phosphorus. The organic phosphorus is present in high protein foods, such as meat, dairy products, eggs, cereals, legumes and nuts. The intestinal absorption of P from vegetal proteins is less than from animal proteins. Specially relevant is the inorganic P which basically is totally absorbed. The inorganic P is included in additives of processed foods and soft drinks. Any strategy to control dietary P intake must consider not only the phosphorus content in food but also its absorption (bioavailability); this issue is important due to the increased use of processed dietary products and beverages.
Since the amount of P in the diet is directly related with the amount of protein in the diet, P restriction could be associated with a decrease in protein intake which may compromise nutrition, survival and quality of life of CKD patients.106,107 In this regard, the quotient P/Cr in urine or the recently described P/NUU in urine, may represent a better measurement of the intestinal absorption of phosphate than the simple assessment of phosphorus content in the ingested food.74,107 There is great variability in the quotient P/protein in the different protein sources. The white egg and animal proteins excluding dairy products are a source of protein with a low quotient phosphorus/protein. By contrast, the egg yolk, the proteins derived from milk, vegetables and fast food have a high quotient P/protein. The P from vegetables is organic it is mostly associated with phytates; thus phytases are required to separate P from Phytate. Humans, as opposed to herbivorous animals do not have phytases so the P absorbed y limited,4,108 so it could be also contemplated the phytate content in the calculations of the different diets in patents with CKD.109
The inorganic P is not bound to proteins, and therefore is absorbed practically a 100%, so it should be insisted on the need to avoid processed foods due to their high content of inorganic P in the form of additives..
In any case, restriction of P intake never must be done at the expense of an excessive restriction of protein; the risks associated with malnutrition may reduce the benefits derived from a reduced P load.
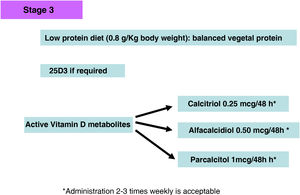
Native vitamin D/calcifediolAdequate levels of 25(OH)D are important since it is the substrate of 1-25(OH)2D and its deficiency aggravates SHPT. In early stages of CKD, 25(OH)D deficiency may be the only cause of SHPT and, therefore, its measurement seems advisable, as well as starting its supplementation following the recommendations of the general population110 to cover the pleiotropic effects of vitamin D beyond SHPT control.
It is well known that VDR is ubiquitous and it is expressed in many tissues. Aging is associated to a decreased expression of VDRs in skin and muscle, which reinforces the need of optimal levels of this hormone. Vitamin D repletion is especially important when implementing treatments for osteoporosis in patients with CKD, as it increases the efficacy of these drugs and avoids unwanted side effects such as hypocalcaemia (mainly induced by denosumab).111
However, there is no complete agreement on the need for supplementation with native vitamin D (cholecalciferol/ergocalciferol ) or calcidiol/ calcifediol in the general population or CKD patients particularly in advanced CKD),59,112–114 nor in the target levels in CKD.56,57 We have indicated already that our recommendation is based on the possibility of extrarenal hydroxylation and its auto- or paracrine pleiotropic effects and not only as an instrument the control SHPT.
CalciumA sufficient supply of Calcium is essential to reduce the PTH secretion. However, it should be kept in mind that patients with CKD have a reduction in urinary calcium excretion115,116 therefore it should be avoided calcium intakes higher than those recommended in the general population.110
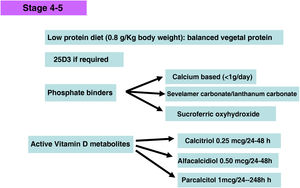
Phosphate binders- -
The ability to bind phosphate of Calcium acetate is similar to that of calcium carbonate but with less supply of calcium, so it would have certain advantage besides his greater phosphate binding capacity over a range of pH. Calcium carbonate is the least expensive of the phosphate binders.
- -
At present lanthanum carbonate and sevelamer carbonate are indicated in the predialysis stage (although the data sheet specifies its use in patients with phosphate values >1.78 mmol/L (5.5 mg/dL)).
- -
Sevelamer, (generic compounds are already available) is a phosphate binder that does not contain calcium or aluminum. It is a polymer that binds phosphorus in the intestinal tract and prevents its absorption. Several prospective studies and meta-analyses show that it is capable of attenuating the progression of calcifications in coronary and aortic arteries117 and also reduces lipid levels and improves the inflammatory profile118 among others multiple pleiotropic effects as demonstrated by clinical and experimental studies.119,120
Sevelamer was initially presented as sevelamer hydrochloride and has the drawback of having limited efficacy; and, in many cases it is necessary to take a large number of tablets that may be poorly tolerated. It is possible that dosages in the form of sevelamer carbonate (powder for oral suspension even tripling the dose) mitigate this problem. The powder can be mixed with a small amount of food.
It is noteworthy that one study and several meta-analyses have shown an improvement in survival in incident dialysis patients treated with sevelamer compared with calcium based phosphate binders,121–123 although other prospective studies and meta-analyses limit the improvement in survival to certain subpopulations, patients over 65 years of age, or even question such an improvement in mortality.37,124
- -
Lanthanum carbonate, is a powerful phosphorus binder that does not contain aluminum or calcium and offers the possibility of improving phosphorus control without relevant secondary effect as shown in different studies.125 Observational studies and meta-analyses also show beneficial effects on the attenuation of the progression of vascular calcification and also patient survival as compared with calcium based phosphate binders.126,127
- -
After the prolonged clinical experience with the use of lanthanum carbonate, the initials concerns about possible toxicity have largely disappeared. Presently it is an effective alternative for the treatment of hyperphosphatemia. It has a powder formulation that can be mixed easily with food and could facilitate adherence to treatment.
- -
The association of calcium acetate with magnesium carbonate, reduces calcium intake, shows good results and has not been associated with problems derived from possible hypermagnesemia, and has also been accompanied by a decrease in PTH levels.128 Potential anticalcifying effects of magnesium have also been described, both in experimental studies and in preliminary clinical studies.129,130 Serum magnesium and ECG monitoring is advised in patients treated with dogoxin. It may be the binder of choice in patients with hypomagnesemia (a parameter also associated with increased mortality and not uncommon in dialysis patients).131 In addition, magnesium could decrease phosphate toxicity.132
- -
Iron-based binders. Iron-based phosphate binders lack the aforementioned pleiotropic effects of other binders. They are relatively well tolerated (mainly due to the use of fewer tablets, e.g. compared to sevelamer hydrochloride); dark stool color and diarrhea (unlike other binders) are their most frequent side effects and may restrict their use in some patients. There are significant differences between the two iron-based phosphate binders (sucroferric oxyhydroxide and ferric citrate), especially in relation to their potency as phosphorus binders (higher for oxyhydroxide), iron absorption (higher for citrate) and number of tablets (lower for oxyhydroxide). At the moment, only sucroferric oxyhydroxide is available in Spain. Phosphate binding to the complex is strong and poorly soluble and has an excellent phosphate binding capacity.133 Sucroferric oxyhydroxide has a lower risk of drug–drug interactions, although like all phosphate binders it interferes with the absorption of levothyroxine.134
To date, there is no work that convincingly demonstrates which binder should be the first choice, so the characteristics, limitations and preferences of the patient should also be taken into account on an individual basis.135 The use of any phosphate binder (except those based on aluminum) has been associated with improved survival in multiple studies in dialysis patients,136,137 although it cannot be ruled out that this effect is due to the fact that the use of phosphate binders allows a somewhat more liberal diet and therefore could be associated with a better nutritional status. Regarding the use of aluminum phosphate binder it is considered that, given the large number of alternatives available and the inability to determine a safe dose of aluminum, the prolonged use of phosphate binders with aluminum should be avoided.4
Hyperphosphatemia is frequently treated with an association of several of these binders to reduce the cost associated with the use of binders without calcium, although there is no clear evidence of greater effectiveness of their combined use.136 In any case, we continue to consider it reasonable that the choice of binders should take into account the stage of CKD, the presence of other components of the CKD–MBD complex (i.e. vascular calcification), concomitant therapies (vitamin D derivatives, calcimimetics, drug–drug interactions) and the side effect profile 4. It should be highlighted the increasing evidence in the 2017 KDIGO guidelines suggesting the need to restrict the use of calcium-based binders in adults with CKD4 given the publication of numerous studies and meta-analyses favoring the use of calcium-free binders.122,123,138 This restriction is especially necessary in patients with persistent or recurrent hypercalcemia, suspected ABD and/or if serum PTH levels are persistently low. Likewise, the last KDIGO 2017 guidelines4 suggest that decisions about the use of phosphorus-lowering treatments should be based on persistent or progressive increases in phosphataemia. Although we can recognize the presence of a body phosphate overload before hyperphosphatemia appears (i.e. based on elevated levels of FGF-23 with normal plasma phosphorus), in light of current knowledge, “preventive” treatment is not yet justified.4,139–141 However, it is noteworthy that the reduction of FGF23 is more evident with the use of calcium free phosphate binders.
