Post-transplantation proteinuria is a risk factor for graft failure. A progressive decline in renal graft function is a predictor for mortality in kidney transplant patients.
ObjectivesTo assess the development and the progression of urinary protein excretion (UPE) in the first year post-transplant in recipients of kidney transplants and its effect on patient and graft outcomes.
Materials and methodsWe analysed 1815 patients with 24-h UPE measurements available at 3 and 12 months post-transplant. Patients were divided based on their UPE level: below 300mg, 300–1000mg and over 1000mg (at 3 and 12 months), and changes over time were analysed.
ResultsAt 3 months, 65.7% had UPE below 300mg/24h, 29.6% 300–1000mg/24h and 4.7% over 1000mg/24h. At one year, 71.6% had UPE below 300mg/24h, 24.1% 300–1000mg/24h and 4.4% over 1000mg/24h.
In 208 patients (12%), the UPE progressed, in 1233 (70.5%) it remained stable and in 306 (17.5%) an improvement was observed.
We found that the level of UPE influenced graft survival, particularly if a progression occurred.
Recipient's age and renal function at one year were found to be predictive factors for mortality, while proteinuria and renal function were predictive factors for graft survival.
ConclusionsProteinuria after transplantation, essentially when it progresses, is a marker of a poor prognosis and a predictor for graft survival. Progression of proteinuria is associated with poorer renal function and lower graft survival rates.
La proteinuria después de un trasplante renal constituye un factor de riesgo para el fallo del injerto. Una disminución progresiva de la función del injerto renal es un predictor de la mortalidad en los pacientes trasplantados renales.
ObjetivosAnalizar la aparición y la progresión de una excreción urinaria de proteínas (EUP) en el primer año siguiente al trasplante en pacientes trasplantados renales, y su efecto sobre la evolución del paciente y del injerto.
Material y métodosAnalizamos un total de 1815 pacientes en los que se dispuso de determinaciones de la EUP de 24 horas a los 3 y a los 12 meses del trasplante. Dividimos a los pacientes según el nivel de EUP, de la siguiente forma: inferior a 300mg, 300-1000mg y más de 1000mg (a los 3 y 12 meses), y analizamos los cambios a lo largo del tiempo.
ResultadosA los 3 meses, el 65,7% presentaban una EUP inferior a 300mg/24h, el 29,6% 300-1000mg/24h y el 4,7% más de 1000mg/24h. A un año, el 71,6% tenían una EUP inferior a 300mg/24h, el 24,1% 300-1000mg/24h y el 4,4% más de 1000mg/24h.
En 208 pacientes (12%), la EUP mostró una progresión, en 1233 (70,5%) se mantuvo estable y en 306 (17,5%) se observó una mejoría.
Observamos que el nivel de EUP influía en la supervivencia del injerto, en especial si se producía una progresión.
La edad y la función renal del receptor al año del trasplante fueron factores predictivos de la mortalidad, mientras que la proteinuria y la función renal lo fueron de la supervivencia del injerto.
ConclusionesLa proteinuria después del trasplante, fundamentalmente cuando muestra una progresión, es un marcador de mal pronóstico y un factor predictivo de la supervivencia del injerto. La progresión de la proteinuria se asocia a una peor función renal y a una tasa de supervivencia del injerto inferior.
In the world of transplants, the introduction of new immunosuppressants has led to an improvement in short- and medium-term graft survival1–3; nevertheless, long-term graft loss remains a problem.4 The many factors associated with these long-term losses have been analysed by a large number of studies.5 Among them, recent studies6 show that proteinuria is an independent risk factor predictive of graft failure for recipients of all ages.7 At the same time, continued renal graft function decline is a strong predictor of mortality in renal transplant patients.6
The prevalence of proteinuria one year after transplantation ranges from 11% to 45%,8 or even higher in patients treated with proliferation signal inhibitors (PSI). Two mechanisms for the development of proteinuria after transplantation are described: tubular origin, due to inadequate protein reabsorption in the proximal tubule cells damaged by ischaemia/reperfusion phenomena, rejection or tubular toxicity, or glomerular origin due to increased passage of higher molecular weight proteins such as albumin through the glomerular barrier due to de novo or transplant glomerular disease, chronic rejection or drug toxicity.
Proteinuria in transplantation is usually indicative of some type of graft disease and generally tends to occur in relation to chronic graft nephropathy (histological substrate with interstitial fibrosis and tubular atrophy), acute rejection, transplant glomerular disease or recurrence of primary kidney disease.8–10 It can be associated with immunological or non-immunological factors11 and factors related to both the donor and the recipient. Degree of HLA sensitisation, age, obesity, hypertension and other cardiovascular risk factors can all contribute to the development of post-transplant proteinuria. Immunosuppressive therapy has also been associated with proteinuria,12 with reports that PSI in particular can induce it or make it worse if it already exists.13,14
Nephrotic-range proteinuria is associated with a worse renal graft outcome, as occurs in other diseases in the native kidney. However, urinary protein excretion usually considered as mild (<500mg/day) from the first months after transplantation15 is also associated with worse transplantation outcomes and leads to much poorer graft and patient survival rates, and may even provide information on the graft.16–17 However there are no studies that focus on the evolution of urinary protein excretion and its repercussion on renal graft survival.
ObjectivesTo analyse the prevalence of proteinuria in the renal transplant population in a particular regional area (Andalusia in southern Spain). To identify contributory factors and the consequences of proteinuria, particularly for graft and patient outcome. To evaluate the impact of the evolution of urinary protein excretion over time on graft survival.
Patients and methodsWe present data from the Renal Transplant Registry of Andalusia, which forms part of a general registry of replacement treatment for chronic kidney disease in the region that has been in operation since 1984.
For this review, we have analysed the data from 1 January 2000 to 31 December 2009, continuing the observation up to 25 May 2012 (12.25 years or 147 months). Data were collected retrospectively for the years 2000–2003 and prospectively from 2004 to 2009. We performed the analysis on the urinary protein excretion records available for 1815 patients after a median follow-up of 77.89±32.94 months (12–147) during this period.
The urinary protein excretion, or simply proteinuria, was quantified as mg/day, categorising the patients for the analysis into three levels according to severity:
- I.
Urinary protein excretion below 300mg a day
- II.
Urinary protein excretion 300–1000mg a day
- III.
Urinary protein excretion over 1000mg a day
The urinary protein excretion data were collected at two patient follow-up points, three months and one year after transplantation, leading to the creation of 6 groups of patients according to severity of urinary protein excretion and time of determination: I3m, II3m and III3m, according to the classification at 3 months and I1y, II1y and III1y, for one year after transplant.
Statistical analysis: The SPSS v21 statistical package was used.
