According to current guidelines, kidney donor candidates with controlled hypertension using 1 or 2 antihypertensive drugs may be considered as donor. However, this recommendation is based on the study that antihypertensive drug was initiated in mainly “after donor registration” and this may be white-coat hypertension because of donation-related anxiety. We compared the follow-up eGFR between kidney donors with preexisting hypertension and matched nonhypertensive donors.
MethodsThis single-center retrospective study classified 97 living hypertensive donors previously receiving antihypertensive drugs into two groups: 1 drug group (61 donors) and 2 drugs group (36 donors). We compared the follow-up eGFR between each donor previously receiving antihypertensive drugs and three matched nonhypertensive donors in terms of age, sex, and follow-up duration.
ResultsAt a mean (range) of 51 months (12–214) in the 1 drug group, and 54 months (12–175) in the 2 drugs group after donation, there was no significant difference in follow-up eGFR between hypertensive donors previously receiving antihypertensive drugs and matched controls in each group and in total donors. There was no difference in the incidence of the patients with follow-up eGFR<45mL/min/m2 in each group and their matched controls. Multiple linear regression analysis showed that baseline eGFR was the only independent predictor for the final follow-up eGFR in the total donors.
ConclusionOur results support the current guidelines that donor candidates with controlled hypertension using 1 or 2 antihypertensive drugs may be considered as donors, and may increase the strength of this recommendation.
Según las guías actuales, los candidatos a donantes con hipertensión controlada que utilicen 1 o 2 antihipertensivos pueden considerarse donantes. Sin embargo, esta recomendación se basa en el estudio en el que el fármaco antihipertensivo se inició principalmente «después del registro del donante» y esto puede ser hipertensión de bata blanca debido a la ansiedad relacionada con la donación. Comparamos la TFGe de seguimiento entre donantes de riñón con hipertensión preexistente y donantes no hipertensos compatibles.
MétodosEste estudio retrospectivo de un solo centro clasificó a 97 donantes hipertensos vivos que recibieron previamente fármacos antihipertensivos en dos grupos: 1 grupo de fármacos (61 donantes) y 2 grupos de fármacos (36 donantes). Comparamos la TFGe de seguimiento entre cada donante que recibió previamente fármacos antihipertensivos y tres donantes no hipertensivos compatibles en términos de edad, sexo y duración del seguimiento.
ResultadosA una media (rango) de 51 meses (12-214) en el grupo de un fármaco y 54 meses (12-175) en el grupo de 2 fármacos después de la donación, No hubo diferencias significativas en la TFGe de seguimiento entre los donantes hipertensos que recibieron previamente fármacos antihipertensivos y los controles emparejados en cada grupo y en el total de donantes. No hubo diferencia en el número de pacientes con TFGe de seguimiento <45ml/min/m2 en cada grupo y sus controles emparejados. El análisis de regresión lineal múltiple mostró que la TFGe basal fue el único factor de riesgo independiente para la TFGe de seguimiento final en el total de donantes.
ConclusiónNuestros resultados apoyan las directrices actuales de que los candidatos a donantes con hipertensión controlada que utilizan 1 o 2 fármacos antihipertensivos pueden considerarse donantes y pueden aumentar la fuerza de esta recomendación.
Several studies have shown an increased risk of end stage renal disease (ESRD) in the long term in living kidney donors compared to that in controls matched for baseline health. Mjøen et al.1 examined a Norwegian database and reported that 0.47% (9/1,901) of donors and 0.07% (2/32,621) of demographically matched healthy controls developed ESRD (adjusted hazard ratio [aHR] 11.38) after a median follow-up of 15.1 years. This study reported that an increased risk of developing ESRD in associated with hereditary factors, since most donors are first-degree relatives. Muzaale et al.2 compared national ESRD data between 96,217 US donors and healthy controls from the National Health and Nutrition Examination Survey (NHNES) III, and reported a 15-year cumulative incidence of ESRD of 30.8 per 10,000 in donors compared with that of 3.9 per 10,000 in matched healthy controls (donation-attributable risk of 26.9 per 10,000).