Aluminum hydroxide can be used for a short period of time in those patients with persistent hyperphosphatemia with a limited response to the rest of phosphate binders. It should administered only in meals whose abundant phosphorus content justifies its use. The safe amount of aluminum binders is unknown. Serum aluminum should be measured twice yearly in those patients receiving aluminum containing phosphate binders. Baseline serum aluminum values <20 µg/L indicate a likely absence of aluminum overload. Repeated values between 20–60 µg/L are difficult to interpret. Values consistently greater than 60 µg/L indicate aluminum overload which does not always imply aluminum bone disease.50 In patients with iron depletion, the risk of tissue incorporation of aluminum is greater, therefore, values much lower than those already mentioned may indicate a significant pathological damage.142
Active vitamin D metabolitesIf PTH shows a progressive and persistent increase despite correction of pathogenic factors (hyperphosphatemia, excessive phosphorus intake, hypocalcemia, vitamin D deficiency), treatment with active vitamin D metabolites or selective vitamin D receptor activators (AsRVD) may be indicated. Low doses of active metabolites do not usually cause hypercalcemia or hyperphosphatemia and have been shown in experimental studies to even slow progression of CKD among many other pleiotropic effects.143 One method to determine that the dose of vitamin D is inappropriately high may be to determine calciuria and one should be more careful in patients taking calcium phosphate binders. The administration of vitamin D has been associated with increased survival despite increasing FGF23 levels, but there are no clinical studies that can inform whether measuring FGF23 values might help in deciding a more appropriate dose of vitamin D. Moreover, it is difficult to generalize about the different functions of the various vitamin D metabolites, as well as to separate their endocrine from their paracrine or autocrine activities. In this regard, both the use of calcitriol and α-calcidol have been associated, in observational studies, with better survival of patients with CKD not on dialysis.144–146
The affinity of Paricalcitol (19-nor-vitamin D2) for the VDR, is not the same in all cell types; it has a greater affinity at the level of parathyroid cells as compared to osteoblasts, intestinal wall cells and vascular smooth muscle cells. For this reason, it seems to be somewhat less hypercalcemic and hyperphosphatemic than calcitriol; this has been demonstrated in different experimental studies.147 Furthermore, in others studies, paricalcitol has been shown to be capable of inducing fewer vascular calcifications compared to equipotent doses of calcitriol,147–149 probably due to its differential effect on bone morphogenic proteins and the Wnt pathway.149
The use of paricalcitol has been associated not only with the control of SHPT but also with other pleiotropic effects such as a decrease in proteinuria in diabetic patients,150 presumably associated with its anti-renin effect, although the results of these studies are still inconclusive.151 Nevertheless, although all the active derivatives of vitamin D are associated with improved survival in patients with CKD in majority of published studies (observational and meta-analyses in both stages 3a-5 and 5D)152,153 the best associations with survival have been observed in dialysis patients treated with paricalcitol.154 Not all studies show that administration of vitamin D is associated with a reduction in the progression of CKD and some studies have shown that that the rate of decline in glomerular filtration rate in CKD patients decreased after the discontinuation of active vitamin D administration.155
It is noteworthy that the 2017 KDIGO guidelines,4 due to the high frequency of hypercalcemia episodes in the PRIMO and OPERA studies with the use of high doses of paricalcitol (1–2 µg/day) and/or calcium based phosphate binders in patients with mild–moderate SHPT156,157 have suggested that calcitriol or vitamin D analogues should not be used routinely in adults with CKD 3a-5 and should be reserved for patients with severe and progressive hyperparathyroidism.4
The authors of the present recommendations do not fully agree with this last criterion, since in the mentioned studies excessive doses of paricalcitol were used to assess its possible beneficial effect on left ventricular hypertrophy and not on SHPT. Furthermore, it would be a mistake not to prevent the progression of SHPT and apply treatment when it becomes severe. Molecular studies of severe hyperparathyroidism,158,159 as well as recent clinical studies160 suggest that prevention of the development of severe SHPT is reasonable and beneficial for the patient.
However, the authors of the present guidelines believe that a complete normalization of iPTH levels is not advisable in CKD given the presence of hyporesponsiveness or resistance to the action of PTH (as occurs with other hormones –insulin, growth hormone, FGF23, vitamin D–)103,104 and that, as previously mentioned, a slight increase in PTH may have beneficial actions by increasing phosphaturia, calcitriol synthesis or maintaining the rate of bone formation. However, we recognize that, especially in CKD stages 3a-5, the optimal level of iPTH is not yet known,1,4,50 which does not justify having to wait for a “severe” SHPT to start treatment.
Calcimimetics: cinacalcet and etelcalcetideCinacalcet is an oral calcimimetic that acts on the CaSR of parathyroid cells and allosterically modifies it, making it more sensitive to the actions of extracellular calcium. Etelcalcetide is a small synthetic peptide administered intravenously at the end of the dialysis session that activates the CaSR even in the absence of calcium; however, signaling is greater in the presence of calcium, so its allosteric action also seems to be present.161
Calcimimetics significantly reduce serum concentration of PTH and, as a consequence, reduces calcemia and eventually also decreases phosphatemia and circulating FGF-23.162–166 At the experimental and clinical level, different pleiotropic effects have also been described, among which it stands out its attenuating effect on the progression of vascular and valvular calcification.101,167,168 In addition, in various clinical studies, the use of cinacalcet has been associated with a significant decrease in parathyroidectomies, fractures, hospitalizations for vascular causes, and a nominally significant improvement in survival, at least in some subgroups (≥65 years) of dialysis patients.38,169–172 Likewise, a possible reduction in the size and vascularization of the parathyroid glands has been described.173
The main side effects of cinacalcet are the gastrointestinal intolerance which in some cases has forced the drug to be discontinued and switch to etelcalcetide (calcimimetic given iv), and the potential incidence of symptomatic hypocalcemia,38,172 so caution should be exercised in patients with risk factors to present a interval QT prolongation or patients with epilepsy.172 Gastrointestinal intolerance improves with administration after the main meal and, in some cases, after dinner or in divided doses. Intravenous etelcalcetide did not show significant differences with respect to cinacalcet,174 but clinical experience show that patients intolerant to cinacalcet can tolerate etelcalcetide and the use of etelcalcetide which is given at the end of hemodialysis allows the detect patients that were non-adherent to oral cinacalcet.175
Cinacalcet is metabolized through cytochrome P450, so the inhibition of this enzyme may cause an increase in the levels of cinacalcet (ketaconazole, itraconazole, cimetidine, clarithromycin, ritonavir, grapefruit juice), while its activation (by barbiturates, phenytoin, carbamazepine, dexamethasone, rifampicin) would result in a decrease of the calcimimetic. The dose adjustment of drugs metabolized by CYP2D6 with a narrow therapeutic margin and that require individual dose adjustment should also be assessed (flecainide, quinidine, tricyclic antidepressants, vinblastine, thioridazine, propafenone, metoprolol, etc.). Etelcalcetide does not present these inconveniences.
