INTRODUCTION
The sustainability of our health system is an increasingly debated topic, and not just because of the current financial crisis. Nephrologists have long been sensitive to this problem,1,2 leading to the NEFROLOGÍA Special Edition in 2010 and other recent articles.3-5
Table 1 gives an idea of the magnitude of the problem.
The annual cost per patient on dialysis renal replacement therapy (RRT) is much higher than for many other chronic diseases. Its impact on the Spanish health system budget is considerable, with 0.1% of the population (46 000 patients) consuming 2.5% of the health budget.
Achieving that sustainability is a priority for the heads of nephrology departments, scientific societies, public and private health care managers, the government and, of course, patients.
However, there are certain aspects that must be considered. It is true that Spain is a leader in kidney transplantation, but this therapy is not applied to 80% of –increasingly elderly– patients who start dialysis. Although much less newsworthy, the most efficient therapy for chronic kidney (CKD) disease is secondary prevention to reduce the number of patients requiring dialysis or transplantation. Renal transplantation is the most efficient replacement therapy and dialysis, however, is the treatment of choice for 80% of RRT incident patients, so we are going to try to clarify some misunderstandings about its cost and effectiveness.
EFFECTIVENESS OF RENAL REPLACEMENT THERAPY
Putting renal transplantation to one side, the majority of records from around the world show that haemodialysis (HD) and home peritoneal dialysis (PD) have very similar long-term survival. PD has better results than HD during the first years of treatment,6,7 which is probably related to the better maintenance of residual renal function.
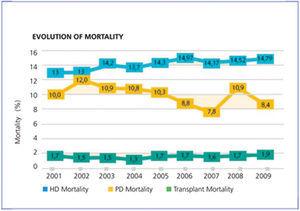
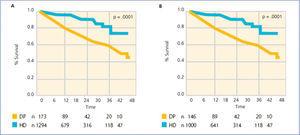
Something similar can be seen in Spain. Figure 1 shows the evolution of mortality from the most recent Spanish registry. Consistently, year after year, increasing survival can be observed for transplant patients, compared to those on dialysis modalities. However, survival for home PD is always greater than for HD. For vital organ replacement therapy, only quality of life-adjusted survival is a definite indicator. It could be argued that the Spanish registry data are not adjusted by are. However, recently this journal published Canary Islands registry data for all incident patients between 2006 and 2009 (Figure 2, reference 7). It can be seen that PD patients have improved survival at the start of treatment and at 46 months follow-up. Moreover, this situation is maintained for all subgroups analysed: those older and younger than 65 years, men and women, and diabetics and non-diabetics.
QUALITY OF LIFE IN DIALYSIS
The perception of quality of life by patients is usually better in those on PD, even in the elderly.8,9 It is also better for home HD, and in both cases there is a close relationship with patient selection bias.10 In the NECOSAD11 study, the quality of life coefficients were 59.1 and 54.0, but it strictly considered health-related quality of life (not social, family or work-related adaptation, which are better for therapies at home).
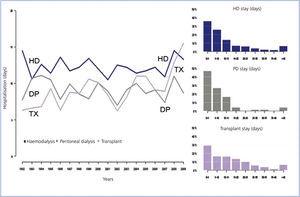
Two of the most important factors in adjusting for quality of life are number of hospitalisations and length of stay. During this time, the quality of life adjustment factor for calculating years of life gained is zero, and home PD has about 3 days less per patient-year of hospital stay (Figure 3).
CALCULATION OF DIALYSIS COSTS
Leaving aside the kidney transplant RRT mode, which should be used whenever organ availability and patient characteristics allow, home PD is the most cost-efficient alternative, as shown in multiple publications in Spain and throughout the world.5,12,13
The cost analyses usually have several methodological problems, as summarised in a recent “Editorial”.14 The first point to note is that all costs involved must be included when evaluating the various dialysis costs, where available. In vital organ replacement therapy, whose alternative is death (with zero health costs, of course), almost all the health costs of the patient can be attributed to dialysis, as it is “responsible” for patient survival and therefore comorbidity costs. In addition, there are the costs of access (also unplanned and temporary access, and the consequent hospitalisations), depreciation, consumables, nephrology department operating expenses, equipment maintenance, outsourced services, inpatient and outpatient medication, the costs of complications (at least those directly derived from the RRT or kidney failure), transport, training, indirect costs of patient mortality and morbidity, especially ordinary hospital admissions and stays and those in intermediate and intensive care units, and the costs of the dialysis sessions themselves.
