Peritoneal dialysis is a treatment alternative in patients with advanced chronic kidney disease. The infusion of liquid into the peritoneal cavity leads to an increase in intra-abdominal pressure, which can sometimes produce leaks to the chest, giving rise to pleuroperitoneal communication. This is not a common complication, but it brings about high drop-out rates among patients using the technique. Diagnosis is easy and must be suspected in patients with sudden dyspnoea with low ultrafiltration and pleural effusion in the chest x-ray. Peritoneal rest and a temporary transfer to haemodialysis, and pleurodesis can be effective treatment strategies.
La diálisis peritoneal es una alternativa de tratamiento en los pacientes con enfermedad renal crónica avanzada. La infusión de líquido en la cavidad peritoneal conlleva un aumento de presión intraabdominal que, en algunas ocasiones, puede producir la fuga del mismo hacia el tórax dando lugar a una comunicación pleuro-peritoneal. Es una complicación poco frecuente, pero supone una alta tasa de abandono de la técnica. El diagnóstico es sencillo y se debe sospechar ante la existencia de disnea súbita con baja ultrafiltración y derrame pleural en la radiografía de tórax. El descanso peritoneal, con transferencia temporal a hemodiálisis, y la pleurodesis pueden ser estrategias eficaces para su tratamiento.
INTRODUCTION
Peritoneal dialysis (PD) is a replacement therapy option for patients with advanced chronic kidney disease. During this procedure there is an increase in intra-abdominal pressure1,2 due to the accumulation of dialysate in the peritoneal cavity. Among the complications secondary to this increase in pressure is the leakage of peritoneal fluid through the diaphragm to the thorax, known as pleuroperitoneal communication3 or secondary hydrothorax, described in 1967 by Edward and Unger.4 The mean incidence has been estimated at between 1.6% and 10%,5,6 although it may be higher as hydrothorax involving small amounts of fluid are difficult to diagnose. Its aetiopathogenesis is not fully understood, but it has been related to congenital or acquired pleuroperitoneal defects7 and to lymphatic drainage disorders.
The aim of this article is to analyse the incidence of this complication in our hospital setting and expound our experience in diagnosing and treating it.
CASE REPORTS
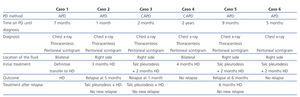
We have reviewed the medical records of all the patients treated with PD in our unit between 1997 and 2010. During this time, 328 patients began this therapy in the La Paz University Hospital in Madrid. Six were diagnosed with pleuroperitoneal communication. Here, we present the cases in chronological order, from the first to be diagnosed to the most recent. Table 1 shows a summary of the cases including the method of diagnosis and the initial treatment.
Case 1
A 55 year old woman with chronic renal failure (CRF) secondary to reflux nephropathy who began renal replacement therapy with automated PD (APD) in 1983. She received a kidney transplant 4 years later from a cadaveric donor, developing chronic graft nephropathy requiring renal replacement therapy 11 years later. She began APD again and 7 months later presented symptoms of dyspnoea with a significant volume overload and a loss of ultrafiltration in the preceding days. A chest x-ray showed mainly right-sided bilateral pleural effusion. In view of suspected pleuroperitoneal communication a peritoneal scintigraphy was performed which confirmed the diagnosis. Given the severity of her condition, the patient was transferred to haemodialysis (HD) on a definitive basis.
Case 2
A 60 year old man with CRF of unknown origin under treatment with APD since 2000. A month after beginning treatment he presented symptoms of progressive dyspnoea, loss of ultrafiltration and volume overload. A chest x-ray was performed showing massive right-sided pleural effusion. A thoracentesis was performed, the analysis of the pleural fluid showed a glucose concentration higher than the plasma concentration, suggesting possible pleuroperitoneal communication. Peritoneal scintigraphy with 99Tc-labelled albumin was carried out, confirming the diagnosis of pleuroperitoneal communication. The patient was transferred to HD for 3 months, and then he began APD again. After 5 months' treatment with APD there was evidence of a relapse of the pleuroperitoneal communication, so talc pleurodesis was carried out. After 2 months on HD the patient started PD again and had no other related complications in the following 3 years.
