Q fever is a worldwide zoonosis caused by Coxiella burnetii acquired mainly by inhalation.1 Clinical manifestations of acute Q fever include self-limited flu-like illness, pneumonia, and mild hepatitis. Chronic infection usually presents as a culture-negative endocarditis, but other uncommon forms of persistent focalized infection and secondary non-Hodgkin lymphoma are also possible.2,3
We present the case of a 70-year-old man with a history of hypertension, type 2 diabetes mellitus, and chronic ischemic dilated cardiomyopathy. The patient was also being monitored by Hematology due to a monoclonal gammopathy of undetermined significance and had stage V chronic kidney disease, secondary to mesangiocapillary glomerulonephritis with heavy chain deposition. A Quantiferon-TB Gold plus had been performed with positive results and therefore he had been taking rifampicin 600mg QD for 4 months as treatment for latent tuberculous infection. Regarding the immunosuppressive treatment, in December 2022 the patient received induction treatment with methylprednisolone pulses and a single Rituximab dose, due to progressive kidney failure. He finally started chronic hemodialysis program 2 days/week through a tunneled central venous catheter at the end of December 2022. A blood test in January 2023 revealed low IgG (488mg/dL) with normal IgM and IgA levels.
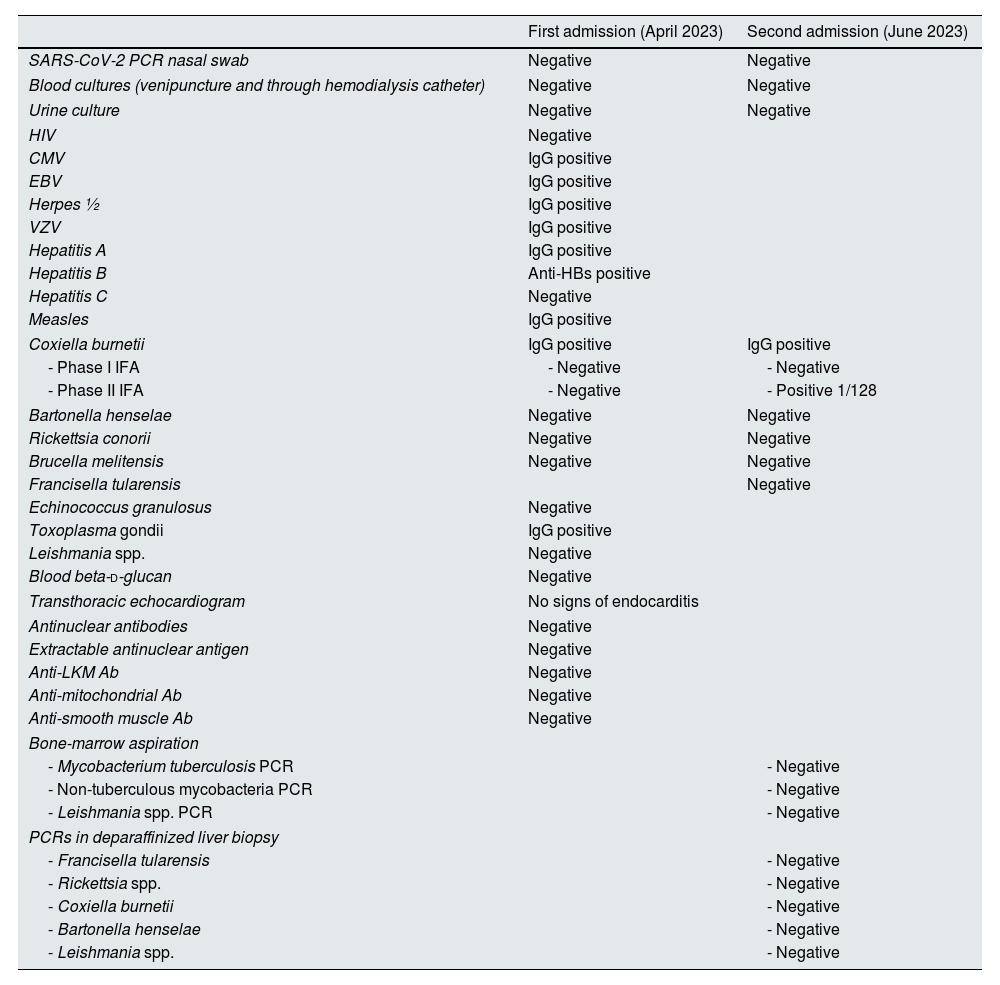
In April 2023, he consulted after a hemodialysis session complaining of general malaise, impaired mobility, pain in the right hypochondrium and moderate fevers for 2 weeks. The analytical findings suggested an infectious process: C-reactive protein (CRP) 364mg/L, procalcitonin 1.71ng/mL, leukocytosis 19,200/mm3, Hb 11.9g/dL, LDH 300U/L, GGT 211U/L, ferritin 1452ng/mL. He was referred to the emergency department (ED) where an abdominal ultrasound and CT scan were performed, describing multiple small nodular liver images, with a hypodense center and ring enhancement, suggestive of microabscesses. Chest-X ray was normal. The patient was then admitted to our Infectious Diseases Unit and antibiotics stopped. Initial microbiological and analytical results are included in Table 1. After all these diagnostic proceedings yielded no result, a liver biopsy was performed. The patient was sent home pending results and he felt much better without any antimicrobial being started. CRP and procalcitonin declined spontaneously and the patient remained on close monitoring as an outpatient.