Since a reduction of PTH leads to hypocalcemia, patients receiving calcimimetics should have more frequent controls of calcemia, mainly at the beginning of treatment.
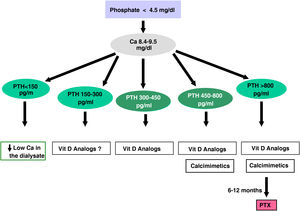
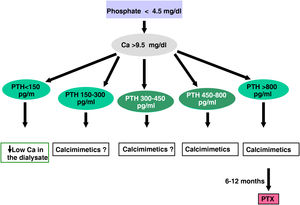
ParathyroidectomyParathyroidectomy should be considered if all of the above measures are ineffective in controlling PTH. Today, with the introduction of new medical treatment alternatives, the indications could be reduced to:
- -
Severe secondary hyperparathyroidism (PTH >800–1000 pg/mL –without hypocalcaemia–) on dialysis without response to combined treatment medical (association of calcimimetics, phosphate binders and vitamin D derivatives) for more than 6 months
- -
Hyperparathyroidism tertiary resistant to calcimimetics or combined treatment
- -
Refractory severe hyperphosphatemia.
- -
Primary Hyperparathyroidism primary (non-iatrogenic hypercalcaemia with unsuppressed PTH) in patients with CKD (especially young people with surgical criteria). In these cases there is the possibility of surgery minimally invasive if sestamibi scintigraphy, parathyroid ultrasound, or PET-scan are positive indicating more active glands.
- -
Patient with calciphylaxis and iPTH above 500 pg/mL who do not respond rapidly to treatment with calcimimetics.
- -
Complications associated with SHPT such as:
- •
Tendon rupture,
- •
Severe bone pain or
- •
Refractory anemia
Imaging techniques prior to parathyroidectomy are recommended to assess glandular size, location, and especially the presence of ectopic glands.176 The association of scintigraphy-MIBI and/or SPECT-CT and/or cervical ultrasound show great sensitivity and specificity. The use of PET-scan with different tracers (11C-methionine, 18F-fluorocholine) is considered an adequate second-line imaging modality to allow minimally invasive parathyroidectomy.
There is controversy about which is the most appropriate technique: total, subtotal, or total parathyroidectomy with autotransplantation. Currently the most used is subtotal parathyroidectomy because it is the one that usually presents the lowest rate of post-surgery recurrence, although it depends largely on the experience of the surgical team of each center.
It is not recommended to leave as residual tissue glands showing hypercaptation in the scintigraphy (in the case of subtotal parathyroidectomy or for use as an autologous transplant).
It should be remembered, however, that parathyroidectomy is not free of complications177; it is associated with an increase in early mortality and increased hospitalization rates during the first year, although it presents good long-term results in observational studies.178 The appearance of hungry bone syndrome (>25% of patients) should be adequately monitored, with a nadir between 1 and 3 weeks postoperatively. Risk factors are age <45 years, postoperative calcium <8.4 mg/dL or alkaline phosphatase levels >120 U/L, among others.178,179 Its treatment requires high doses of oral and intravenous calcium, calcitriol and temporarily increase the calcium concentration in the dialysate(one of its few indications).178,179 It has been suggested that the use of bisphosphonates prior to parathyroidectomy could minimize the severity of the hungry bone syndrome.179,180
Antiresorptive drugs/favouring bone formationBisphosphonatesIn the different studies performed in the general population, there were patients included that had impaired renal function and it has been observed in post-hoc analysis, that patients with reduced renal function had an improvement in BMD and a reduction in the risk of fractures, regardless of the degree of renal dysfunction.4,181 Thus, these benefits have been described with the use of with alendronate, risendronate, (and also with the selective estrogen receptor modulator, raloxifene) in patients women with osteoporosis with CKD stages 1–4 (with apparently normal creatinine or <1.6 mg/dL), with no known prior diagnosis of CKD, and with normal values of calcium, phosphorus, PTH, and alkaline phosphatase.4,181
Likewise, in the few published studies of dialysis patients, improvement in BMD has also been observed, especially when the patients had elevated PTH.182 However, it must be taken into account that, according to the data sheet, bisphosphonates are not recommended due to lack of experience for creatinine clearance <35–30 mL/min, although they have been used successfully in patients affected by calciphylaxis.182 Contrary to what might have been expected, it has not been clearly demonstrated that bisphosphonates cause adynamic bone disease.4,63
Although there have been very few negative effects of bisphosphonates on the kidney, if given intravenously it is important to lengthen the infusion time, reduce the dose by half and limit the exposure time (i.e. two years) to avoid side effects. Cases of tubular necrosis, interstitial nephritis, and focal glomerulosclerosis with nephrotic syndrome have been described. Oral administration does not appear to alter renal function.
Special mention deserves osteonecrosis of the jaw, which has been related in recent years to treatment with antiresorptive agents, although its incidence is very low. This lesion is defined as the presence of one or several ulcerated lesions with bone exposure in the upper jaw and/or mandible of a duration of more than eight weeks. It is rare to observe these lesions in patients on oral bisphosphonates. Risk factors for the development of osteonecrosis include the administration of intravenous bisphosphonates for a long period of time, neoplasms, chemotherapy, radiotherapy, high doses of steroids, alcohol and/or tobacco abuse, and especially local factors such as periodontal disease, tooth extraction, and maxillofacial surgery. This is why good oral hygiene and temporary suspension of these drugs are recommended in the event that these treatments are required, after assessing the risk/benefit (suspension only if the risk of fracture is relatively low). Atypical femoral (diaphyseal) fracture has also been described in very few cases. It is possible that the benefits of reducing a high risk of fracture and associated mortality justify the low risk of these very rare complications.