Case 3
A 52 year old man with CRF secondary to membranoproliferative glomerulonephritis (GN) under treatment with continuous ambulatory PD (CAPD) since February 2001. Two months after beginning treatment he presented acute respiratory failure with volume overload. He underwent a chest x-ray, thoracentesis and peritoneal scintigraphy, resulting in a diagnosis of pleuroperitoneal communication. Talc pleurodesis was performed with peritoneal rest for 2 months. A month after beginning CAPD again, he suffered a relapse of pleuroperitoneal communication, so pleurodesis was performed again. He began CAPD again 3 months later, continuing the treatment for 6 months until receiving a kidney transplant. The patient suffered no new complications during that period.
Case 4
A 74 year old man with CRF secondary to nephroangiosclerosis under treatment with CAPD since September 1998. In October 2001, after 2 years of treatment, he began to suffer symptoms of dyspnoea and a chest x-ray showed bilateral pleural effusion. Peritoneal scintigraphy provided confirmation of the diagnosis with tracer uptake in the base of the left hemithorax. He was transferred to HD and after 4 months peritoneal scintigraphy was performed showing that a small amount of tracer still entered both hemithoraxes. PD was begun again as it was requested by the patient. After 6 months he was transferred to definitive HD due to Candida albicans peritonitis, before which there had been no evidence of a relapse.
Case 5
A 51 year old man with CRF secondary to chronic pyelonephritis due to a neurogenic bladder and vesicoureteral reflux. After 10 years' treatment with HD he was transferred to APD in June 2006 because of vascular access problems. After 9 months' treatment with DP he began to suffer from progressive dyspnoea and a CT scan of the abdomen and thorax confirmed moderate right-sided pleural effusion. A diagnostic thoracentesis was performed revealing higher levels of glucose in relation to plasma in the peritoneal fluid. The diagnosis of pleuroperitoneal communication was confirmed with peritoneal scintigraphy with 99Tc-labelled albumin. Talc pleurodesis was performed and the patient was transferred temporarily to HD. After 2 months' peritoneal rest he began APD again with a dry day. Six months later, after beginning continuous cyclic peritoneal dialysis (CCPD), he presented symptoms of dyspnoea and a chest x-ray revealed right-sided pleural effusion, probably related to a relapse of the prior pleuroperitoneal communication, so he was transferred to HD for 6 months. At present he is under APD treatment with a dry day, with no new complications after 2 years' follow-up.
Case 6
A 47 year old woman with CRF secondary to nephroangiosclerosis under treatment with CCPD since June 2009. After 5 months of treatment, she presented symptoms of dyspnoea with minimal effort and was unable to tolerate the decubitus position. A chest x-ray was carried out showing massive right-sided pleural effusion. The diagnosis was confirmed using peritoneal scintigraphy, which showed the flow of peritoneal fluid from the abdominal cavity to the right lung field. Talc pleurodesis was performed and the patient transferred to HD for 2 months. Then, she returned to DP without relapse of the pleuroperitoneal communication after 10 months on this treatment.