An overall descriptive analysis was performed on the entire study population. Comparative analysis was carried out according to proteinuria groups, for which we performed a bivariate analysis using ANOVA, applying Welch's test for those groups in which there was a lack of homoscedasticity in the variances of the groups. For analysis of multiple comparisons in quantitative variables that showed statistically significant differences with respect to the proteinuria groups, we used multiple comparison tests indicated for groups with unequal variances and Bonferroni for groups with equal variances. Pearson's Chi-square test was used to compare qualitative variables.
The level of significance was considered to be p<0.05.
Patient and graft survival censored for death was studied by a Kaplan–Meier analysis and a multivariate analysis was performed using a Cox regression model by means of manual stepwise regression.
The following variables were introduced into this model: donor age; donor gender, cause of donor death; recipient age; recipient gender; pre-transplant cytotoxic antibodies; maximum cytotoxic antibodies; HLA-A, -B and -DR incompatibility; ACE inhibitors (3 months); ARA2 (3 months); creatinine (3 months); creatinine (1 year); proteinuria (3 months); and proteinuria (1 year).
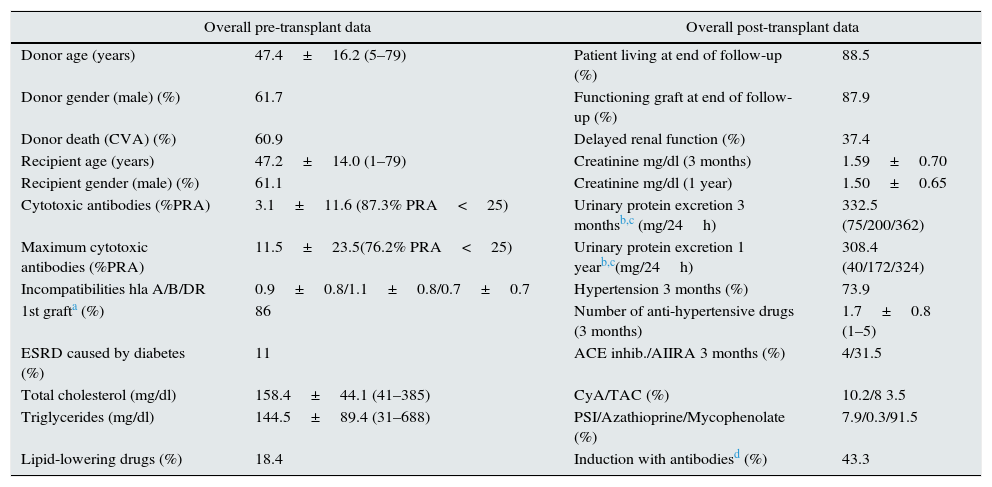
ResultsEpidemiological data and overall descriptive analysisPre-transplant and post-transplant data are shown in Table 1.
Pre-transplant variables considered overall (the first column) and overall post-transplant data in all patients studied (the second column).
| Overall pre-transplant data | Overall post-transplant data | ||
|---|---|---|---|
| Donor age (years) | 47.4±16.2 (5–79) | Patient living at end of follow-up (%) | 88.5 |
| Donor gender (male) (%) | 61.7 | Functioning graft at end of follow-up (%) | 87.9 |
| Donor death (CVA) (%) | 60.9 | Delayed renal function (%) | 37.4 |
| Recipient age (years) | 47.2±14.0 (1–79) | Creatinine mg/dl (3 months) | 1.59±0.70 |
| Recipient gender (male) (%) | 61.1 | Creatinine mg/dl (1 year) | 1.50±0.65 |
| Cytotoxic antibodies (%PRA) | 3.1±11.6 (87.3% PRA<25) | Urinary protein excretion 3 monthsb,c (mg/24h) | 332.5 (75/200/362) |
| Maximum cytotoxic antibodies (%PRA) | 11.5±23.5(76.2% PRA<25) | Urinary protein excretion 1 yearb,c(mg/24h) | 308.4 (40/172/324) |
| Incompatibilities hla A/B/DR | 0.9±0.8/1.1±0.8/0.7±0.7 | Hypertension 3 months (%) | 73.9 |
| 1st grafta (%) | 86 | Number of anti-hypertensive drugs (3 months) | 1.7±0.8 (1–5) |
| ESRD caused by diabetes (%) | 11 | ACE inhib./AIIRA 3 months (%) | 4/31.5 |
| Total cholesterol (mg/dl) | 158.4±44.1 (41–385) | CyA/TAC (%) | 10.2/8 3.5 |
| Triglycerides (mg/dl) | 144.5±89.4 (31–688) | PSI/Azathioprine/Mycophenolate (%) | 7.9/0.3/91.5 |
| Lipid-lowering drugs (%) | 18.4 | Induction with antibodiesd (%) | 43.3 |
The analysis was performed with the three-month urinary protein excretion determination in 1815 patients.
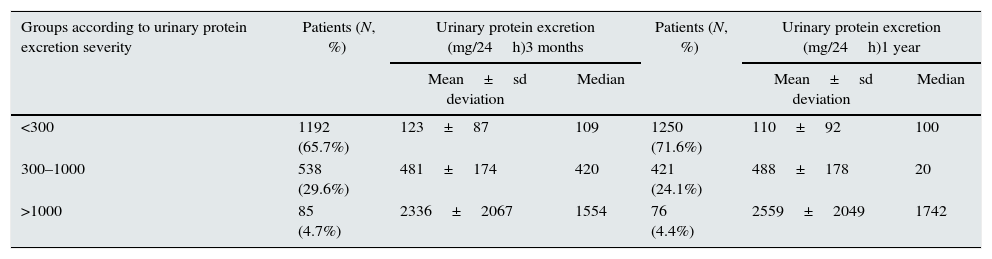
The distribution of cases by degree of urinary protein excretion at three months and one year and the mean determinations are shown in Table 2. The three levels had similar proportions.
Urinary protein excretion at three months and one year in each of the classified groups.
| Groups according to urinary protein excretion severity | Patients (N, %) | Urinary protein excretion (mg/24h)3 months | Patients (N, %) | Urinary protein excretion (mg/24h)1 year | ||
|---|---|---|---|---|---|---|
| Mean±sd deviation | Median | Mean±sd deviation | Median | |||
| <300 | 1192 (65.7%) | 123±87 | 109 | 1250 (71.6%) | 110±92 | 100 |
| 300–1000 | 538 (29.6%) | 481±174 | 420 | 421 (24.1%) | 488±178 | 20 |
| >1000 | 85 (4.7%) | 2336±2067 | 1554 | 76 (4.4%) | 2559±2049 | 1742 |
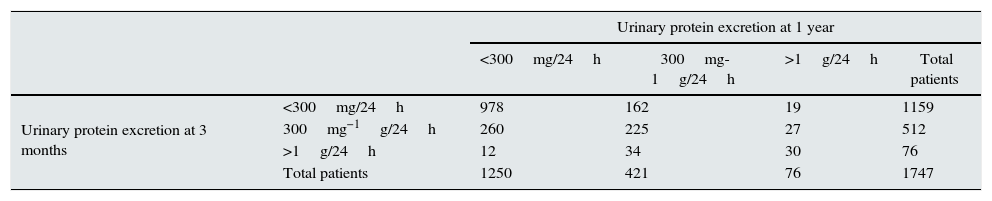
At one year, in the majority of patients, urinary protein excretion remained in the same range as at three months or had decreased. The urinary protein excretion range had increased (progression) in only 208 patients (12%) at one year, 1233 (70.5%) remained stable with the same degree (stable) and in 306 (17.5%), the range had improved (improvement) (Table 3).