There is a concern that hypertensive donors may face an increased risk of worsening hypertension and kidney failure (e.g. nephrosclerosis) in the future.3 Talseth et al.4 reported that a higher pre-donation blood pressure was associated with a larger decrement in glomerular filtration rate (GFR) after donation. Fehrman-Ekholm et al.5 reported diagnosis of 4 out 6 living donors who had reached ESRD was nephrosclerosis, 1 histological and 3 clinical, which may be as a result of long-standing hypertension. We also reported that kidney donors with hypertension were significantly more likely to have a Modification of Diet in Renal Disease(MDRD)-GFR ≤60mL/min/1.73m2.6 In a combined cohort of donors and matched healthy non-donors in Norway, each 1mmHg increase in systolic blood pressure (SBP) was associated with a small increase in ESRD incidence at 2.5 years of follow-up.1,7
The 2010 Kidney Health Australia – Caring for Australasians with Renal Impairment (KHA-CARI),8 the 2012 British Transplantation Society Guideline,9 the 2013 European Renal Best Practice Guideline,10 and the 2017 KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors7 all recommended that donor candidates with controlled hypertension (usually <140/90mmHg) receiving 1 or 2 antihypertensive drugs without target organ damage may be acceptable as donors. We speculated that the recommendation “using 1 or 2 antihypertensive drugs” was based on the study by Textor et al.3 In this study, antihypertensive drug was initiated in 14 out of 24 hypertensive donors “after donor registration”. To avoid donors who might have had only white-coat hypertension due to donation anxiety,3 donors who were on previous antihypertensive drugs should be differentiated from those who were not. However, little is known about the effect of preexisting hypertension on post-transplantation residual renal function.11,12
This study was performed to compare follow-up eGFR between living hypertensive and matched normotensive donors.
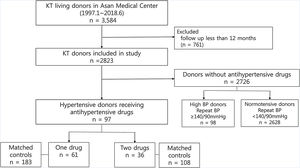
Patients and methodsStudy populationThis single-center retrospective study reviewed 3584 participants who underwent living donor nephrectomy from January 1997 to June 2018 at Asan Medical Center, a 2,743-bed tertiary referral university hospital in Seoul, South Korea. Of these, 761 donors with a follow-up period shorter than 12 months were excluded from the study. This is because previous studies have suggested that renal function at 1-year post-donation remains stable for at least the next decade.13 Hypertension was defined as antihypertensive drug use.11,14 Of the remaining 2823 donors, there were 97 hypertensive donors who received antihypertensive drugs, while 98 were donors who did not receive any antihypertensive drugs, but had an SBP≥140mmHg and/or a diastolic blood pressure (DBP)≥90mmHg after repeated measurement. There were 2,672 normotensive donors who were not previously receiving any antihypertensive drugs and had an SBP<140mmHg and a DBP<90mmHg. We classified these 97 hypertensive donors into two groups according to the number of class of antihypertensive drugs, not the number of tablets or dose. Finally, 61 donors were included in 1 drug group, and 36 donors in 2 drugs group. (Fig. 1).
Normotensive donors who were not previously receiving antihypertensive drugs and had an SBP<140mmHg and DBP<90mmHg after donor registration were classified as matched controls in this study. To avoid including untreated hypertensive donors as controls, high BP donors not receiving antihypertensives but had an SBP of ≥140mmHg and/or a DBP of ≥90mmHg from repeated measurements were excluded. Department of Biostatistics performed 1:3 matching for age, sex, duration of follow-up among controls who were not receiving antihypertensive drugs.
MeasurementsThe protocol for donor evaluation and selection was similar to that used in our previous study.6 Data on the following parameters were collected from medical records: donor age, sex, smoking history, height, weight, body mass index (BMI), SBP, DBP, serum creatinine, hemoglobin A1c (HbA1c), GFR based on urinary clearance of 99mTc-Diethylenetriaminepentaacetic acid (DTPA), left ventricular hypertrophy (LVH) prevalence in electrocardiography (ECG), and echocardiography before donor nephrectomy.
At the out-patient clinic, blood pressure (BP) was measured at least three times using a manual mercury sphygmomanometer or an automated device and the average taken for donor evaluation. Donors with an average BP>140/90mmHg were further evaluated using ambulatory blood pressure monitoring (ABPM) or home BP measurements and fundus examination. If repeated blood pressure values were within the normal range and there was no evidence of end-organ damage, the individuals were accepted as donors. Target organ damage may manifest as prior occurrence of a cardiovascular event such as myocardial infarction or stroke, urine albumin/creatinine ratio>30mg/g, hypertensive retinopathy, and/or evidence of LVH.7 However, we included the donors with LVH on echocardiography, because normotensive populations have LVH on echocardiography of 2.1%.15 We excluded the patients with other factors as donor.