DenosumabDenosumab is a humanized monoclonal antibody with high affinity and specificity against the receptor activator of nuclear factor kappa B (RANKL) that is administered subcutaneously every 6 months. By inhibiting the proliferation and activity of osteoclasts, denosumab decreases bone resorption and consequently increases BMD and therefore the risk of fractures are reduced.183 It is not eliminated by the kidney, which may be an advantage in patients with CKD since it does not require dose correction. However, it may cause severe hypocalcaemia with transient worsening of SHPT, especially in patients with CKD. Rebound bone loss has been described after discontinuation of denosumab.184
Although there is no conclusive evidence, both antiresorptive agents (bisphosphonates, denosumab) could exacerbate adynamic bone disease, or simply not be effective. It reasonable to stop the treatment with bisphosphonates during a period of time; this strategy should not be used with denosumab since a recent post hoc analysis has shown that the benefits of denosumab are rapidly lost and the risk of fractures is increased.185 The use of “sequential therapies” with these agents would be indicated. There is increasing experience of its prolonged use in patients with persistent high risk of fracture or with corticosteroid-induced osteoporosis.186–188
Regarding the risk of osteonecrosis of the jaw and atypical femoral fracture, the same principles mentioned with bisphosphonates could be applied.
TeriparatideTeriparatide is a fragment of human PTH including the first 34 amino acids of N-terminal molecule. This PTH fragment interacts with the type 1 PTH receptor, which is located primarily on osteoblasts and renal tubular cells. Intermittent teriparatide therapy increases the number of osteoblasts and subsequent augments bone formation. This anabolic effect is mediated by a decrease in osteoblast apoptosis and an increased activation of osteoblasts and pre-osteoblasts. It could have an indication in ABD, but its use is limited to two years due to the risk of osteosarcoma.189
Treatment by stagesSTAGES 1–2The K/DOQI and the KDIGO Guidelines do not recommend any treatment for CKD stages 1–2 patients. However, considering the data obtained in several studies, several strategies should be implemented for prevention rather than for treatment.
DietIt is known that patients with moderate decrease in renal function already have phosphate retention, with undetectable decreases in total calcium and an increase in PTH if they are exposed to phosphate overload, so starting with discrete dietary phosphate restriction seems adequate. Furthermore the patient will be more receptive to undergo a diet restriction when he has just been diagnosed with kidney rather than years after the diagnosis was made and no one has told him about the benefit of a diet. It could be started with a diet containing 0.8 g protein/kg of ideal body weight/day (new WHO recommendation for the general population); this would produce 2 benefits, a decrease in phosphorus intake with the consequent reduction of stimulus for PTH and FGF23 and, second, a decrease in the detrimental effect of glomerular hyperfiltration.190–192 It should also be taken into account that, in general, the degree of evidence presented in different CKD guidelines is variable.4,107
25(OH)D3 (calcidiol)Measurement of calcidiol levels is suggested in patients with CKD from stage 3 or in patients who, for whatever reason, are diagnosed with SHPT. Vitamin D deficiency or insufficiency should be corrected following the strategies recommended in the general population.110 It is considered that the recommended level should be >20 ng/mL to cover the daily needs of 97.5% of the population or 30 ng/mL to avoid increased levels of PTHi.110,193
Exact doses are not well defined. Clinical trials in the general population have used doses of 300–800 IU/day, considering a maximum of 4000 IU/day.110
In Spain we do not have a pharmacopoeia for vitamin D2 (ergocalciferol) except in multivitamin preparations. However, we have vitamin D3 (cholecalciferol) in the form of drops (in bottles of 10 mL = 20,000 IU/bottle = 2000 U/mL = 30 drops; 1 drop = 67 IU) or even in single-dose vials of 25,000 IU (to usually administer monthly –830 IU/day– or every 15 days). Although the dosage must be individualized, in general cholecalciferol doses of less than 600–800 IU/day (i.e. 9–12 drops) are usually ineffective. There are also several preparations that contain 200–800 IU of vitamin D3 + various amounts of calcium. It is recommended to be cautious with the use of these associations, especially in patients with vascular calcifications or at risk of developing vascular calcifications.
Calcifediol is easier to use is (ampoules or capsules of 0.266 mg = 266 µg = 16,000 IU of 25-(OH) vitamin D) generally administered monthly and exceptionally every two weeks, with closer control and during short periods. There is also a presentation in drops, in bottles of 10 and 20 mL, where 1 drop = 240 IU. Attention should be paid in obese patients as it is a fat-soluble vitamin.
The potency of calcifediol (µg to µg) appears to be about 5 times that of cholecalciferol194 and its half-life is longer. The administration of calcifediol on a monthly basis (exceptionally every 15 day), with the aforementioned controls of the levels of calcidiol, creatinine, calcium and phosphorus, is a convenient alternative to adjust the nutritional intake in patients with CKD or osteoporosis.195 For patients with CKD stages 3–4 with SHPH and vitamin D deficiency, a formulation with extended/prolonged release of calcifediol has already been marketed in some countries.196
CalciumBetween 15–20 mg/kg/day of calcium m in the diet would be sufficient to ensure the coverage of this element. The recommended daily amount in the general population varies according to age and sex between 1000 and 1300 mg/day.110 It is not known whether these amounts should be limited in patients with vascular calcifications.
Antiresorptive agentsThere is a limited information about the use of antiresorptive agents in patients with early CKD stage. This could consider paradoxical, especially if it is considered that this type of patients have a higher risk of fractures than the general population. However, in patients with CKD stages 1–2 with osteoporosis and/or high risk of fracture it is currently recommended, according to the WHO criteria, the same management as in the general population.4
STAGE 3 (Fig. 5)During this period, it is usually observed an elevation in PTH values starting to show an exponential increase. The recommendations on this issue change with respect to the previous CKD stages and there is no uniformity of the suggestions from the different guides.4,107
DietProtein restriction will seek to avoid phosphorus intake and hyperfiltration, but with adequate monitoring to avoid malnutrition. The patient will easily tolerate the change if he had already adapted to the diet from the previous stages.
Some authors only limit products with disproportionate phosphorus content compared to protein content (abuse of dairy products, sodas and especially products prepared with additives), since many patients spontaneously lose appetite for protein as kidney disease progresses.
25(OH)D3 (calcidiol)The levels of 25(OH)D3 will continue to be monitored to ensure that they are normal with the administration of cholecalciferol or calcifediol in its various forms if it is required.110
Phosphorus bindersWith this degree of renal function and dietary intervention, it is not difficult to maintain normal serum phosphate levels, at least until advanced CKD stage 3b. If this is not the case, phosphate binders may be started, although calcium binders should be used with caution in patients with vascular calcifications.
Active metabolites of vitamin DIn case of progressive and significant increase in iPTH levels, the suggested initial dose of calcitriol is 0.25 µg 2–3 times a week or every 48 h, and the dose of α-calcidiol is 0.25 µg every 24–48 h.197 These doses should be adjusted according to regular biochemical controls without trying to normalize PTH levels for the adaptive reasons explained above. It may be advisable to give it at night because it is followed by an 8-h fast and thus has less effect on intestinal absorption of calcium.
The suggested dose of paricalcitol in this stage is 1 mcg 2−3 times a week or every 48 h depending on the iPTH levels. This dose should be adjusted according to the results of periodic biochemical controls. Conversion to paricalcitol typically follows a relationship of 1 mcg paricalcitol ≈ 0.25 mcg calcitriol ≈ 0.50 α-calcidiol.
Antiresorptive agentsIn patients with CKD 3a–3b with PTH in the normal range and with osteoporosis and/or high risk of fracture according to WHO criteria, the treatment suggested is as in the general population.4,198 The indication of bisphosphonates or denosumab should be carefully weighed in cases with suspected EOA. In patients with CKD 3a onwards (3a-5D) with biochemical abnormalities of the CKD–MBD complex and decreased BMD and/or fragility fractures, it is suggested that the decision about treatment options should be made taking into account the magnitude and reversibility of the biochemical abnormalities and the progression of CKD, considering the possibility (not mandatory) of a bone biopsy.4,91,198
STAGE 4 (Fig. 6)In this phase, the elevation of the PTH value is considerable and it is fast, so diet and treatment must be strict.
