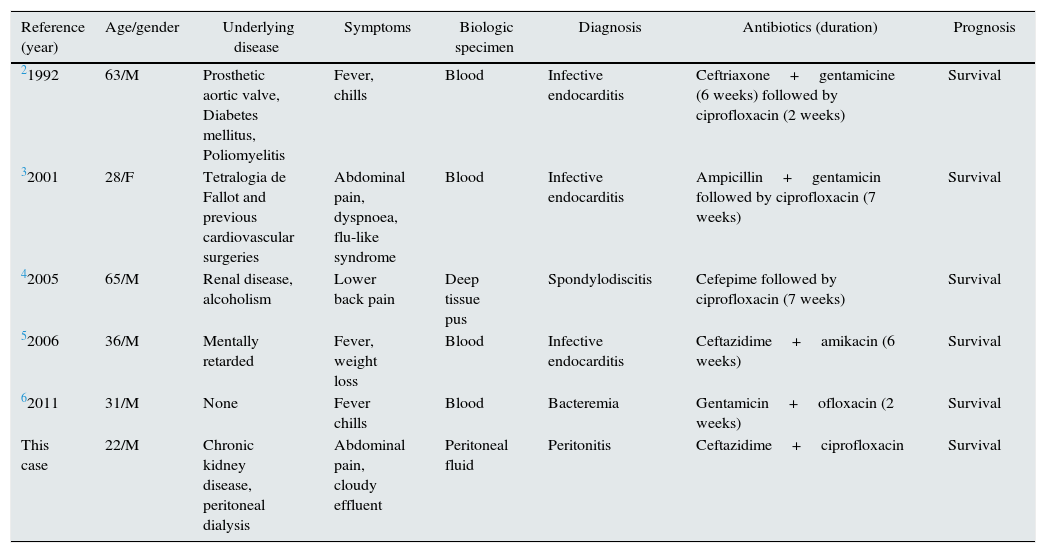
Peritonitis is the leading complication of peritoneal dialysis (PD), contributing to technique failure and hospitalisation.1Pseudomonas mendocina is a gram-negative non-fermentative rod that was first isolated by Palleroni and others in 1970 from soil and water samples.2 It is a low-virulence organism and is rarely encountered in clinical specimens or reported as a human pathogen. Aragone et al.3 reported the first case of P. mendocina, as a human pathogen, in a 63-year-old man with endocarditis. Since this report, four cases of infection have been reported4–7: three of endocarditis,3,4,6 one of spondylodiscitis5 and one of bacteremia7 (Table 1).
Clinical manifestations and outcomes of reported cases of P. mendocina infection.
| Reference (year) | Age/gender | Underlying disease | Symptoms | Biologic specimen | Diagnosis | Antibiotics (duration) | Prognosis |
|---|---|---|---|---|---|---|---|
| 21992 | 63/M | Prosthetic aortic valve, Diabetes mellitus, Poliomyelitis | Fever, chills | Blood | Infective endocarditis | Ceftriaxone+gentamicine (6 weeks) followed by ciprofloxacin (2 weeks) | Survival |
| 32001 | 28/F | Tetralogia de Fallot and previous cardiovascular surgeries | Abdominal pain, dyspnoea, flu-like syndrome | Blood | Infective endocarditis | Ampicillin+gentamicin followed by ciprofloxacin (7 weeks) | Survival |
| 42005 | 65/M | Renal disease, alcoholism | Lower back pain | Deep tissue pus | Spondylodiscitis | Cefepime followed by ciprofloxacin (7 weeks) | Survival |
| 52006 | 36/M | Mentally retarded | Fever, weight loss | Blood | Infective endocarditis | Ceftazidime+amikacin (6 weeks) | Survival |
| 62011 | 31/M | None | Fever chills | Blood | Bacteremia | Gentamicin+ofloxacin (2 weeks) | Survival |
| This case | 22/M | Chronic kidney disease, peritoneal dialysis | Abdominal pain, cloudy effluent | Peritoneal fluid | Peritonitis | Ceftazidime+ciprofloxacin | Survival |
M, male; F, female.