Donor GFR of 90ml/min/1.73m2 or greater (previously 80) using one or more of the following measurements was considered to indicate an accepted level of kidney function:
- 1)
eGFR using the Chronic Kidney Disease-Epidemiology Collaboration equation (CKD-EPI)7 (previously Cockcroft-Gault or MDRD equation)
- 2)
Measured creatinine clearance
- 3)
Measured GFR using urinary clearance of 99mTc-DTPA
Those with diabetes mellitus, persistent hematuria, or proteinuria (current spot urine albumin/creatinine ratio>30mg/g; previous 24h urinary protein excretion>250mg) were rejected as donors.
Donors underwent routine follow-ups every year with nephrologists (52%), urologists (23%), or kidney transplant surgeons (25%). However, donors with first post-donation eGFR<60mL/min/1.73m2 underwent follow-ups more frequently. The follow-up frequency for donors with eGFR≥60mL/min/1.73m2 was 1.3±0.9 per year, and for those with eGFR<60mL/min/1.73m2, it was 2.2±1.2 per year.
Final follow-up parameters included SBP, DBP, serum creatinine, MDRD-eGFR, use of antihypertensive drugs, and morbidity including coronary artery disease (CAD), cerebrovascular accidents (CVA), and congestive heart failure (CHF).
Although CKD-EPI is more accurate and has a better correlation with GFR measured using 99mTc-DTPA in renal function assessment of the kidney donors,16 we chose MDRD-eGFR for GFR measurement because the laboratory code of CKD-EPI has been used since 2013 in our hospital. This study was approved by an ethics review board of Asan Medical Center (2019-0249). The need for informed consent was waived owing to the retrospective design of the study.
Statistical analysisData were analyzed using SPSS software version 21 (SPSS Inc., Chicago, IL, USA), with results presented as mean±SD or median and range. The prevalence of categorical variables between hypertensive donors and matched controls were analyzed using the Pearson chi-squared test. If the expected cell frequency was<5, we used Fisher's exact test instead of Pearson's chi-squared test. Differences in age, BMI, SBP, DBP, duration of follow-up, and MDRD-eGFR between hypertensive donors and matched controls were evaluated using the Student's t-test. Multiple linear regression analysis using age, sex, smoking, use of antihypertensive drugs, SBP, DBP, BMI, and pre-donation eGFR was performed to identify the independent factors affecting eGFR at the final follow-up in the 388 donors. P-values less than 0.05 were considered statistically significant.
ResultsAntihypertensive drugs used in 1 drug group were as follows: calcium channel blocker (CCB) in 37 donors, angiotensin receptor blocker (ARB) in 17 donors, and others in 7 donors. Two donors in 1 drug group received 2 kinds of CCB (both amlodipine and diltiazem). Antihypertensive drugs used in 2 drugs group were as follows: CCB+ARB in 16 donors, ARB+diuretics in 4 donors, CCB+β-blockers (BB) in 4 donors, and others in 12 donors.
Table 1 shows a comparison of the pre-donation clinical characteristics between the hypertensive donors and matched controls. BMI, SBP, DBP and LVH on echocardiography in 1 drug group were higher than in matched controls. There were no differences in age, sex, smoking, HbA1c, MDRD-eGFR, GFR using isotope, and LVH on ECG between the donors previously receiving 1 antihypertensive drug and matched controls. BMI was higher in 2 drugs group than in matched controls. There were no differences in age, sex, smoking, SBP, DBP, HbA1c, MDRD-eGFR, GFR using isotope, LVH on ECG, and LVH on echocardiography between the donors previously receiving 2 antihypertensive drugs and matched controls.