DietProtein restriction will be slightly more important (maximum 0.8 g/kg of weight/day) but only if adequate nutrition can be ensured.
25(OH)D3 (calcidiol)The levels of 25(OH)D3 will continue to be monitored to ensure they are normal with the administration of cholecalciferol or calcifediol in its various forms if required.
Phosphate bindersWith this reduction in kidney function, it becomes somewhat difficult to maintain normal phosphate levels despite dietary restriction. In the event of a progressive and persistent increase in phosphorus above normal levels, measures of phosphate restriction should be increased and/or a higher dose of binders should be used with meals, which, if they contain calcium, should not be exceeded in any case the 1000 mg/day. In any case, we must remember that the KDIGO 2017 guidelines4 suggest restricting the doses of calcium-based binders in adults from CKD stage 3a, having increased the degree of evidence as compared to previous guidelines, despite the limitations in the data sheets for lanthanum carbonate and sevelamer carbonate. Interestingly, and accepted in the data sheet, aluminum hydroxide could be used (if available) for a short period, administering it only in meals whose phosphorus content justifies it. There is experience about the effectiveness use of oxyhydroxide sucroferric before starting dialysis.
Active metabolites and analogs of vitamin DAt this stage, depending on PTH levels, the recommended initial dose of calcitriol is 0.25–0.50 mcg every 24–48 h. These doses should be adjusted to the results obtained with regular biochemical controls. The more reduced effect of paricalcitol on calcium and phosphorus absorption, as well as its potentially lesser effect on cardiovascular calcifications, may make its use recommendable in this stage, especially trying to avoid high doses of calcitriol or α-calcidol.
The suggested starting dose of paricalcitol at this stage is 1 mcg every 24–48 h. This dose should be adjusted based on periodic biochemical controls.
Antiresorptive agentsAs previously mentioned, in patients with CKD 3a and above (3a-5D) with biochemical abnormalities of the CKD–MBD complex and decreased BMD and/or fragility fractures, it is suggested that treatment options take into account the magnitude and reversibility of biochemical abnormalities and progression of CKD, considering the possibility (not mandatory ) of a bone biopsy. If treatment with antiresorptive agents is considered due to the existence of a high risk of fracture in patients with creatinine clearance <30 mL/min and with adequate control of the biochemical values of CKD–MBD, the possibility of treatment with antiresorptive drugs such as denosumab could be considered even though they are eliminated by the kidneys. But of course, following the warnings mentioned above.111,199 The use of bisphosphonates is not strictly contraindicated, but they are not recommended since the experience is insufficient.
STAGE 5 (Fig. 6)In this stage of CKD the control of SHPT is more difficult. Renal function is markedly reduced and both excretory and endocrine functions are deficient, biochemical variability is great and the situation can change in a short time, making treatment more difficult to standardize. If the patient has been controlled since the early stages, the regimen for the previous stage is generally sufficient.
DietProtein restriction should be maintained, taking care not to compromise adequate nutrition.
25(OH)D3 (caldidiol)The serum Calcidiol values will continue to be monitored, to ensure that they are greater than 20 ng/mL with cholecalciferol or calcifediol.
Phosphorus bindersWith this degree of renal function the administration of phosphate binders with meals are usually required to maintain normal phosphate levels. The guideline is the same as in the previous stage, but doses are usually higher. It is advised a greater restriction of calcium binders.
Active metabolites of vitamin DIn this stage, the recommended doses are the same as in the previous stage, although they may be modified depending on calcemia, phosphataemia and serum PTH values. The suggested initial dose of paricalcitol in this stage is 1 µg every 24 h if the iPTH is less than 500 pg/mL and increase to 1−2 µg every 24 h if the iPTH is above 500 pg/mL, the dose should be modified depending on calcemia and phosphatemia.
STAGE 5D (dialysis) (Fig. 7)The control of phosphateThe increase in serum phosphate levels is one of the main problems presented by CKD patients on dialysis. Avoiding hyperphosphataemia has two purposes: one, to achieve an adequate control of bone-mineral metabolism, mainly to avoid the development, progression and complications of SHPT, and the other objective is to reduce cardiovascular risk and the high rate of morbidity and mortality in these patients. The independent association between hyperphosphatemia and mortality has been demonstrated by retrospective analysis of several large databases. More recently, the COSMOS study has shown a significant reduction in the risk of mortality associated with decreases in serum phosphorus.52 Therefore, maintaining serum phosphorus within normal limits (i.e. <4.5 mg/dL), with reasonable therapeutic measures is a priority. The COSMOS study showed that the lowest mortality is observed with a serum phosphorus level of 4.4 mg/dL (range 3.6–5.2); in another study, the “Dialysis Outcomes and Practice patterns Study “(DOPPS), the survival improved in those patients with a low number of serum phosphate above 4.5 mg/dL during a period of 6 months.200 Likewise, the area under the curve of the serum phosphorus concentration of the patients (with serial measurements, as recommended by the KDIGO) was also a better predictor of cardiovascular death than the most recent serum phosphorus level.200 Additionally, those types of patients with phosphorus levels greater than 5 mg/dL were associated with a higher rate of mortality and hospitalization, regardless of whether the serum levels of calcium and PTH are high, normal, or low.201
In this case, the treatment of hyperphosphatemia is based on three fundamental pillars:
- a)
Restriction of dietary intake of foods with a high content of phosphorus without compromising the necessary protein intake.
- b)
Modifications of the characteristics and dialysis scheme to optimize the elimination of this solute.
- c)
Administration of phosphorus binders.
- d)
In most cases it is required combination of these three therapeutics alternatives.
In hemodialysis, protein–energy requirements must be higher than those recommended for the general population, given the catabolic condition and the disease itself. Understandably, protein–energy requirements are also higher than those recommended in patients with CKD who are not yet on dialysis.
The priority must be to guarantee an adequate caloric, protein and mineral support. The price to pay for a presumably adequate diet should never be insufficient nutrition. Common sense sets the standards for a balanced diet. Four meals daily, balanced in carbohydrates, fats and proteins. A recent meta-analysis shows that diet (and 20−30 min per month of therapy by a dietitian) may reduce phosphorus levels without compromising nutritional status, but the evidence is low and probably this therapy will be useless if it is not maintained.202
It is considered that the optimal protein intake should be 1–1.2 g/kg/day (of which 50% should be of high biological value, that is, animal proteins), and the caloric intake should be 30–35 kcal/kg/day (35 for younger and 30 for older than 65 years). In peritoneal dialysis, the recommendation is even higher (1.2–1.3 g/kg/day).
Less restriction of dietary protein may result in an increases in phosphorus intake. Therefore, the price to pay to ensure enough protein intake may be the need for higher doses of phosphate binders.
DialysisThe ideal duration of the dialysis session is a highly controversial topic. Currently it is considered that the duration of dialysis should be individualized according to the requirements of each patient. Although there is no absolute evidence that there is an independent effect of dialysis time on phosphorus control, except for patients with high residual renal function. In general terms, an increase in dialysis time and/or frequency improves solute removal.
- -
The duration of the dialysis session may be decisive in the elimination of small solutes, mainly located in the space intracellular, as is the case with phosphorus.