Relevant results of the two hospital admissions.
| First admission (April 2023) | Second admission (June 2023) | |
|---|---|---|
| SARS-CoV-2 PCR nasal swab | Negative | Negative |
| Blood cultures (venipuncture and through hemodialysis catheter) | Negative | Negative |
| Urine culture | Negative | Negative |
| HIV | Negative | |
| CMV | IgG positive | |
| EBV | IgG positive | |
| Herpes ½ | IgG positive | |
| VZV | IgG positive | |
| Hepatitis A | IgG positive | |
| Hepatitis B | Anti-HBs positive | |
| Hepatitis C | Negative | |
| Measles | IgG positive | |
| Coxiella burnetii | IgG positive | IgG positive |
| - Phase I IFA | - Negative | - Negative |
| - Phase II IFA | - Negative | - Positive 1/128 |
| Bartonella henselae | Negative | Negative |
| Rickettsia conorii | Negative | Negative |
| Brucella melitensis | Negative | Negative |
| Francisella tularensis | Negative | |
| Echinococcus granulosus | Negative | |
| Toxoplasma gondii | IgG positive | |
| Leishmania spp. | Negative | |
| Blood beta-d-glucan | Negative | |
| Transthoracic echocardiogram | No signs of endocarditis | |
| Antinuclear antibodies | Negative | |
| Extractable antinuclear antigen | Negative | |
| Anti-LKM Ab | Negative | |
| Anti-mitochondrial Ab | Negative | |
| Anti-smooth muscle Ab | Negative | |
| Bone-marrow aspiration | ||
| - Mycobacterium tuberculosis PCR | - Negative | |
| - Non-tuberculous mycobacteria PCR | - Negative | |
| - Leishmania spp. PCR | - Negative | |
| PCRs in deparaffinized liver biopsy | ||
| - Francisella tularensis | - Negative | |
| - Rickettsia spp. | - Negative | |
| - Coxiella burnetii | - Negative | |
| - Bartonella henselae | - Negative | |
| - Leishmania spp. | - Negative | |
6 weeks thereafter (June 2023) the patient visited the ED again complaining of right hypochondrium pain, general discomfort, hematuria, and low-grade fever. Blood CRP was >480mg/L and procalcitonin 6.73ng/mL. He was readmitted to our Infectious Diseases Unit again. Second abdominal CT was carried out with similar results than the first one. Table 1 shows all microbiological results.