DISCUSSION
Pleuroperitoneal communication is a rare complication in patients on DP, but it results in a high drop-out rate. In our series we observed an incidence of 1.84%, which is a slightly lower percentage than in other series in the literature.5,6 Although most of the cases traditionally described have been women, only one patient in our series was female, with the added peculiarity that she had been treated with DP for 4 years in a previous stage without any complications. The cause of the CRF has also been associated with this complication, it being more common in patients with hepatorenal polycystic disease9 due to an added increase in intra-abdominal pressure. In our series there were no patients with polycystic kidney disease. With regard to the location, 2 of our patients suffered from bilateral pleuroperitoneal communication, which is uncommon according to the medical literature, where a higher prevalence of right-sided only hydrothorax is reported.10 It has been suggested that this could be related to a malformation in this location.1,6,11 Gagnon and Daniels proposed the existence of embryonic remnant, the persisting pneumatoenteric recess, which allows fluids to pass from the peritoneal cavity to the right pleural space.3
Diagnosing this problem is simple but clinical suspicion is crucial. It is necessary to rule out pleuroperitoneal communication when faced with a patient treated with DP presenting with dyspnoea of more or less sudden onset, loss of ultrafiltration and pleural effusion.7 If a pleural fluid sample obtained from a thoracentesis shows that the glucose concentration is higher than that of the plasma, positive diagnosis can be suspected.12,13 Peritoneal scintigraphy has been shown to be very effective at confirming the diagnosis of this anatomic disorder.14,15 It shows how the radioactive isotope passes from the peritoneal cavity through the pleura to the thorax. Figure 1 shows the different images corresponding to a peritoneal scintigram of a patient with this complication.
There are several treatment options: the conservative option, pleurodesis or surgery. None of these has been shown to be better than the others so the decision depends on the patient's clinical condition and their preference. Patients should be previously informed of the risks and benefits of the different options.16 Pleuroperitoneal communication is a clinical situation with little relevance outside the context of PD, thus, conservative treatment may be the most suitable option for patients who will be transferred to haemodialysis. In our series, one of the patients has not had a relapse since beginning HD.
Once the diagnosis has been confirmed, the most important step to take is the temporary, or even permanent, interruption of PD, which is determined by the magnitude of the communication and the needs of the individual patient. Conservative treatment has been seen to be effective in approximately 50% of patients,5 however, our attempt to treat one patient with peritoneal rest was unsuccessful. In some patients with preserved residual diuresis low-volume CAPD could be continued17,18 or low volume APD and a dry day.19,20 However, this option could lead to under-dialysis and is not viable with anuric patients. In our hospital, patients were treated with peritoneal rest with a temporary transfer to HD,21 which is an essential decision, particularly if the pleural effusion results in breathing conditions. Talc pleurodesis is a safe and effective treatment for pleuroperitoneal communication.22 In our series, it was performed on 3 patients, 2 at onset and one after a relapse. With one patient it was necessary to carry out the procedure twice, but there were no complications during the procedure in any of the cases. These patients wanted to continue on PD and pleurodesis was chosen for this reason. PD was never maintained because there was a contraindication for a definitive transfer to HD, as would be the case if there were no vascular access. There are other treatment options such as tetracycline pleurodesis, which provides similar results to talc pleurodesis,23 or pleurodesis with autologous blood, which has had inconsistent results.24-26
Video-assisted thoracoscopic surgery allows for the direct visualisation of the diaphragm and malformations in this area.27 If the condition is associated with a morphological disorder, a thoracotomy and direct repair is mandatory. Although it is an aggressive treatment, it offers a high rate of success. However, although surgery is very effective,28 it is reserved as the last treatment option as it is not devoid of risks.
In general, with both conservative or surgical treatment, up to 58% of patients can continue on PD treatment.21 However, the relapse rate is generally high, which is why the results with the different treatments are not very encouraging6,29 and a high percentage of cases require a definitive transfer to HD30. This means that it is not possible to give clear directions in favour of one treatment or the other.
CONCLUSION
Pleuroperitoneal communication is a rare complication in patients on PD, but it leads to a high drop-out rate from the treatment. Its diagnosis is easy and its clinical suspicion is extremely important. The treatment involves peritoneal rest accompanied or not by pleurodesis.
Figure 1a. Peritoneal scintigram with Tc-99m nanocolloid tracer.
Figure 1b. Peritoneal scintigram with Tc-99m nanocolloid tracer
Figure 1c. Peritoneal scintigram with Tc-99m nanocolloid tracer
Table 1. Description of the series