Cadmium (Cd) is an ubiquitous element in nature and high levels of Cd exposure is considered a risk factor for renal injury; however, their nephrotoxic effects at low-environmental exposure levels are debated.1,2
Cd accumulates in the proximal tubule renal cells where inhibits the mitochondrial respiratory chain and this results in mitochondrial dysfunction and free radical formation.3
Some of the urinary markers used to evaluate Cd nephrotoxicity are N-acetyl-β-d-glucosaminidase (NAG), α1-microglobulin (α1M), β2-microglobulin (β2M) and the kidney injury molecule (KIM-1).4 The aim of this study was to search for the effects of urinary cadmium excretion on markers of early renal injury in population living in suburban communities in central Mexico.
The study was done in 7 communities located close to Queretaro city in Central Mexico; farming and agricultural practices are common but these areas are rounded by manufacturing activities. We evaluated 90 voluntary healthy subjects (≥20 years old), using a simple probabilistic sampling procedure on every of the communities studied. Those with current urinary tract infection, previous diagnosis of kidney disease, liver disease, cancer, or other chronic disease, as well as pregnant women were excluded.
A questionnaire was used to obtain information on personal health history, and risk factors to Cd exposure. Blood pressure measurements were obtained with an aneroid sphygmomanometer after a 5min resting in sitting position, and blood samples were taken after a fasting ≥8h during the same visit.
GFR was calculated with the CKD-Epi formula and spot urine samples for albumin, α1-microglobulin and cadmium analysis were collected in cadmium-free containers. Albumin and α1M were creatinine adjusted.
Cd measurements were done at the Department of Environmental Toxicology Laboratory (San Luis Potosi Medical School); and quantification was carried out with a Perkin-Elmer 3110 atomic absorption spectrometer.5
We categorized urinary Cd excretion in two groups according to CdU excretion and multivariate analysis was performed to identify risk factors for high Cd levels, albuminuria and higher urinary α1M. A p value ≤0.05 was considered as statistically significant and data were analyzed using the SPSS 23.0 software.
The overall analysis included 90 adults with no antecedent of occupational exposure to Cd, 66.6% of all participants were women and the mean age was 41±12 years; CdU median levels were 0.37±0.41μg/gCr and few patients (n=3) had CdU ≥1μg/gCr.
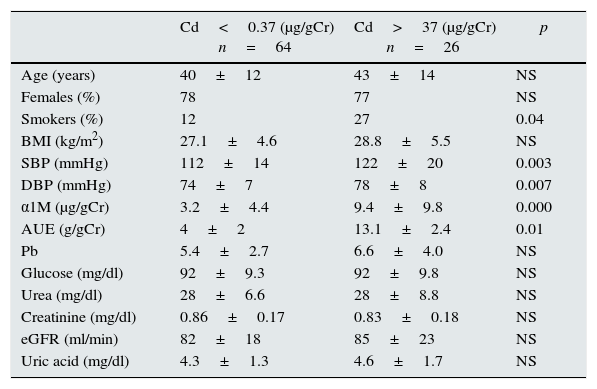
Those subjects with CdU≥0.37μg/gCr had higher levels of α1M (9.4±9 vs 3.2±4μg/gCr, p=0.001) and albumin excretion (13.1±24 vs 3.9±2.5g/gCr, p=0.001). Those patients with higher CdU excretion had a higher risk for α1M≥10 (OR 5.0, CI95 1.4–18.6, p=0.01) and micro-albuminuria (OR 20, CI95 1.0–39, p=0.04).
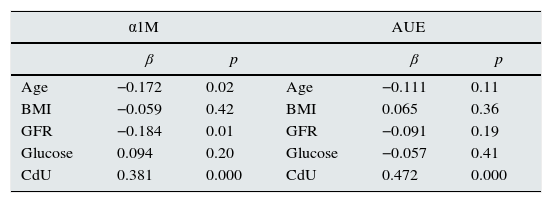
In multivariate analysis, CdU was the most important risk factor associated with higher α1M excretion and albuminuria after adjustments for age, BMI, smoking status, blood pressure, lead concentration and GFR.