Changes in urinary protein excretion over time: number of patients in each group at 3 months and 1 year.
| Urinary protein excretion at 1 year | |||||
|---|---|---|---|---|---|
| <300mg/24h | 300mg-1g/24h | >1g/24h | Total patients | ||
| Urinary protein excretion at 3 months | <300mg/24h | 978 | 162 | 19 | 1159 |
| 300mg−1g/24h | 260 | 225 | 27 | 512 | |
| >1g/24h | 12 | 34 | 30 | 76 | |
| Total patients | 1250 | 421 | 76 | 1747 | |
Comparing the resulting groups, we found:
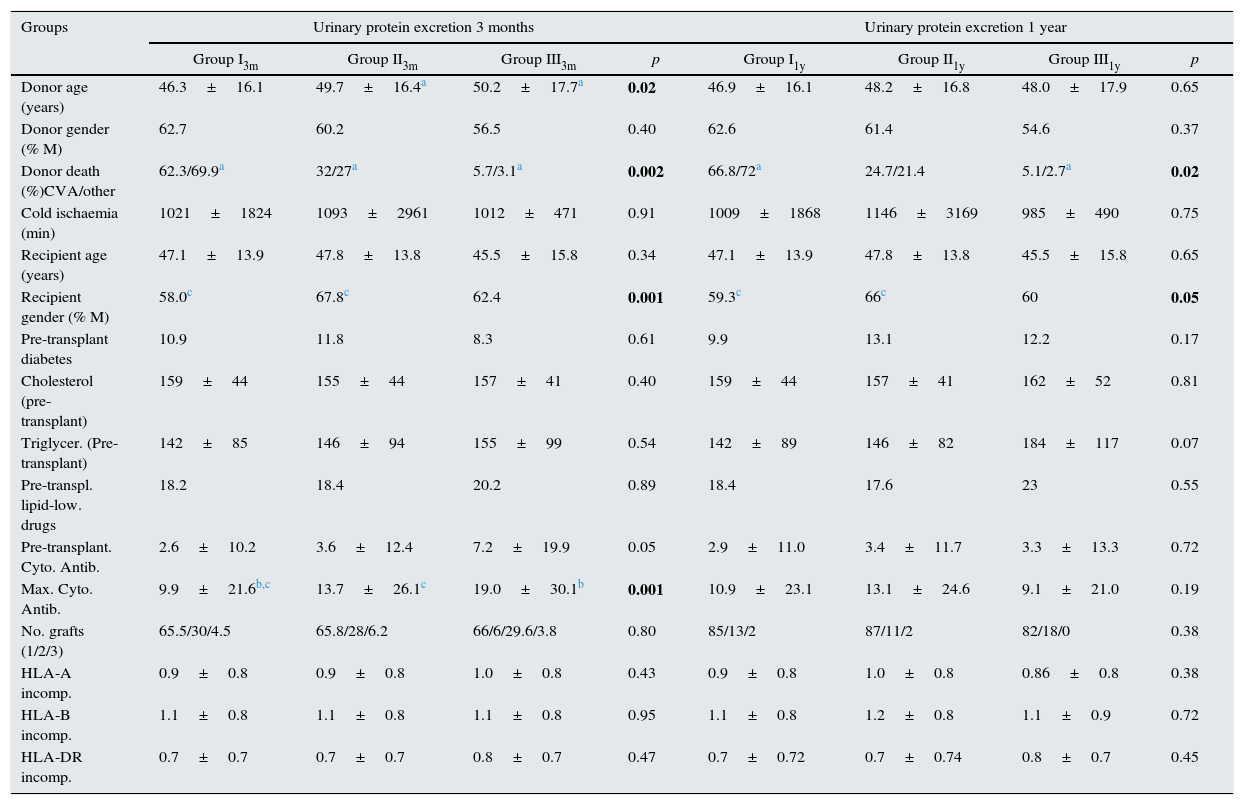
(a) Pre-transplant variables. Shown in Table 4.
Pre-transplant variables. Variables found to be statistically significant are shown in bold.
| Groups | Urinary protein excretion 3 months | Urinary protein excretion 1 year | ||||||
|---|---|---|---|---|---|---|---|---|
| Group I3m | Group II3m | Group III3m | p | Group I1y | Group II1y | Group III1y | p | |
| Donor age (years) | 46.3±16.1 | 49.7±16.4a | 50.2±17.7a | 0.02 | 46.9±16.1 | 48.2±16.8 | 48.0±17.9 | 0.65 |
| Donor gender (% M) | 62.7 | 60.2 | 56.5 | 0.40 | 62.6 | 61.4 | 54.6 | 0.37 |
| Donor death (%)CVA/other | 62.3/69.9a | 32/27a | 5.7/3.1a | 0.002 | 66.8/72a | 24.7/21.4 | 5.1/2.7a | 0.02 |
| Cold ischaemia (min) | 1021±1824 | 1093±2961 | 1012±471 | 0.91 | 1009±1868 | 1146±3169 | 985±490 | 0.75 |
| Recipient age (years) | 47.1±13.9 | 47.8±13.8 | 45.5±15.8 | 0.34 | 47.1±13.9 | 47.8±13.8 | 45.5±15.8 | 0.65 |
| Recipient gender (% M) | 58.0c | 67.8c | 62.4 | 0.001 | 59.3c | 66c | 60 | 0.05 |
| Pre-transplant diabetes | 10.9 | 11.8 | 8.3 | 0.61 | 9.9 | 13.1 | 12.2 | 0.17 |
| Cholesterol (pre-transplant) | 159±44 | 155±44 | 157±41 | 0.40 | 159±44 | 157±41 | 162±52 | 0.81 |
| Triglycer. (Pre-transplant) | 142±85 | 146±94 | 155±99 | 0.54 | 142±89 | 146±82 | 184±117 | 0.07 |
| Pre-transpl. lipid-low. drugs | 18.2 | 18.4 | 20.2 | 0.89 | 18.4 | 17.6 | 23 | 0.55 |
| Pre-transplant. Cyto. Antib. | 2.6±10.2 | 3.6±12.4 | 7.2±19.9 | 0.05 | 2.9±11.0 | 3.4±11.7 | 3.3±13.3 | 0.72 |
| Max. Cyto. Antib. | 9.9±21.6b,c | 13.7±26.1c | 19.0±30.1b | 0.001 | 10.9±23.1 | 13.1±24.6 | 9.1±21.0 | 0.19 |
| No. grafts (1/2/3) | 65.5/30/4.5 | 65.8/28/6.2 | 66/6/29.6/3.8 | 0.80 | 85/13/2 | 87/11/2 | 82/18/0 | 0.38 |
| HLA-A incomp. | 0.9±0.8 | 0.9±0.8 | 1.0±0.8 | 0.43 | 0.9±0.8 | 1.0±0.8 | 0.86±0.8 | 0.38 |
| HLA-B incomp. | 1.1±0.8 | 1.1±0.8 | 1.1±0.8 | 0.95 | 1.1±0.8 | 1.2±0.8 | 1.1±0.9 | 0.72 |
| HLA-DR incomp. | 0.7±0.7 | 0.7±0.7 | 0.8±0.7 | 0.47 | 0.7±0.72 | 0.7±0.74 | 0.8±0.7 | 0.45 |
There were significant differences between proteinuria groups in donor age, cause of donor death, recipient gender, maximum cytotoxic antibody values (% PRA) and induction therapy received.