Comparison of pre-donation clinical characteristics between hypertensive donors and matched controls.
| Control (n=183) | Hypertensive donors-1 drug (n=61) | P value | Control (n=108) | Hypertensive donors-2 drugs (n=36) | P value | |
|---|---|---|---|---|---|---|
| Age (years)a | 50.0±8.3 | 50.3±8.6 | 0.805 | 60.0±6.7 | 56.0±6.8 | 0.989 |
| Male gender | 108 (59.0%) | 36 (59.0%) | 1.000 | 57 (52.8%) | 19 (52.8%) | 1.000 |
| Smoking | 43 (23.5%) | 16 (26.2%) | 0.666 | 27 (25.0%) | 8 (22.2%) | 0.736 |
| BMI (kg/m2) | 24.5±3.6 | 26.4±3.4 | <0.01 | 24.6±2.4 | 26.1±3.1 | 0.014 |
| SBP (mmHg) | 123±15 | 139±21 | <0.01 | 125±16 | 127±16 | 0.567 |
| DBP (mmHg) | 78±11 | 87±12 | <0.01 | 84±50 | 80±9 | 0.657 |
| HbA1c (%) | 6.4±8.5 | 5.8±0.6 | 0.661 | 5.6±0.3 | 5.7±0.3 | 0.277 |
| MDRD-eGFR (mL/min/1.73m2) | 98.9±17.2 | 98.9±17.6 | 0.993 | 99.9±14.9 | 100.0±15.0 | 0.985 |
| GFR using 99mTc-DTPA | 96.1±17.0 | 102.0±18.5 | 0.061 | 93.3±20.8 | 87.0±17.9 | 0.190 |
| LVH on EKG | 15 (8.2%) | 8 (13.1%) | 0.255 | 6 (5.6%) | 5 (13.9%) | 0.145 |
| LVH on echocardiography | 4 (4.2%) | 7 (15.9%) | 0.036 | 1 (1.8%) | 3 (9.1%) | 0.138 |
Table 2 shows a comparison of outcomes between the donors at final follow-up. The mean follow-up was 51 months (range 12–214) in 1 drug group, and 54 months (range 12–175) in 2 drugs group. There were no significant differences in follow-up eGFR and percentage change in eGFR between hypertensive donors and matched controls in each group and in total donors. No statistical difference was observed between the hypertensive groups and each matched control for the incidence of donors with follow-up eGFR≤45mL/min/1.73m2.
Comparison of outcome between hypertensive donors and matched controls at the time of final follow-up.
| Control (n=183) | Hypertensive donors-1 drug (n=61) | P value | Control (n=108) | Hypertensive donors-2 drugs (n=36) | P value | |
|---|---|---|---|---|---|---|
| Follow up (months)a | 54 (12–263) | 51 (12–214) | 0.700 | 51 (12–226) | 54 (12–175) | 0.776 |
| SBP (mmHg) | 123±15 | 130±13 | 0.006 | 126±13 | 130±17 | 0.567 |
| DBP (mmHg) | 74±10 | 78±9 | 0.014 | 74±11 | 79±12 | 0.038 |
| Antihypertensive drugs | 3 (1.6%) | 61 (100%) | <0.001 | 0 (0.0%) | 36 (100%) | <0.001 |
| Increased number of classes of antihypertensive drugs | 12 (19.7%) | 3 (8.3%) | ||||
| MDRD-eGFR (mL/min/1.73m2) | 70.6±14.6 | 73.5±13.6 | 0.172 | 71.2±11.4 | 69.9±21.1 | 0.650 |
| MDRD-eGFR (% change) | 27.9±13.1 | 24.9±11.3 | 0.101 | 28.1±11.5 | 30.0±18.1 | 0.546 |
| MDRD-eGFR<45mL/min/1.73m2 | 5 (2.7%) | 0 (0.0%) | 0.335 | 0 (0.0%) | 2 (5.6%) | 0.061 |
| Morbidity after donation | ||||||
| CAD | 1 (0.5%) | 3 (4.9%) | 0.049 | 0 (0.0%) | 0 (0.0%) | |
| CVA | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 1.000 | |
| CHF | 0 (0.0%) | 1 (1.6%) | 0.250 | 0 (0.0%) | 0 (0.0%) | |
The SBP, DBP, use of antihypertensive drugs, and prevalence of CAD were higher in 1 drug group than in the controls. There was no significant difference in the duration of follow-up and prevalence of CVA and CHF between hypertensive donors previously receiving 1 antihypertensive drug and matched controls.
The DBP and prevalence of antihypertensive drugs were higher in 2 drugs group than in the controls. There were no significant differences in follow-up duration, SBP, and morbidity after donation between hypertensive donors previously receiving 2 antihypertensive drugs and matched controls.