In non-smoking subjects, those with CdU≥0.37μg/gCr had higher urinary excretion of α1M (7.5±7.2 vs 3.3±4.7μg/gCr, p=0.002) and albumin (11.1±18 vs 3.9±2.5g/gCr, p=0.003); CdU was associated with a higher risk for α1M≥10μg/gCr (OR 4.1, CI95 1.1–19, p=0.03), and CdU was the most important factor associated with higher α1M excretion.
This is the first study done in Mexico to evaluate the effects of Cd on kidney injury markers such as albumin and α1M, and in this study we showed that in this population living in central Mexico the non-critical levels of urinary Cd excretion had an important effect on markers of tubular injury, though association with low GFR was not found.
Cadmium levels in this study were just above to those reported in other countries such as the United States (media 0.26μg/gCr), Spain (media 0.28μg/gCr), or Korea (media 0.30μg/gCr)6,7; however, comparing the results with other studies that had shown nephrotoxicity associated to CdU, the threshold reported for CdU is higher (0.8–1.0μg/gCr),8,9 so our finding is of interest because the CdU levels analyzed in our study are in a range considered as non-nephrotoxic.
Tubular injury and urinary excretion of tubular injury markers are the first clinical manifestations of Cd nephrotoxicity, and some studies have found association between urinary α1M and tubular atrophy, GFR decline, and higher mortality; however its role in progressive kidney disease is controversial.10
Some limitations of this study are the small number of patients, the high representation of women, the lack of Cd environmental levels and of reliable epidemiological data about the prevalence of low GFR in the communities studied.
In conclusion, our study shows that non-critical Cd excretion is a risk factor associated with an increased excretion of markers of tubular injury and further work need to be done to test Cd as a possible toxin in the occurrence of CKD of unknown etiology in Mexico (Tables 1–2).
Comparison of demographic and laboratory characteristics according to CdU excretion.
| Cd<0.37 (μg/gCr) n=64 | Cd>37 (μg/gCr) n=26 | p | |
|---|---|---|---|
| Age (years) | 40±12 | 43±14 | NS |
| Females (%) | 78 | 77 | NS |
| Smokers (%) | 12 | 27 | 0.04 |
| BMI (kg/m2) | 27.1±4.6 | 28.8±5.5 | NS |
| SBP (mmHg) | 112±14 | 122±20 | 0.003 |
| DBP (mmHg) | 74±7 | 78±8 | 0.007 |
| α1M (μg/gCr) | 3.2±4.4 | 9.4±9.8 | 0.000 |
| AUE (g/gCr) | 4±2 | 13.1±2.4 | 0.01 |
| Pb | 5.4±2.7 | 6.6±4.0 | NS |
| Glucose (mg/dl) | 92±9.3 | 92±9.8 | NS |
| Urea (mg/dl) | 28±6.6 | 28±8.8 | NS |
| Creatinine (mg/dl) | 0.86±0.17 | 0.83±0.18 | NS |
| eGFR (ml/min) | 82±18 | 85±23 | NS |
| Uric acid (mg/dl) | 4.3±1.3 | 4.6±1.7 | NS |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; α1M: α1-microglobuline; AUE: albumin urinary excretion; GFR: glomerular filtration rate.
Multivariate analysis of variables associated with α1M and albumin urinary excretion.
| α1M | AUE | ||||
|---|---|---|---|---|---|
| β | p | β | p | ||
| Age | −0.172 | 0.02 | Age | −0.111 | 0.11 |
| BMI | −0.059 | 0.42 | BMI | 0.065 | 0.36 |
| GFR | −0.184 | 0.01 | GFR | −0.091 | 0.19 |
| Glucose | 0.094 | 0.20 | Glucose | −0.057 | 0.41 |
| CdU | 0.381 | 0.000 | CdU | 0.472 | 0.000 |
α1M: α1-microglobuline; AUE: albumin urinary excretion; BMI: body mass index; GFR: glomerular filtration rate.
The authors declare that they do not have conflicting interest.
The authors acknowledge support from grant Fondos Mixtos del Estado de Querétaro (FOMIX-CONACYT QRO-2009-C01-118970). The authors wish to thank Dr. Alberto Vázquez-Mellado for albumin determinations.