With regard to the cause of donor death, we found that organs from older donors and donors who died as a result of a CVA (ischaemic or haemorrhagic) had more urinary protein excretion than those from donors who died from any other cause.
Regarding immunosuppressive induction therapy received, we found no difference in urinary protein excretion at three months between patients who received no induction therapy (urinary protein excretion 314mg/24h), those who received antithymocyte globulin (ATG) (387mg/24h) and those who received anti-CD25 antibodies (basiliximab or daclizumab) (350mg/24h).
However, one year after transplant, patients who had received thymoglobulin induction had higher levels of urinary protein excretion than those who had received anti-CD25 and those with no induction: 650mg/24h vs 280mg/dl and 325mg/24h, respectively.
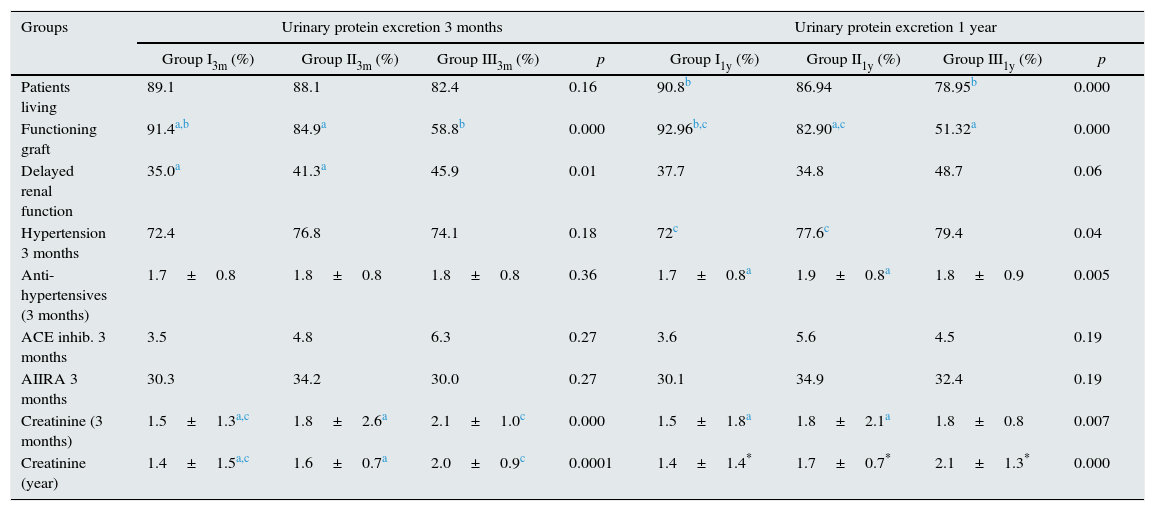
(b) Post-transplant variables: See Table 5.
Post-transplant variables according to urinary protein excretion group at three months and one year. Comparison of qualitative variables using Pearson's Chi-square test.
| Groups | Urinary protein excretion 3 months | Urinary protein excretion 1 year | ||||||
|---|---|---|---|---|---|---|---|---|
| Group I3m (%) | Group II3m (%) | Group III3m (%) | p | Group I1y (%) | Group II1y (%) | Group III1y (%) | p | |
| Patients living | 89.1 | 88.1 | 82.4 | 0.16 | 90.8b | 86.94 | 78.95b | 0.000 |
| Functioning graft | 91.4a,b | 84.9a | 58.8b | 0.000 | 92.96b,c | 82.90a,c | 51.32a | 0.000 |
| Delayed renal function | 35.0a | 41.3a | 45.9 | 0.01 | 37.7 | 34.8 | 48.7 | 0.06 |
| Hypertension 3 months | 72.4 | 76.8 | 74.1 | 0.18 | 72c | 77.6c | 79.4 | 0.04 |
| Anti-hypertensives (3 months) | 1.7±0.8 | 1.8±0.8 | 1.8±0.8 | 0.36 | 1.7±0.8a | 1.9±0.8a | 1.8±0.9 | 0.005 |
| ACE inhib. 3 months | 3.5 | 4.8 | 6.3 | 0.27 | 3.6 | 5.6 | 4.5 | 0.19 |
| AIIRA 3 months | 30.3 | 34.2 | 30.0 | 0.27 | 30.1 | 34.9 | 32.4 | 0.19 |
| Creatinine (3 months) | 1.5±1.3a,c | 1.8±2.6a | 2.1±1.0c | 0.000 | 1.5±1.8a | 1.8±2.1a | 1.8±0.8 | 0.007 |
| Creatinine (year) | 1.4±1.5a,c | 1.6±0.7a | 2.0±0.9c | 0.0001 | 1.4±1.4* | 1.7±0.7* | 2.1±1.3* | 0.000 |
We found that most patients with more urinary protein excretion had more delayed renal function, more hypertension at three months and required a greater number of antihypertensives to control it. The groups with more proteinuria also had worse renal function. At the end of the observation period, there were a lower proportion of functioning grafts in groups with higher proteinuria. In addition, with the urinary protein excretion measured at one year, we found a higher proportion of deaths among patients with proteinuria above 1g/24h.
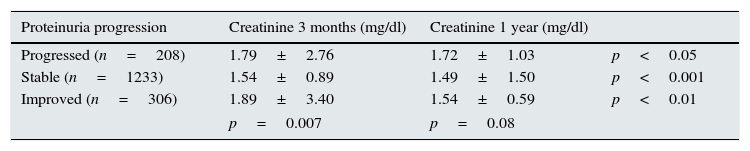
(c) Lastly, we studied changes in urinary protein excretion between three months and one year. Based on this, we analysed renal function, measured as serum creatinine, at three months and one year after transplantation; Table 6 shows patients in whom urinary protein excretion progressed, remained stable or improved. We found differences in renal function at three months according to whether the urinary protein excretion later progressed, stabilised or improved (p=0.007); however, there was no difference between groups one year after transplant (p=0.08).