- -
There are not controlled and randomized prospective studies that confirm definitively that an increase in the time of dialysis has a positive effect on control of hyperphosphatemia. However, most published studies describe that increasing the duration of the hemodialysis session and/or nocturnal intermittent a hemodialysis have beneficial effect on phosphorus removal.203
- -
Another alternative is to Increase the frequency of hemodialysis sessions. Again, there are no adequate studies to assess the effect of an increase in the frequency of dialysis on phosphorus clearance. Most of these the studies are observational, with a limited number of selected patients, followed by a short time period.
- -
Currently, to achieve a significant reduction in phosphorus levels there is a tendency to increase frequency and time of hemodialysis with schedules of 2.5−3.0 h 5–6 times a week.204,205 The increase in time and frequency, can be an effective procedure for the treatment of refractory hyperphosphatemia. With long nocturnal dialysis daily (5–6 sessions of 6−10 h duration per week) there is a marked decrease in phosphorus levels, which allows a reduction in the doses of phosphate binders. Even it may be necessary to add phosphate to the dialysis bath, despite having realyze that the patients had increased the daily intake of phosphorus.205
- -
Techniques with high convective transport can be considered a therapeutic alternative for hyperphosphatemia. As compared with low flow membranes, the high flow membranes have a greater phosphorous removal capacity. However, currently there is no clear evidence of the potentials advantage of the new membranes or hemodiafiltration regarding the phosphorus clearance.
As already mentioned, most hemodialysis patients have a positive phosphorus balance, so they will require additional treatment with intestinal phosphate binders to prevent hyperphosphatemia. The potential limitations of calcium binders (associated or not with magnesium) and aluminum hydroxide are the same as in previous stages; it is suggested the restriction of calcium binders in adults and it is recommended avoiding the use of aluminum binders (in addition to avoiding contamination of dialysis fluid with aluminum).4 There is extensive experience with both sevelamer and lanthanum carbonate and less with oxyhydroxide sucroferric, although with encouraging results with the latter due to the low number of tablets needed to control serum phosphorus. A new recent clinical trial shows how strict control (versus 5.0–6.0 mg/dL) of plasma phosphorus with lanthanum carbonate or oxyhydroxide Sucroferric could delay the progression of coronary calcification in these patients,206 while in another trial whose objective was to maintain plasma phosphorus between 3.5–6.0 mg/dL, no differences were observed in cardiovascular events between lanthanum carbonate and calcium.207
Calcium controlCurrently, as a general recommendation on calcium levels, it is considered that the objective is to avoid hypercalcaemia (usually >9.5 mg/dL). A European study in dialysis patients52 showed that the lowest mortality was observed in patients with calcium levels between 7.9–9.5 mg/dL.
It is important to avoid the association of high calcium with low PTH levels, as well as the association of high calcium and phosphorus levels, combinations that have been related to increased mortality or the presence of vascular calcifications. A certain degree of asymptomatic hypocalcemia induced by calcimimetics is considered tolerable and could even be beneficial. In addition, with a relatively low calcium, FGF23 decreases, as long as P is controlled.208
DietThe proportional increase in calcium with the increase in protein recommendations varies depending on the amount of dairy products. As a guideline, a diet of 1–1.2 gr/kg/day of protein already contains between 550 and 950 mg of calcium.
The total intake of elemental calcium per day should not exceed 1000 milligrams, including both dietary calcium and that included in phosphorus binders or ion exchange resins.
DialysisAdjustments in the calcium concentration in the dialysis fluid can contribute to optimizing the calcium balance in these patients. There is no consensus on what should be the calcium content in the dialysis fluid. Values of 1.25 mM (2.5 mEq/L; 5 mg/dL) have been associated with a negative calcium balance and a tendency to increase the PTH. In addition, with 1.25 mM there could be a worse hemodynamic tolerance to ultrafiltration, which is enhanced if the magnesium content is not adequate. Higher levels of calcium such as 1.75 mM (3.5 mEq/L; 7 mg/dL), reduce the secretion of PTH but produce a positive calcium balance and should be reserved only for severe symptomatic hypocalcemia or cases of hungry bone syndrome post-parathyroidectomy.
Following the 2017 KDIGO,4 we insist that hypercalcemia must be avoided and a persistent increase in calcium in the dialysis fluid does not seem advisable patients with to calcimetic-induced hypocalcemia. Furthermore, it is important the control of P in the presence of hypocalcemia. In addition, in patients with low PTH, a Ca 1.25 mmol/L (even 1 mmol/L) in the dialysis fluid with may be indicated to stimulate PTH secretion and prevent adynamic bone disease (ABD). But, it should be taken into consideration that low concentrations of Ca Calcium in the bath may predispose to arrhythmias and hemodynamic instability.
If possible, the calcium content in the dialysis fluid should be individualized according to the characteristics of each patient and the evolution of PTH.209
Taking into account all the factors mentioned, the recommended concentration that is generally best suited to the needs in situations of normocalcaemia and controlled PTH is 1.5 mmol/L (3 mEq/L; 6 mg/dL). One proof of the lack of consensus is that the KDIGO guidelines4 have not reached the same conclusion and suggest the use of fluid in the bath between 1.25 and 1.50 mmol/L (2.5−3 mEq/L). The calcium balance according to the dialysis fluid in patients with hypocalcaemia secondary to the use of calcimimetics is still unknown. All these considerations are applicable to peritoneal dialysis, where the use of fluids with a high calcium content is very common and frequently erroneous in patients who already have a greater probability of suffering from ABD and a longer exposure time to ABD.210,211
PTH monitoringIt is suggested that initial goal is to maintain the serum PTH values in the range of 150−300 pg/mL (approximately 2–5 times the upper limits of normality) corrected for the kit used as the initial target for iPTH. Concentrations of PTH outside this range have been associated with increased morbidity and mortality in hemodialysis patients.212–214 It is especially advised to avoid values below 100−120 and above 500–600 pg/mL as It was already mentioned in the previous guides. These latter values approximately coincide with the values considered as “extreme risk” expressed in the KDIGO guidelines (avoid values <2X and >9X in relation to the upper limits of normality of the kit used for measuring PTHi) (3.4). The recent COSMOS study establishes a PTH of 398 pg/mL as the point of greatest survival of the population.52 For this reason, we mention an initial therapeutic objective of PTH between 2X–5X–7X the upper limit of normality for the kit used.