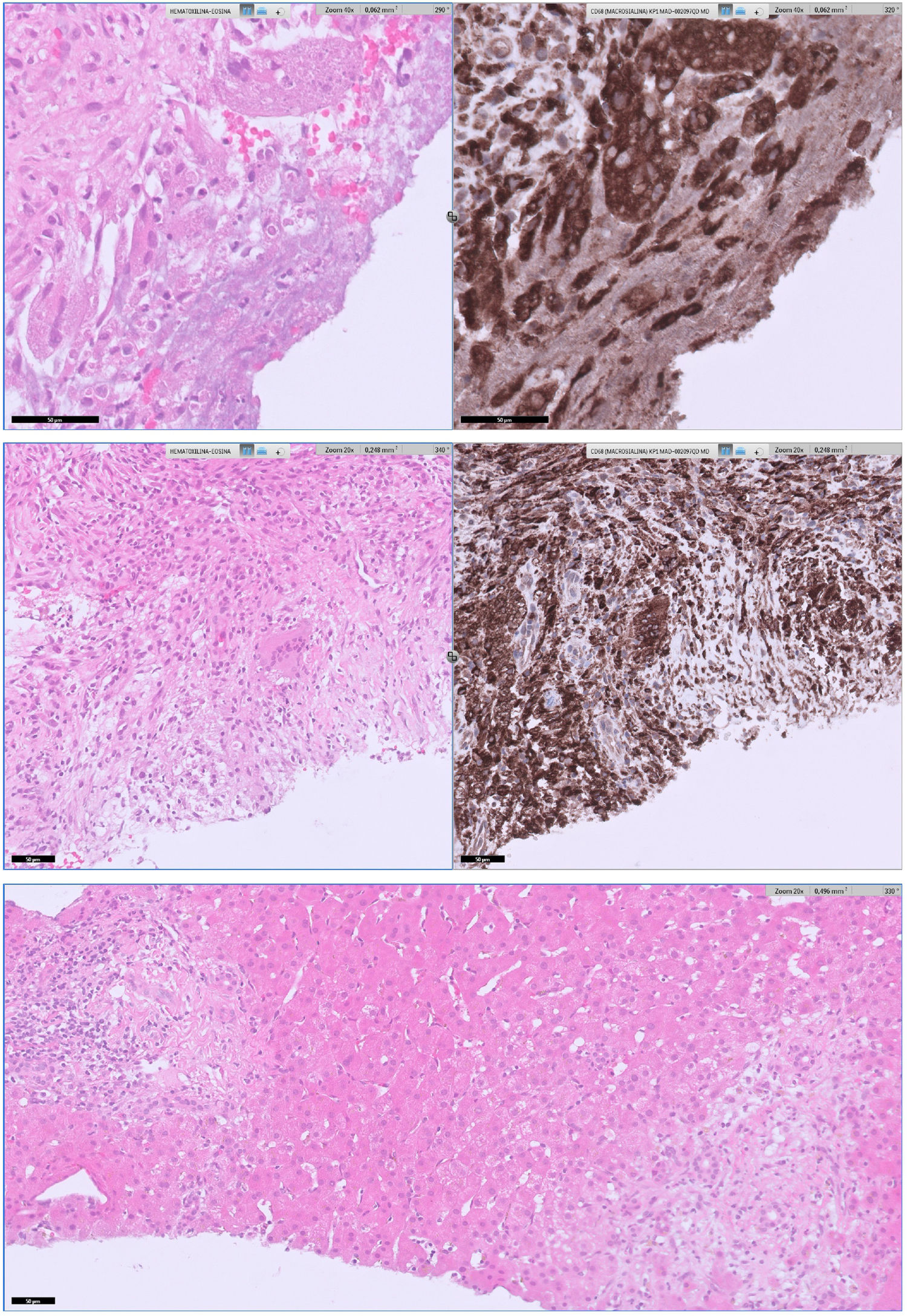
The patient was diagnosed of Q fever based on serological results and the result of the liver biopsy (Fig. 1). According to this, doxycycline was started (100mg BID for 28 days). He became afebrile, and another blood analysis had normal parameters including CRP and procalcitonin. Follow-up serology 7 months after first admission was performed with negative phase I and positive phase II with a title of 1/200. All liver lesions resolved in a control CT-scan.
Upper section: areas of necrosis with portal and lobular involvement produced an alteration in the hepatic architecture. A histiocytic ring surrounding the necrosis is highlighted with CD68 immunostaining, configuring histiocytic granulomas of variable size. No fibrin-ring granulomas were not observed on the serialized slides nor ancillary techniques performed. Middle section: giant cells were scarce, localized in the lobule, and their presence was more detectable with CD68 staining. Lower section: in addition, some portal-periportal inflammation with T lymphocytes prevalence, scarce B lymphocytes and very few plasma cells, with focal extension to interphase and lobules was present.