Renal function according to changes in the urinary protein excretion between three months and one year.
| Proteinuria progression | Creatinine 3 months (mg/dl) | Creatinine 1 year (mg/dl) | |
|---|---|---|---|
| Progressed (n=208) | 1.79±2.76 | 1.72±1.03 | p<0.05 |
| Stable (n=1233) | 1.54±0.89 | 1.49±1.50 | p<0.001 |
| Improved (n=306) | 1.89±3.40 | 1.54±0.59 | p<0.01 |
| p=0.007 | p=0.08 | ||
Analysing changes in renal function measured as serum creatinine, we found that it improved significantly between three months and one year after transplantation in all groups, but the degree of significance was greater in patients in whom the urinary protein excretion stabilised or improved and lower in patients whose urinary protein excretion progressed.
Analysis of patient survivalIn our population considered as a whole, the patient survival rate at 5 years was 93.1%, at 10 years 82% and at the end of the study (12.25 years), 78%.
Patient survival according to urinary protein excretion:
- (1)
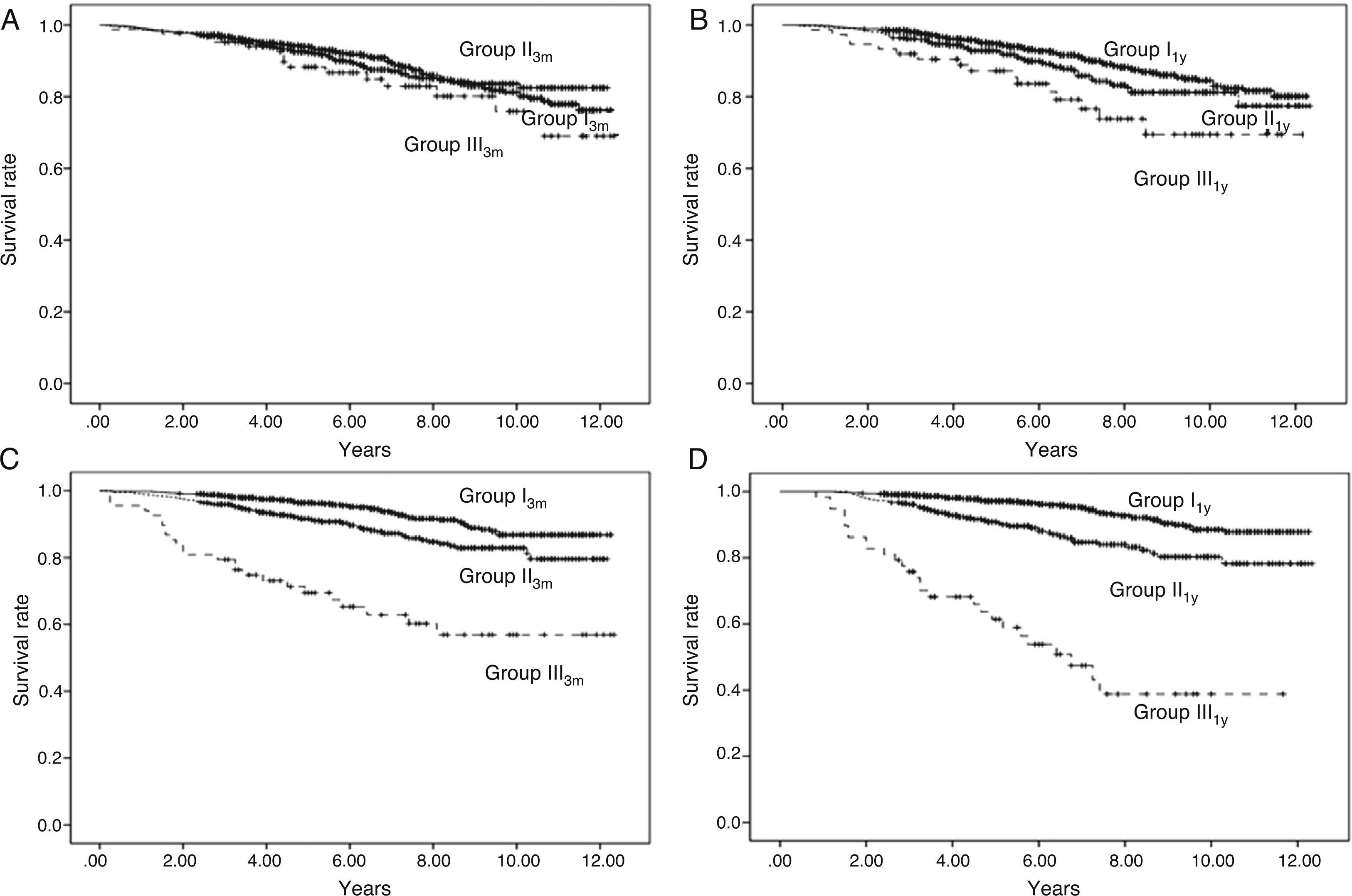
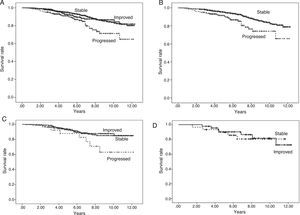
According to level of urinary protein excretion at 3 months, we found no differences in survival among the 3 groups (log rank, p=0.389) (Fig. 1A).
Fig. 1.Patient survival and graft survival censored for death. Kaplan–Meier survival: Shows patient survival according to urinary protein excretion group at three months (Panel A) and one year (Panel B) and graft survival censored for death according to urinary protein excretion group at three months (Panel C) and one year (Panel D). There are no differences in patient survival at three months with urinary protein excretion. With urinary protein excretion groups at one year, there are significant differences between groups I and II (p<0.05) and between I and III (p<0.001); continuous line – group I (urinary protein excretion<300mg/day), short dashed line – group II (urinary protein excretion 300–1000mg/day) and long dashed line – group III (urinary protein excretion>1000mg/day). With proteinuria groups at both three months and one year, there are significant differences in graft survival among the three groups (p<0.001); continuous line – group I (urinary protein excretion<300mg/day), short dashed line – group II (urinary protein excretion 300–1000mg/day) and long dashed line – group III (urinary protein excretion>1000mg/day).
- (2)
According to level of urinary protein excretion at 1 year, we found significant differences in survival rates between groups (log rank, p=0.001) (Fig. 1B).
- (3)
Analysing patient survival according to progression of urinary protein excretion, i.e. whether urinary protein excretion progressed between three months and one year compared to those who remained stable or improved, we found significant differences.