However, sometimes simplifications made about dialysis costs can be confusing. Below are some examples to clarify some important issues:
Often data on average dialysis costs are incomplete and biased (in the interests of the source). For example, by comparing continuous ambulatory peritoneal dialysis (CAPD), automated peritoneal dialysis (APD) and all their supplements with HD outsourced to private hospitals without any supplement (on-line haemodiafiltration, more than 3 sessions per week, etc). The costs of night duty or reserving HD monitors at referral hospitals, dialysis accesses, training, admissions, medication, etc. are not evaluated.
It is often assumed that all PD patients, both on CAPD and on APD, use supplements (bicarbonate and icodextrin solutions). In fact, a large number of patients do not use supplements. For example, icodextrin is not available in 2 of the 3 companies performing these services. This deviation would erroneously increase the estimated annual cost for all home PD modalities by between €2000 and €3000. Similarly, it is assumed that all APD patients use a high volume APD, when the case is exactly the opposite.
In Spain, the majority of patients starting RRT on PD do so with CAPD, which has the lowest price. There are patients who begin treatment with APD due to lifestyle or social needs preferences (e.g., to continue at work or school). When this happens, almost all use a much cheaper APD prescription, the low volume APD. The annual cost is more than €4000 cheaper than that reported in a recent “Editorial”.3
However, when the same study is performed with HD, it is assumed that the transportation cost for all HD patients is €10 per session: €5 each for the outward and return journeys. Obviously, this will be the cost for some patients, but in general this is not the case and many patients have to manage with a significantly higher cost. For example, another recent study15 revealed much higher costs. It estimated an average transport cost of 8.1% of the total (€2700 per patient-year), and of 9.7% (€3200 per patient-year) for services outsourced to private centres, which is twice that indicated by “the industry”.3
In fact, we believe that even these latter costs are underestimated, considering the differences between autonomous communities in geographical extension, outsourcing charges, and medical transport systems.
Some autonomous communities still apply a surcharge for bicarbonate in HD, which can cost between €8 and €15 per session, which is between €1248 and €2340 per patient-year. Some apply surcharges for using high-flow or biocompatible membranes, which cost between €6 and €23 per session, i.e. between €936 and €3588 per patient-year.
In some communities, on-line haemodiafiltration (HDF) requires a supplementary fee of between €25 and €33 per session, i.e., 20% more for each HD session, between €3900 and €5148 per patient-year.
Many cost studies assume that all HD patients undergo dialysis 3 times a week, which is not the case. Although it is incomplete, according to the latest registry of the Spanish Society of Nephrology (S.E.N.), 687 patients undergo dialysis 4 times a week, 138 five sessions a week, and 200 six sessions per week (about 1000 patients in total). Each additional session increases the costs for these patients by 30%, €7120 per patient-year.
OTHER INACCURACIES AND INCORRECT ESTIMATES WHEN CALCULATING COSTS
When comparing HD and PD, we often forget to include the cost of the public HD, which is absolutely necessary. Outsourcing HD to private centres relies heavily on public resources, without which it could not be done.
For all sorts of problems that HD patients may have, e.g., vascular access, the public resources, doctors, nurses and other public staff, beds etc. are always there 24 hours a day, 365 days a year. This entire clinical arsenal (buildings, equipment, other specialists) is available to outsourced HD patients, and must be included when calculating the costs. The counter-argument might be that this type of infrastructure costs should also be included in home techniques; however, as previously stated, the use of these resources (with the main cost being hospital admissions) is much lower in the case of PD.
Therefore, in calculating HD costs (and also PD), an average of the HD costs in public hospitals must also be calculated, because they support the outsourcing of HD to private centres. If there were no public hospitals to treat patients with HD and PD, then neither outsourced HD nor home PD could be done.
In the last most comprehensive comparative study performed in Spain,13 HD was estimated at 47% more expensive than PD on average. However, this study was also missing some data.
MORE INFORMATION TO ADD TO COST DIFFERENCE
As noted above, when comparing all HD costs against PD in Spain, with the current public-private distribution for PD and the existing APD-CAPD distribution, the result is more favourable to PD (as it is worldwide), by more than €12 000 per patient-year.
The publication quoted13 suggested that this difference is even greater, by more than €2300 per year, considering the cost of incident patients and indirect costs, making it an annual saving of €14 300 per patient-year for home PD. A currently unpublished ALCER survey among patients in working age showed that the difference in indirect costs is highly significant since it shows that more APD patients are employed compared to HD patients.