C. burnetii infection is a well-known cause of prolonged fever all over the world. Acute infection usually manifests as an atypical pneumonia or mild hepatitis, although most patients experience an acute self-limiting infection.4,5 Granulomatous hepatitis with fibrin-ring granuloma is the classic pathological form in liver biopsy but this is non-specific and non-sensitive because Q fever can produce other forms of granulomatous hepatitis.6,7 We found a report of a kidney transplant patient who suffered acute Q fever presenting liver and spleen abscesses with a splenic biopsy that showed extensive abscess formation and focal poorly formed non-necrotizing granulomatous inflammation.8
There is a wide differential diagnosis for a patient with granulomatous liver disease and prolonged fever. Diagnostic options may be grouped into several categories: sarcoidosis, autoimmune (primary biliary cirrhosis above all), infectious diseases (tuberculosis being the main, Mycobacterium avium-intracellulare complex in immunocompromised patients, fungal infections like histoplasmosis, unfrequently viral infections, and zoonosis like Rickettsia conorii and C. burnetii), drugs, cancer and idiopathic.9
Some diagnostic issues may be highlighted with respect to Q fever: gold standard is still IFA serology, and it usually takes 2–4 weeks to turn positive. C. burnetii has a peculiarity called antigenic phase variation: phase I has the more complex lipopolysaccharide (LPS) and it is the virulent-infectious phase. C. burnetii quickly turns into phase II, with a loss in the LPS complexity, virulence and infectivity.10
Phase II serology positivity is found in acute infection. Serological diagnosis of acute Q fever can be made either with phase II IgG≥1/128 or IgM≥1/32 or a 4-fold increase between two serum samples taken 3–6 weeks apart.1,11 IFA serologies used by our reference laboratory do not differentiate IgG from IgM.
On the contrary, high phase I serology (usually IgG≥1/800) is suspicious of chronic Q fever and should lead to further testing (echocardiogram, PET-CT). The general recommendation is to perform serological follow-up until 1–2 years after acute infection in patients at risk of progression (immunocompromised, chronic valvular disease, vascular graft, etc.).1
Some patients can be diagnosed by PCR in blood or tissue samples, but PCR is not widely available. PCR in blood specimens has high specificity but sensitivity is only good enough during the first days of the disease and when antimicrobials have not yet been started. PCR for C. burnetii targets the IS1111a insertion element.12 We performed a PCR in deparaffinated liver biopsy which resulted negative but deparaffination could reduce sensitivity of this technique. We didn’t start doxycycline until a positive phase II serology because we still had tuberculosis in our differential diagnosis and the patient became suddenly afebrile and asymptomatic before his second hospital admission.
An interesting question is whether anti-CD20 therapy could justify a longer duration of the febrile process and delay C. burnetii serology turning positive. There is a clear negative association between anti-CD20 therapies and SARS-CoV-2 seroconversion after SARS-CoV-2 mRNA vaccine.13 There is also an association between rituximab and low immunoglobulins just like the case of our patient. It would be reasonable to hypothesize of a low level of phase II antibodies in patients under anti-CD20 therapy who suffer acute Q fever.
To conclude we would like to suggest an initial protocol for the diagnosis and management of patients with prolonged fever (>7 days). In stable patients a first set of explorations should be performed: chest-X ray, serial blood cultures (also through catheters if available), urine culture, and an initial round of viral PCRs (influenza, RSV, SARS-CoV-2) and serologies (basic are EBV, CMV and HIV). If all these results are negative and the patient has a slight elevation of liver enzymes, LDH and CRP/procalcitonin, we recommend a therapeutic trial with doxycycline. Doxycycline is an antibiotic with a good efficacy/security profile. It is administered at a dose of 100mg twice daily and do not require adjustment in any grade of renal or hepatic failure.14 Confirmative serology for C. burnetii (and other zoonosis like Rickettsia spp., Leptospira interrogans and Bartonella henselae) will always take time, and maybe longer in immunosuppressed patients who may also suffer from atypical or more prolonged forms of the disease. A high index of suspicion is required to better reach this diagnosis.
FundingAll authors declare no financial interest that could influenced the work presented in this paper.
Authors’ contributionEmilio Guirao-Arrabal: Conceptualization, writing-original draft. Ana Delgado-Ureña: supervision, collected patient data and informed consent, writing-review and editing. Elena Borrego-García: conceptualization, writing-review and editing. Rosa Ríos-Pelegrina: writing-original draft, collected pathological figures. All authors revised, read, and approved the final manuscript.
Informed consent statementWritten informed consent was obtained from the patient.
Conflict of interestAll authors declare no competing interest that could influenced the work presented in this paper.