- (3.1)
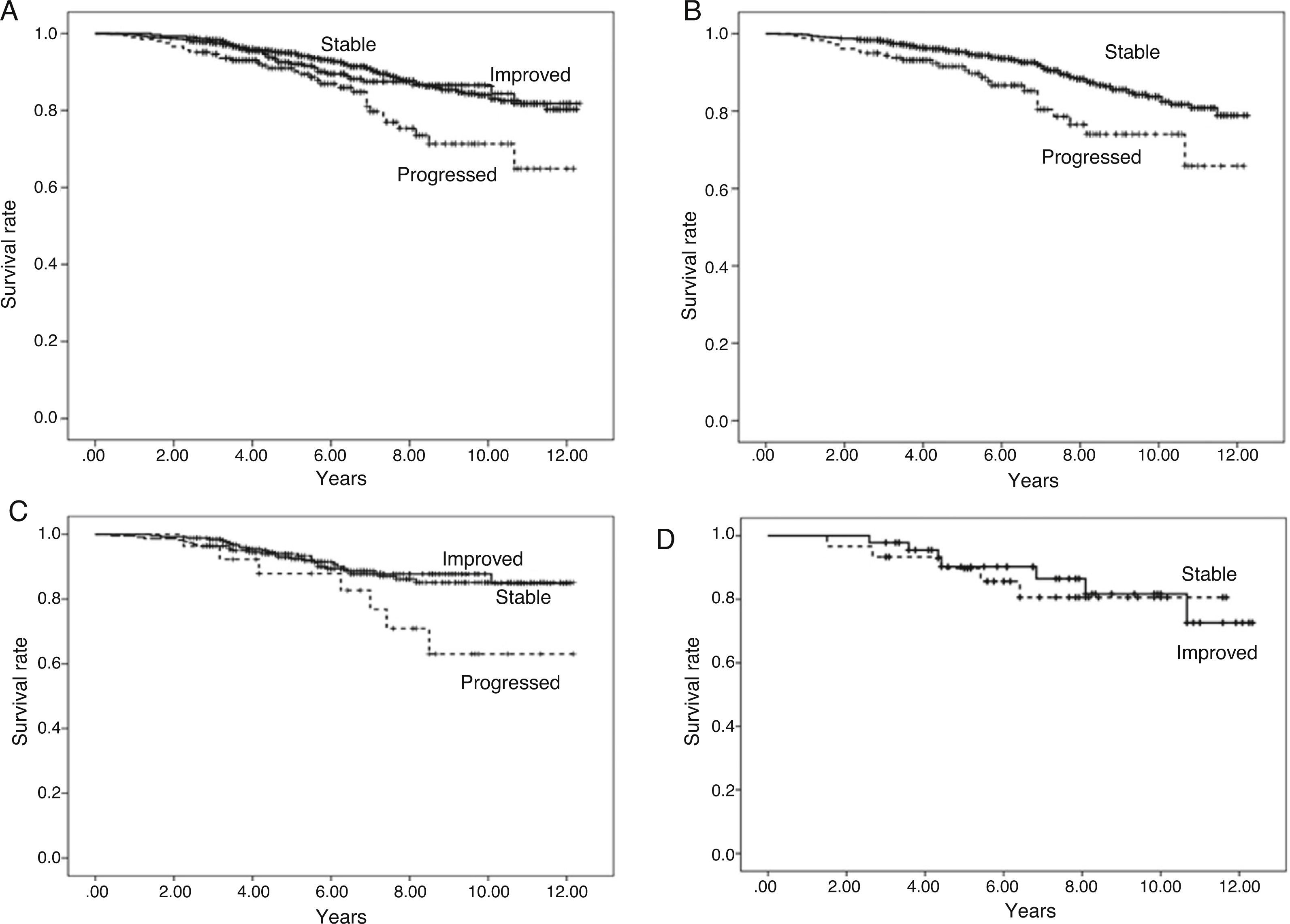
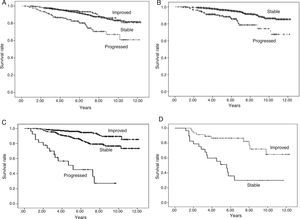
Overall patient survival is shown in Fig. 2A: for those in whom urinary protein excretion progressed, the difference in survival is significant, becoming more apparent from the 5th year on. There were no differences in survival between the groups of patients whose urinary protein excretion remained stable or improved.
Fig. 2.Patient survival according to progression of urinary protein excretion. Panel A shows overall survival of all the patients according to progression of urinary protein excretion from three months to one year post-transplant. Survival is worse (p=0.000) for those with progression (who change to a higher proteinuria level group) compared to those with no change (stable) or who improve (change to a lower urinary protein excretion level group). There were no differences in survival between the groups of patients whose proteinuria remained stable or improved. Panel B shows patients with urinary protein excretion of less than 300mg/day at three months, who remained at that level (stable) at one year or progressed (changed to a higher proteinuria level group), in which case, survival worsened. Panel C shows patients with urinary protein excretion of 300–1000mg/day at three months, and differences in survival according to whether their urinary protein excretion range improved, remained stable or progressed at one year. Panel D shows the survival of the group of patients with proteinuria above 1g/day at three months according to whether their urinary protein excretion remained within the same range (stable) or improved at one year.
- (3.2)
We then analysed each urinary protein excretion group at three months and their progress at one year.
- (3.2.1)
Taking the urinary protein excretion <300mg/24h group as reference and analysing those whose urinary protein excretion progressed and those who remained within that range, we found differences in survival (p=0.000) (Fig. 2B).
- (3.2.2)
Taking the urinary protein excretion 300mg−1g/24h group and analysing outcomes for those whose urinary protein excretion worsened, remained stable or improved, it can be seen from Fig. 2C that there are differences in survival at 10 years between the group that progressed and the other two groups (p=0.000). There were no differences in survival between patients whose urinary protein excretion remained stable or improved.
- (3.2.3)
In the group with proteinuria more of 1g/24h at three months, 40% remained in the same range at one year and 60% improved. In these two cases patient survival remained unchanged (p=0.793) (Fig. 2D).
- (3.2.1)
- (3.1)
- (1)
Carrying out the analysis censoring the 209 deaths with functioning graft, the graft survival rate at 5 years was 93.8%, at 10 years 84.3% and at the end of the study (12.25 years), 83.3%.
- (2)
The K–M curves for graft survival censored for death for the three groups according to level of urinary protein excretion at 3 months and one year are shown in Fig. 1C and D (log rank, p=0.000), with differences among the three groups.