The cost of drugs is another important part of the costs for dialysis patients. Usually, only hospital costs are considered, among them, EPO costs, which are double in HD than in PD (€2382 compared to €1245 per year), although the difference between non-calcium and calcium binders can be up to €2000.16-18 However, these calculations are published only in HD. No reference to the costs of phosphate binders in PD was found, but it is well known that sevelamer is rarely used in PD, where calcium binders at low doses are preferentially used. This may provide an economic advantage for PD over HD of up to €2000 per year, depending on the use of non-calcium binders in the HD unit evaluated. Also, antihypertensive drugs, which are also less commonly used in PD, may involve an additional €500 reduction.
The cost of hospitalisation is another factor which is insufficiently analysed. As noted in the Basque Country registry throughout the whole 15-year period of data collection, home PD has always involved less days of hospitalisation per patient-year than HD (Fig. 3). In addition, in recent years, it has less hospitalisation days than transplant patients. While hospital stay length for patients on HD and PD have been constant, with a difference of 3 or 4 days between the 2 techniques, the hospital stay for patients undergoing transplantation has been showing a rising trend, probably due to the increasing age of this group.
Using an average cost of €600 per patient per day staying in a public hospital, home PD would save another €1800 annually, compared to HD, i.e., all the differences in costs between HD and PD might be more than €18 000 in savings per patient-year for home PD.
PERITONEAL DIALYSIS AS A SHORT-TERM THERAPY
This is a mistake that is constantly seen in the literature. Without any references, or at least any recent references, it is still claimed that PD can only last 2 years, due to the loss of capacity of the peritoneum, peritoneal infections or exhaustion of the patient or caregiver.
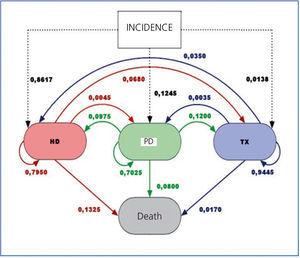
However, when analysing the transitions between different states (or therapies) –see Figure 4– in the Markov model used for cost analysis (data taken from the Spanish Registry), we see that PD mainly leads to transplantation (at twice the rate than from HD). Also, although the rate of transfer from PD to HD is twice that of the reverse, patients who transfer from PD to HD have good survival, as opposed to those moving from HD to PD.19,20 Mortality is also lower in PD.
The median survival time in the PD in Spain (Baxter's internal data) is 65 months. Without adjusting for age and comorbidity, it seems similar to that of HD. The drop-out from PD, except for those receiving a kidney graft (which is good and desirable) has a well established correlation with peritoneal infections. It seems strange that in 2011 we still have to remind ourselves that the peritoneal infection rate in PD is less than 1 event every 2 years per patient, and that most patients have no such event over a number of years.
The weariness of the caregiver and other social problems are no more frequent in PD patients than in HD, but are “perceived more” in PD patients. However, health professionals in hospital do not look at the harmful effects of HD on the working life of young patients who, after a period in HD, undergo a transplant and continue unemployed due to HD limitations. When calculating social costs, the difficulty of finding employment after a cure is not taken into account. This is because cost calculation methods are developed in countries without unemployment rates as high as Spain.
FOCUSING ON THE PATIENT, NOT ON THE TREATMENT
In any case, the phrase heard many times, “focus on the patient”, is shedding more light on a better way forward. Each individual patient must be provided with the best quality of life, the best treatment at the lowest cost at all times and at every stage in the evolution of a chronic treatment. This will help further the sustainability of the system.
Predialysis visits with minimal resources (a doctor, a nurse and 2 offices on part-time) generate huge savings for the national health system: reducing the progression of chronic renal failure to advanced stages,21 offering the opportunity to undergo a transplant without first undergoing dialysis and choosing a home dialysis technique. It also improves survival if dialysis is not started on an unscheduled basis.
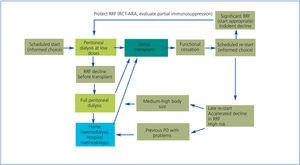
Therefore, the best way forward is to perform a transplant as soon as possible for eligible patients, try to include them on the waiting list in the predialysis stage using an advanced-stage rnal disease programme and use efficient models recognised by the majority, as summarised in Figure 5, which proposes an orderly strategy for RRT for renal transplant eligible patients.22
Home PD is an excellent form of initial treatment for any patient. It has clear benefits for many, but is highly recommended for that group of patients who are eligible for a kidney transplant. Having the best renal transplantation results in PD patients is also an efficiency factor attributable to the dialysis modality.
There is evidence collected in Spain23-25 suggesting an excess of despotism in prescribing RRT. When patients are adequately informed about their treatment options, half of them choose home therapies. However, the forced use of home therapy logically leads to poor results.26 Treatments must be planned to make them “really” complementary, offering the best treatment at the best price at all times.