The working group considers that establishing values <2X and >9X of the upper limits of normal for the kit used as the only adequate quality control margin as suggested by the KDIGO is possibly inappropriate, since it would inevitably result in that a significant number of patients would fall outside these ranges due to the Gaussian distribution of the populations. Establishing a narrower therapeutic goal as suggested (“aim” initially at 2X–5X), would ensure that a greater number of patients would be in the safety margin away from the extremes of risk. Accepting such wide margins208 can also have not only negative impacts on bone quality, but also favor the progression of parathyroid hyperplasia, decrease the efficacy of therapeutic strategies209 and also make it difficult to control calcemia and phosphatemia.215 Something similar occurs with the increase in PTH before the start of dialysis, since this value predicts uncontrolled SHPT at 12 months of dialysis despite the use of more drugs to reduce PTH secretion 160. The margin suggested by the KDIGO is based on epidemiological studies that associate these extreme values of iPTH with greater specificity for the diagnosis of high or low bone turnover disease, or others that associate these values with higher mortality.214
To maintain patients in this range of PTH, it is important to have an appropriate control of the serum calcium, phosphorus, and probably calcidiol levels, since the pre-dialysis period208; if having achieved this objective, the PTH is significantly elevated or it is increasing progressively (assess increasing trends even within the range between 2X–9X), the use of active metabolites of vitamin D, calcimimetics or a combination of both is suggested to reduce PTH. It should be kept in mind that relatively low PTH levels are associated with increased mortality regardless of the values of calcium and phosphorus (low, normal, or high).201 However, it is possible that this association is not be due to the decrease in PTH, but to the underlying disease or other associated factors such as age, diabetes, malnutrition, inflammatory state, etc.63,216
Although the EVOLVE study was not significant in its primary endpoint (cardiovascular outcome),38 most members of KDIGO 2017 and SEN were reluctant to exclude potential benefits of cinacalcet in patients with CKD 5D based on a series of nominally significant results in pre-specified secondary analyses and post-hoc studies.166,170,171,217,218 However, it is not considered that there is yet enough evidence to prioritize any anti-parathyroid agent219 and that all are acceptable as first-line treatment in dialysis patients.4
Also, it is considered reasonable to base initial therapy on calcium and phosphorus levels,216 as well as other aspects of CKD–MBD (e.g., vascular calcification), and that the dose of phosphate binders be adjusted so that treatments to control PTH do not compromise calcium and phosphorus levels.
25(OH)D3 (calcidiol)There are suggestions that indicate that at this stage it is also necessary to maintain adequate levels of calcidiol.221,222 This could be useful for the control of SHPT, but especially for other pleiotropic, auto- or paracrine effects of vitamin D.
Active metabolites of vitamin DTreatment with active metabolites (calcitriol or α-calcidiol, paricalcitol) may reduced PTH levels, but can often increase the serum phosphorus, calcium, and calcium × phosphorus product and require careful monitoring. In retrospective studies the administration of active metabolites have been associated with increased survival in dialysis patients despite the increase in FGF-23 levels. One possible explanation is the experimental demonstration that treatment with calcitriol, for example, attenuates the FGF-23/FGFR-4/calcineurin/NFAT signaling pathway responsible for the induction of left ventricular hypertrophy by FGF-23,26,223 or that the VDR activators, particularly paricalcitol, attenuate fibrosis heart disease, at least partially, by regulating microRNAs.224
Treatment with active metabolites of vitamin D should be minimized or discontinued if there is an elevation in serum calcium and/or phosphorus (see next paragraph) or if the PTH is less than 100–150 pg/mL (or approximately <2X the upper limit of normal for the kit used). It has been mentioned already that paricalcitol has less effect on the elevation of Ca, P and CaxP and, although it lowers PTH rapidly, this effect has a relative value since the decrease in PTH levels does not constitute a medical emergency. Some authors propose the possibility of activating VDRs with low doses of paricalcitol (i.e. 1 µg /week), even with low PTH levels,225,226 if native vitamin D is not used in these circumstances. All active metabolites of vitamin D can be administered orally or intravenously at doses that will depend on serum PTH levels, and as long as serum calcium and phosphorus levels are controlled (e.g. <9.5 mg/dL and <5 mg/dL, respectively). Occasionally, in some cases of severe SHPT, vitamin D derivatives can lower plasma phosphorus (reducing PTH may decrease bone release of “endogenous phosphorus” from bone), but in cases of manifest hyperphosphataemia, it would be preferable to start treatment with calcimimetics and associate vitamin D analogues if neccesary.
Calcimimetics: cinacalcet and etelcalcetideCalcimimetics should not be started in dialysis patients with a serum calcium concentration (corrected for albumin) below the lower limit of the normal range (<8.4 mg/dL). If the patient is already being treated with calcimimetics, the treatment can be continued if the clinical situation and patient’s safety allows it.
In these patients, the use of cinacalcet could be considered if there in PTH trend towards an increase, even if it has not reached 300 pg/mL, as long as calcaemia and/or phosphatemia are elevated.
The starting dose r starting dose recommended for adults is 30 mg once daily, which should be adjusted every 2–4 weeks but not to exceed the maximum dose of 180 mg once daily. In case of need to reduce the dose, the drug can be given every 48 h. Other intermediate doses are achieved with the administration of different schedules (e.g. 60 mg–30 mg every other day would correspond to a dosage of 45 mg/day).
A therapeutic alternative to cinacalcet for the control of hyperparathyroidism is treatment with intravenous etelcalcetide, which also ensures therapeutic compliance in non-adherent patients.175 The recommended starting dose of etelcalcetide is 5 mg, administered by bolus injection 3 times a week (coinciding with hemodialysis sessions, at the end of the session). Corrected serum calcium should be at or above the lower limit of the normal range prior to administration of the first dose, dose increase, or restart after dose interruption. It should not be administered more frequently than 3 times per week. The dose of etelcalcetide should be adjusted individually between 2.5 mg and 15 mg, according to PTH and calcium levels, and with increments of 2.5 mg or 5 mg not more frequently than every 4 weeks until a dose maximum of 15 mg 3 times per week to reach the target value of PTH.
Blood samples to assess PTH levels should be obtained at least 12 h after the last dose.
In the event of significant hypocalcaemia (i.e. <7.5 mg/dL), it is recommended to reduce the dose of calcimimetic and/or increase the dose of vitamin D derivatives if PTH levels are still elevated.
The calcium concentration in the dialysis bath should not be increased to correct hypocalcemia unless it is symptomatic.
Association of calcimimetics and active metabolites of vitamin DIt is possible that the association of vitamin D metabolites and calcimimetics could have additive and/or synergistic effect on the control of secondary hyperparathyroidism, perhaps due to the upregulation of the parathyroid cell receptors,19,20,227 or to present other beneficial effects (e.g. on vascular calcification).228 It has been observed that the use of calcimimetics has been associated with a decrease in the need for derivatives of vitamin D and vice versa. The combination of treatments may help not only to reduce doses but also biochemical or clinical side effects,88,220 just as it occurs in combined treatment of arterial hypertension or immunosuppression in kidney transplants.
The following graphs (Figs. 7–12) show an indicative algorithm for the management of biochemical alterations of bone-mineral metabolism in patients in stage 5D, based on serum levels of iPTH and phosphorus.
STAGE 5T (kidney transplantation) (Figs. 13–15)After kidney transplantation, persistence of hyperparathyroidism is common, both secondary (elevated PTH with normal calcium and phosphorus) and tertiary (elevated PTH with high calcium, with or without low phosphorus), with elevated levels of FGF-23, and low levels of vitamin D.229 In addition, immunosuppressants, mainly steroids, have deleterious effects on bone. Transplanted patients have different degrees of renal failure, occasionally acidosis, and the negative effect of loop diuretics on calcium balance. All this translates, basically, into the following problems:
- -
Persistent hyperparathyroidism.
- -
Bone loss and fractures.
Persistent tertiary or SHPT is found in 15%–50% of patients after the first year after transplantation, and those with higher serum PTH and calcium values at the time of transplantation will show persistence of SHPT for a longer period of time.230,231
Presently, since the introduction of cinacalcet, the percentage of dialysis patients who access transplantation with controlled PTH has increased notably. Now the problem is to decide whether cinacalcet should be suspended in a specific patient at the time of transplantation.