- (3)
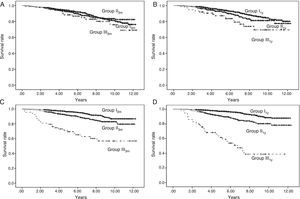
Analysis of graft survival for all patients according to progression of urinary protein excretion:
- (3.1)
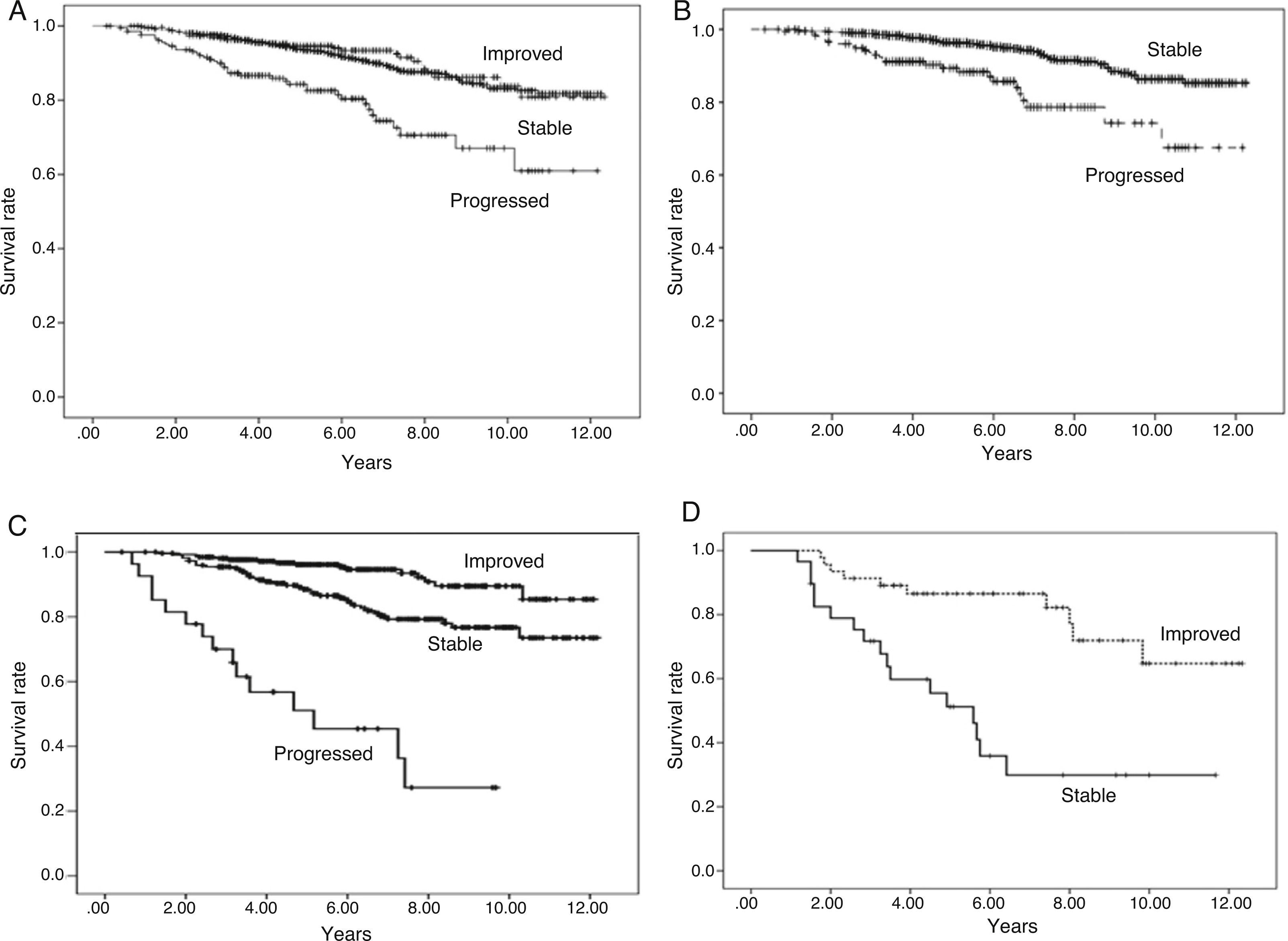
Analysing all patients with urinary protein excretion who progressed, remained stable or improved from three months to one year as a whole, we found significant differences (Fig. 3A).
Fig. 3.Graft survival according to progression of urinary protein excretion: Panel A shows overall survival for all the patients according to progression of proteinuria from three months to one year post-transplant (p=0.000). There were no differences in survival between the groups of patients whose urinary protein excretion remained stable or improved. Those who progressed had worse survival than those who remained at the same level of proteinuria (stable) or improved. Panel B shows patients with urinary protein excretion of less than 300mg/day at three months who remained at that level (stable) at one year or progressed (changed to a higher proteinuria level group), in which case survival worsened, compared to 94.4%, 86.4% and 85.3% for those whose urinary protein excretion remained at <300mg/day at one year (p=0.000). Panel C shows patients with proteinuria of 300–1000mg/day at three months, and differences in survival according to whether their proteinuria range improved, remained stable or progressed at one year (p=0.000). Panel D shows the survival of the group of patients with proteinuria above 1g/day at three months according to whether their proteinuria remained within the same range (stable) or improved at one year (p=0.000).
- (3.2)
We then analysed each urinary protein excretion group at three months and their progress at one year.
- (3.2.1)
Taking the urinary protein excretion <300mg/24h group as reference and analysing those whose urinary protein excretion progressed and those who remained within that range, we found differences in graft survival (Fig. 3B).
- (3.2.2)
Taking the proteinuria 300mg−1g/24h group and analysing outcomes for those whose urinary protein excretion worsened, remained stable or improved, there were differences in survival (Fig. 3C).
- (3.2.3)
In the group with proteinuria more of 1g/24h at three months, 40% remained in the same range at one year and 60% improved. There were differences in survival (Fig. 3D).
- (3.2.1)
- (3.1)
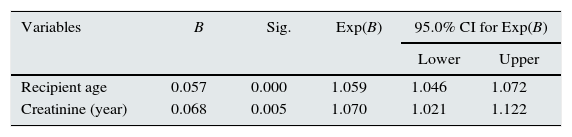
Both the age of the recipient and the age of creatinine at one year were found to be risk factors for the death of the patient (Table 7).
The risk of death is 1059 times greater for every year of the patient's age at transplant and 1070 greater for every milligramme of creatinine at one year after transplantation.
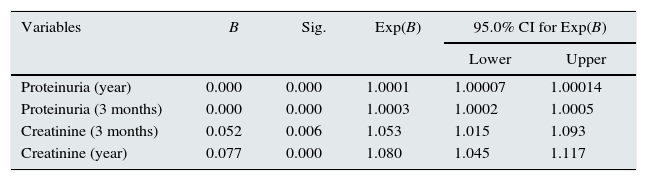
Analysis of factors influencing graft survival timeRisk factors for graft loss were found to be: proteinuria and serum creatinine. The resulting model formed by the variables is shown in Table 8.
- -
Serum creatinine: The risk of graft loss was 1.053 and 1.077 times greater for every milligramme of creatinine at three months and one year, respectively.
- -
Proteinuria: The risk of graft loss was 1.03 and 1.01 times greater for every 100 milligrams of urinary protein excretion at three months and one year, respectively.