IMPROVING PLANNING
Recent simultaneous openings of new hospitals with HD departments –often oversized– are another issue to be considered. The perception of inefficiency resulting from “empty beds” in HD encourages nephrologists to fill them, precisely so that the cost of HD does not increase dramatically. It is not allowed to have half-busy nursing staff attending to 2 HD machines only, or to have machines not being used (it is often ignored that the cost of the machine and its maintenance are a small fraction of the cost of dialysis and that this fraction is less than the cost of transporting patients to HD.)
In addition, regarding HD treatment, decisions are taken which in other fields of health care would be incomprehensible. When new HD facilities or new treatment centres are opened, it is usually done on a massive scale. It is common practice to exceed the real demand when calculating the infrastructure for patients in HD. Therefore, so that costs are not excessive, this oversized capacity must be filled with more staff and more patients, preventing patients from accessing to home therapy in equal conditions.
Over time, more and larger HD centres have been created because HD benefits greatly from economies of scale. Currently, in certain areas, there are too many HD stations; and it is known that the available number of HD stations has a negative influence on home PD use.
A recent “Editorial”27 summarised the structural causes leading to a much greater and unjustified use of HD over PD. We can see that there is a
greater use of PD in the public budget supported health systems throughout the world (United Kingdom, Canada, Australia, New Zealand and Scandinavian countries), in opposition to private practice, based on the fee-for-service reimbursement system (Belgium and Germany).28-30
Another factor in some countries is the expansion of the big providers of (haemo)dialysis.31 For example, the decline in the use of PD in dialysis patients since 1996 in the USA, from 14% to 8%, is parallel to the change in the ownership of dialysis units to major suppliers (from 39% in 1996 to 63% in 2005).28
Home HD has also been adversely affected by these factors. The possibility of RRT is not offered in many centres when empty HD stations, along with nursing staff who could teach the technique and are available to training patients who wish to perform dialysis at home.
DISCUSSION
We do not dispute that a transplant is more efficient than dialysis, even though its actual costs are far from being well known (especially those resulting from hospital admissions). However, while reality shows over and over again that survival and costs favour the use of home PD, as supported by several studies from many other countries, we have to remind ourselves about this form of treatment from time to time, as habits and interests often deny the evidence.
This is probably because the PD modality is not very well known,32-35 and has always been used less than HD. It may also be connected with the domestic and individual nature of using PD at home, compared with the apparently “more technological” HD.36
However, sustainability has not always been considered as a problem. Even 10 years ago, it was argued that one of the qualities of hospital HD was that it created more healthcare jobs.36 We are still in the learning process, so we should repeat once again that in the current situation, home PD is more efficient than HD. According to the data presented above, we could save up to €25 000 by using PD. This would be more than €40 000 by quality-adjusted life-year (QALY). These figures must be taken seriously. Economic concepts such as “limit that Society is willing to pay per life-year saved” are still hard for us to accept. In the Winkelmayer meta-analysis,37 a limit of $55 000 per life-year saved without quality adjustment was suggested, which is an amount exceeded by more than a third of our dialysis patients.
What can be done? Plan, close the circle, and go back to the beginning of this paper. Achieving sustainability for RRT is a priority that should occupy the heads of nephrology departments and scientific societies, public and private health care managers, the government and, of course, patients. The lack of information about alternative therapies is still a complaint of patients and their associations. However, patients cannot be adequately informed about therapies which are not known in depth by their doctors. Again we return to the lack of knowledge about PD, both by many nephrologists and health care managers, and consequently, by patients and the population at large.
PD training of resident doctors, retraining of staff, including PD at an equal level in nephrology conferences, and much more, can all be done by nephrologists. Let’s not lay all the blame on our health authorities. Regional differences and the evolution in PD use in some autonomous communities on the past year suggest that we ourselves are an important part of the problem.
KEY CONCEPTS
The sustainability of our health care system requires a profound reflection on the costs and efficiencies of our prescriptions.
Table 1. Health costs for different chronic diseases in Spain
Figure 1. Crude mortality in different renal replacement therapy (RRT) modalities
Figure 2. Comparison between survival in haemodialysis (HD) and peritoneal dialysis (DP) patients. Registro Canario de Enfermos Renales (Renal Disease Registry of the Canary Islands)
Figure 3. Length of hospital stay for patients undergoing renal replacement therapy (RRT)
Figure 4. Transitions between states (therapies)
Figure 5. Global strategy for renal replacement therapy