We describe the first case of P. mendocina peritonitis in a young adult on PD and discuss its prognostic implications. A 22-year-old male, with chronic kidney disease stage 5d, on automated PD (APD) for 15 months, with no past infectious complications reported, came to our country for a 6 month period. On the 43rd day, he was admitted with peritonitis. Empiric antibiotherapy was initiated, with intraperitoneal cefazolin and ceftazidime in a continuous inpatient PD regimen during 2 days, as the patient was not familiar with intraperitoneal antibiotherapy. His handling regarding PD was evaluated and no mistakes were found. Oral ciprofloxacin (250mg 12/12h), was initiated empirically before discharge and the patient reinitiated his habitual APD regimen, maintaining intraperitoneal ceftazidime and cefazolin. The peritoneal fluid (PF) culture revealed Pseudomonas mendocina. Cefazolin was interrupted and treatment was maintained for 21 days, due to the good clinical evolution in the presence of two anti-pseudomonal antibiotics. The domestic water (in a rented flat with piped water and basic sanitation) was analysed, but contamination was not found.
Six days after the treatment the patient returned to the hospital with relapsing peritonitis. Empirical intraperitoneal cefazolin and ceftazidime was reinitiated, plus ciprofloxacin 500mg 12/12h and fluconazole 50mg 24/24h PO. The Tenckhoff catheter was filled with alteplase. As for the source of infection, we reanalysed the domestic water and contamination with P. mendocina was not found. The bathroom was shared with other colleagues, so suspicion of contamination of a wet shared towel remains the most likely source. Housing conditions were evaluated and sharing of the bathroom and towels with his roommates was discouraged.
PF culture came negative and leucocyte count <10mm3 was observed at 9th day. Empirical therapy was prolonged for 21 days. The patient had a recurrence 46 days after (on his 137th day abroad). Previous therapeutic scheme was initiated, with exception for fluconazole which was increased to 200mg/day. Microbiological, mycobacterium and fungal analysis came negative. He returned to his country one week after and maintained the treatment for 28 days. After 6 months, this patient had no further recurrences or relapses. He was asymptomatic and performing PD. The PF cell count remains routinely negative.
This case entails many self-limited factors that could be perpetuating the source of contamination (staying in a foreign country and different daily routines or constraints to usual aseptic technique), so it was decided not to remove the catheter as he returned home and presented asymptomatic, with clear PF, negative cell count and microorganism growth. He evolved free of infectious complications and the evolution after these 6 months proved that P. mendocina can be treated without removal of the catheter.
Some questions arose, nevertheless. Which was the source of infection for this rare microorganism? Was there a perpetuating focus or inoculum while abroad, as this infection resolved with a passive strategy of maintaining therapy? Could these relapses be due to the antibiotic and dialytic regimens chosen or to the nature of this microorganism, known to produce a bio-film?8 Was the longer course of antibiotics the key to resolution of this relapsing/recurrent cycle? Could the transference to continuous ambulatory PD (CAPD) and a longer course of therapy have been enough to cure the first episode? The availability to form biofilm has addressed by transiently filling the Tenckhoff catheter with alteplase, as it may be a role to prevent relapsing peritonitis.9 Although its benefit has not been definitively established,10 no harm has been held up against.
Transference to CAPD, despite being our normal procedure, was not a choice due to logistic issues concerning supplies of allocation and the fact that this patient was not trained in CAPD technique. Little is known about intermittent dosing requirement in patients treated with APD10 and the treatment regimen was not of easy choice in this particular case. Another remark concerning ceftazidime must be made, as there are no data for this antibiotic for intermittent dosing in APD, and its usage was based on equivalent dosing in CAPD for cefazolin.
Nevertheless, we could concluded that it was not imperative to remove the catheter in this case of low-virulence bacteria belonging to Pseudomonas spp. Further research is needed about P. mendocina, namely its antibiotic sensitivity and therapeutic duration for successful treatment.
DeclarationsInformed consent to publish individual data was obtained from the patient.
Conflict of interestThe authors declare no conflict of interest.