The persistence of SHPT is a risk factor for:
- -
Loss of bone mass and increased risk of fracture.231
- -
Hypercalcemia. Its incidence varies greatly depending on the time elapsed since the transplantation. It has negative cardiovascular effects, and it has also been identified as one of the many factors responsible for renal graft failure in the medium term.232
- -
Hypophosphatemia, probably also secondary to persistently elevated FGF-23 values.231,233
- -
Impaired kidney function and the incidence of tubulo-interstitial calcifications.234
For the management of these abnormalities, it is advisable to wait and observe the evolution, maintaining a close control of the serum levels of calcium, phosphorus and PTH.
Regarding the treatment there are two alternatives:
- -
Parathyroidectomy. It has been shown to be effective in controlling calcemia and improving BMD, although has been described a short-term deterioration in kidney function after parathyroidectomy.235 Considering the wide use of cinacalcet, we believe that parathyroidectomy could be reserved for patients who do not respond to calcimimetics, although in a recent randomized study subtotal parathyroidectomy was superior and more cost-effective than cinacalcet for calcium control if the duration of treatment with cinacalcet had to be prolonged for more than 14 months.236
- -
Calcimimetics (cinacalcet). It has been shown to be effective in correcting hypercalcemia and hypophosphatemia secondary to persistent hyperparathyroidism. No negative effect on renal function or interaction with immunosuppressants (calcineurin inhibitors or m-TOR inhibitors) has been described.237–240 In addition, calcimimetics could have a beneficial effect on BMD,240 although further studies must be performed to confirm this beneficial effect.
In patients with calcemia >10.5 mg/dL and iPTH >100 pg/mL, the most advisable strategy would be to start treatment with cinacalcet 30 mg/day and modify the dose depending on the response.
Bone loss and fracturesDifferent prospective studies have shown that during the first 6 months after kidney transplantation there is a rapid loss of BMD (7%–10%), which mainly affects cancellous bone (trabecular).231 At the level of the lumbar spine, the loss of BMD is 1.5% per month. Significant bone loss has also been described at the level of the proximal femur. The decrease in BMD stabilizes or tends to recover after 12 months.231,241,242
These findings highlight the importance of starting prophylactic measures since the moment of transplantation.
Regarding long-term bone loss, there are discrepancies in the results obtained in different; some reported a rate of bone loss of 1%–2% per year at the level of the lumbar spine, while others show no changes and even a slight increase in BMD. These discrepancies may be due to the use of different maintenance doses of corticosteroids.
The rapid bone loss that occurs after transplantation determines a high prevalence (7%–20%) and incidence (3%–4% per year) of fractures. The rate of hip fracture is 3.3/1000 person-years, which is 34% higher than in patients on the kidney transplant waiting list,243 and according to the USRDS, 22.5% of transplant patients suffered a fracture during the first 5 years after transplantation.244
Fractures resulting as a consequence of initial bone loss usually occur in the late post-transplant period, and although bone loss is preferentially at the trabecular bone level, most fractures affect the appendicular skeleton, particularly the feet and ankles.231,244
Since vertebral fracture is a powerful risk factor for the future development of fractures, its detection provides the opportunity to intervene in secondary prevention. Hence, an imaging technique should be incorporated as part of the follow up to detect asymptomatic vertebral deformities in transplant patients with a higher risk of fracture.
Currently, in transplant patients (stages 1T–5T) with risk factors for osteoporosis, the KDIGO 2017 guidelines suggest measuring BMD to assess the risk of fracture if the results may affect the therapeutic approach.4
Ambiguously, the KDIGO globally suggest that in transplant patients with a GFR >30 mL/min/1.73 m2 and low BMD to treat with vitamin D, calcidiol/alphacalcidol and/or antiresorptive agents during the first 12 months after transplantation and modulate the treatment depending on the serum values of calcium, phosphorus, PTH, alkaline phosphatase and calcidiol.4
Recommendations for the prevention of bone mass loss and post-renal transplant fractures (Figs. 13–15)The prevention of bone loss and fractures begins when the transplant is performed. Apart from reducing the dose of corticosteroids, the level of evidence for the different therapies is low.245
- -
Immunosuppressants
The first measure is to minimize corticosteroid doses and discontinue them as soon as it is considered safe.
- -
Vitamin D and calcium supplements
Normalization of calcium intake is suggested, if possible with calcium from the diet or with the administration of oral calcium supplements (e.g. 0.5 g/day) and vitamin D supplementation of 800–1000 IU/day, either in the form of cholecalciferol or calcidiol. The use of active vitamin D metabolites (calcitriol, α-calcidol or paricalcitol) also prevents bone mass loss in the first few months after transplantation.246–250
It is recommended to measure the levels of 25(OH)D3 after the transplant periodically (every 6 or 12 months), and correct the values if required by administering cholecalciferol or 25(OH)D3 (calcidiol) in the form of a daily dose (800–1000 IU/day), biweekly or monthly basis.
- -
Bisphosphonates
Bisphosphonates, in combination with calcium and vitamin D supplements, have been shown to be effective in preventing post-transplant bone loss, and in the treatment of osteoporosis.251–258 All bisphosphonates: pamidronate i.v., l ibandronate oral montly or i.v. or risendronate oral weekly or alendronate weekly, have been shown to be equally effective.251–257 A recent meta-analysis showed a 6% increase in femoral BMD and a 7.4% increase in lumbar BMD without affecting serum creatinine or plasma calcium, but there were no differences in the incidence of fractures compared to controls, probably due to the small sample and short follow-up period.259
We recommend performing a bone densitometry and a lateral X-ray of the thoracic and lumbar spine during post-transplant hospitalization, at least in recipients with a higher risk of fracture, as assessed by clinical criteria (i.e. sex, age, diabetes, type of donor) or by FRAX.260,261 Instead of X-ray, the measurement of TBS or spinal morphometry obtained with the newer DEXA equipment may be useful.262 The use of bisphosphonates will be based on the BMD and PTH values.
In patients at risk, it is also recommended to repeat the examination after a year; if there is significant worsening in the densitometry, or some new fractures, the standard treatment should be a bisphosphonate (alendronate, risedronate, or ibandronate orally) or another antiresorptive agent if used for a longer period of time.
After more prolonged follow up the DXA could be done every two or three years.
- -
Denosumab
The administration of denosumab in the first year after transplantation increases BMD with a therapeutic effect superior to other previously described treatment alternatives.263,264
Calcemia and PTH should be monitored in about 15 days after the first dose, due to the risk of hypocalcemia and the acute increase in PTH. To prevent these changes in PTH and calcium it is recommended the daily administration of vitamin D and oral calcium.
It has been described a non-severe urinary tract infections, this is something that needs to be monitored.264,265
It is administered subcutaneously at a dose of 60 µg every 6 months. It does not require dose adjustment according to renal function, so it would be mainly indicated in transplant patients with significant deterioration in renal function or intolerance to bisphosphonates.
- -
Parathyroid hormone
Teriparatide has not been shown to be effective in preventing post-transplant bone loss. Its use should be reserved to transplant recipients with a high risk of fracture (previous fractures, severe osteoporosis, particularly with even acute vertebral fractures) and suspected ABD, such as patients with advanced age (>65 years), diabetics and with values very low serum concentration of PTH (<60 pg/mL). It is administered in a daily subcutaneous dose of 20 µg, although it can be used as other weekly doses.266
Teriparatide could be an interesting therapeutic option in renal transplant recipients with hypoparathyroidism (previous parathyroidectomy), in whom hypocalcemia can be further aggravated by the use of steroids231 and pending the approval of other molecules with a specific indication for hypoparathyroidism.
FundingNo funding was received for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.