Cox regression: graft survival.
| Variables | B | Sig. | Exp(B) | 95.0% CI for Exp(B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Proteinuria (year) | 0.000 | 0.000 | 1.0001 | 1.00007 | 1.00014 |
| Proteinuria (3 months) | 0.000 | 0.000 | 1.0003 | 1.0002 | 1.0005 |
| Creatinine (3 months) | 0.052 | 0.006 | 1.053 | 1.015 | 1.093 |
| Creatinine (year) | 0.077 | 0.000 | 1.080 | 1.045 | 1.117 |
We studied a population of 1815 kidney transplants performed since 2000 with a follow-up period of up to 12.25 years to analyse the impact of proteinuria on renal transplantation outcomes.
Analysing factors that can influence the development of proteinuria, we found donor-related factors, such as cause of death (CVA vs non-CVA), to be determinants. In our case, the groups with the highest levels of proteinuria were recipients from donors who had died from cerebrovascular causes. It might be assumed that grafts from donors who have died from a CVA would have a worse cardiovascular profile. Since we believed that this relationship might be influenced by donor age, we analysed the cause of death, whether CVA or not, according to quartiles of donor age and found significant differences: as expected, there were more deaths from CVA in older age groups. We also found differences in proteinuria according to quartiles of donor age, especially at three months after transplantation, where donor grafts of the extreme age quartiles (1st and 4th) were those with most proteinuria. This leads us to believe that the age of the donor also influences the development of proteinuria, although we only found differences in the analysis of donor age at three months and not at one year by proteinuria groups. Recent studies18–21 show that older donor age has a negative effect on renal graft, both in deceased-donor and living-donor donation, but none of these studies analyse proteinuria. Many authors have found a higher degree of delayed renal function in older donors.22 In our case, delayed renal function was associated with higher levels of proteinuria and a greater risk of graft loss.
Moreover, in addition to our group, other authors23 have found that higher pre-transplant levels of cytotoxic antibodies negatively affect graft outcome; in our case, we found that patients with higher antibody levels had more proteinuria and, indirectly, worse graft outcome.
Our patients who had more post-transplant hypertension had more proteinuria and worse graft outcome, with post-transplant hypertension acting as an independent factor for graft loss. This could be explained by the fact that hypertension is an expression of multiple factors, some graft-related, some recipient-related and others related to immunosuppressive therapy or chronic renal disease,24 which continue after the transplant as cardiovascular disease; in this clinical setting, we know that the renin–angiotensin–aldosterone axis (RAAS) is activated and also, that this activation favours the onset of proteinuria.25 Improvement of proteinuria and its consequences have also been reported after treatment with RAAS blockers.26 In our case, the percentage of patients treated with angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (AIIRAs) at three months after transplantation was small and no differences were, therefore, found between proteinuria groups.
Immunosuppressive induction therapy affects the renal graft.27 We analysed induction therapy and, although we did not find it to be a risk factor for graft loss, we did find that patients who had received thymoglobulin had significantly more proteinuria one year after transplantation than those treated with anti-CD25 or those who had received no induction. Although this finding may seem paradoxical, one explanation could be that in our region, use of thymoglobulin induction is rare and confined to patients with high levels of anti-HLA sensitisation pre-transplant.
We found that post-transplantation proteinuria (at three months and one year) is predictive of graft outcome and is associated with poorer renal function, a lower graft survival rate and even with higher patient mortality rates. In the literature, there is evidence that even for the same degree of renal dysfunction, increased proteinuria can increase the risk of cardiovascular death.28 Oblak et al. recently reported that patients who have had an episode of acute rejection followed by increased proteinuria have worse outcomes than those who have suffered rejection but no increase in proteinuria,29 with both worse graft survival and increased risk of death. Some authors, such as Roodnat,30 believe that proteinuria affects not only graft survival, as we found, but also that of the patient. In our multivariate analysis, we failed to demonstrate that severity of proteinuria was associated with worse recipient survival. In the Kaplan–Meier study, higher levels of proteinuria at one year correlated with worse patient survival, but this may only reflect their association with worse renal function, which, as in the majority of published studies, was identified as a predictor of mortality in the adjusted analysis.
However, we did find that the changes in proteinuria over time seemed to significantly affect transplant outcome, worsening patient and graft survival in those in whom proteinuria worsened between three months and one year post-transplantation. Proteinuria remained stable in more than two thirds of our patients between three months and one year post-transplantation, while it worsened in 12% and improved in 18%.
Compared to cases in which proteinuria improved or remained stable between three months and one year, recipient survival was significantly worse in the group of patients with worsening proteinuria, regardless of the level they started with (<300mg or 300–1000mg/day). Nevertheless, improvement in their proteinuria had no effect on patients survival.
We think that in these patients with improvement in proteinuria (18%), this could be related to the problems after transplant and disappears in the first months, but not with graft pathology. This group, which improves proteinuria has not improved survival, possibly because the proteinuria was not a problem relative to the graft.
When analysing graft survival overall for all patients, we found that the factor most likely to worsen survival is progression of proteinuria. However, when survival for each proteinuria group at three months and one year were analysed separately, here we did find that progression of proteinuria was associated with worse survival rates, while improving proteinuria also improved graft survival, in both cases compared to patients who remained stable. It might be expected that the difference in survival would be related to a parallel worsening in renal function, but we found no differences in serum creatinine at one year in terms of whether the proteinuria progressed, remained stable or improved. We found that there were differences at three months, with renal function being worse in the group in which proteinuria subsequently improved. This may be the result of immediate post-transplant factors that could be resolved, allowing both proteinuria and renal function to improve.
We are therefore able to state that the progression of proteinuria in the first year after transplantation, on its own, will predict long-term graft survival. The mechanism underlying this association is beyond the scope of this work. Proteinuria, especially its progression, may indicate one or more ongoing damaging processes, such as chronic rejection, recurrence of underlying disease or overload of a kidney which was inadequate due to donor characteristics. Moreover, high-level proteinuria may itself exacerbate the progress of the organ.
Based on these results, we believe that in addition to analysing the degree of proteinuria at a particular time, we should monitor changes over time.
In another vein, our analysis found that factors that appear to increase the risk of proteinuria at three months, such as donor age, disappear at one year, while the cause of death of the donor remains among the factors that influence the development of proteinuria both at three months and one year.
In our case, as had previously been found by authors of our group,31 the Cox regression showed the age of the recipient as mortality factors. In addition, renal function, as measured by serum creatinine, at one year post-transplant was also a determinant of patient survival.
In the Cox analysis for graft survival, proteinuria, renal function at three months and one year post-transplant were predictive factors for graft loss.
In conclusion, we found that proteinuria is a marker of poor renal graft outcome, especially when it progresses. Although its cause may be multifactorial, it is clear that we can consider it as an epiphenomenon of a series of events that will ultimately lead to a worse outcome for the transplanted patient.
Conflict of interestsThe authors declare no conflict of interest.
We are grateful to Carmen Rosa Garrido and Francisco José Borrego Utiel for statistical advice.