Table 2 shows that 12 of 61 (19.7%) donors in the 1 drug group received additional antihypertensive drugs at final follow-up. In the 2 drugs group, only 3 of 36 (8.3%) donors required an increased number of classes of antihypertensive drugs.
We also compared hypertensive donors receiving 1 and 2 antihypertensive drugs. In pre-donation clinical characteristics, it was observed that hypertensive donors in the 2 drugs group were older than those in the 1 drug group (50.3±8.6 vs. 56.0±6.8, P=0.001). SBP, DBP, and the percentage of LVH on echocardiography were higher for the 1 drug group as compared to the 2 drugs group (mean SBP [SD], 139±21mmHg vs. 127±16, P=0.004; mean DBP [SD] 87±12mmHg vs. 80±9, P=0.003; LVH on echocardiography, 15.9% vs. 9.1%, P<0.001). Hypertensive donors in the 1 drug group showed higher GFR using 99mTc-DTPA than those in the 2 drugs group (102.0±18.5 vs. 87.0±17.9, P=0.003). There was no significant difference in other clinical parameters between the two groups.
At final follow up, DBP was higher in the 2 drugs group (78±9 vs. 79±12, P=0.038). The 1 drug group required more classes of antihypertensive drugs at final follow-up (12 [19.7%] vs. 3 [8.3%], P=0.002). Of the two, donors in the 1 drug group had a higher incidence of CAD at final follow-up (P<0.001). However, it is difficult to appreciate significance owing to a low incidence. No significant difference was identified in MDRD-eGFR, percentage change of MDRD-eGFR, and the incidence of donors with follow-up eGFR≤45mL/min/1.73m2 between the two groups.
Table 3 shows the results of multiple linear regression analysis of factors associated with follow-up MDRD-eGFR in the 388 donors. Multiple linear regression analysis using age, sex, smoking, SBP, DBP, BMI, use of antihypertensive drugs, and pre-donation eGFR showed that pre-donation eGFR was the only independent predictor for eGFR at the final follow-up among the 388 donors.
Multiple linear regression analysis of factors associated with follow-up estimated GFR in total 388 donors.
| B±SE | β | t | P | |
|---|---|---|---|---|
| eGFR at predonation | 0.444±0.040 | 0.510 | 10.992 | <0.001 |
| Use of Anti-hypertensive drugs | 1.711±1.529 | 0.052 | 1.119 | 0.264 |
| Age | −0.088±0.08 | −0.051 | −1.11 | 0.267 |
| BMI | −0.080±0.198 | −0.018 | −0.405 | 0.686 |
| DBP | 0.006±0.023 | 0.012 | 0.256 | 0.798 |
| Sex | 0.23±1.41 | 0.008 | 0.17 | 0.869 |
| SBP | 0.004±0.038 | 0.004 | 0.095 | 0.924 |
| Smoking | −0.125±1.62 | −0.004 | −0.08 | 0.939 |
B±SE, regression coefficient and its SE; β, standardized regression coefficient; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate.
We analyzed 98 donors with high BP (SBP≥140mmHg and/or DBP≥90mmHg) previously not receiving antihypertensive drugs. We compared this group with normotensive donors who were not receiving antihypertensive drugs. The follow-up duration was the same (mean follow-up month (range), 62 (13–241) vs. 52 (12–263), P=0.417). Baseline SBP, DBP were higher in high BP donors not receiving antihypertensive drugs as compared with normotensive donors (mean SBP [SD], 147±7mmHg vs. 121±14, P<0.001; mean DBP [SD], 95±4mmHg vs. 77±9, P<0.001). At final follow-up, untreated donors with high BP also had higher SBP and DBP than normotensive donors (mean SBP [SD], 131±8mmHg vs. 123±15, P=0.004; mean DBP [SD], 78±6mmHg vs. 73±10, P=0.045). Pre-donation eGFR were higher in normotensive donors as compared to high BP donors not receiving antihypertensive drugs (99.92±16.11mL/min/1.73m2 vs. 93.05±17.72, P=0.041). However, there was no difference in the follow-up eGFR between untreated donors with a high BP and the control group (69.11±14.51mL/min/1.73m2 vs. 70.93±12.96, P=0.500).
DiscussionTo the best of our knowledge, this is the first study to compare follow-up eGFR between living hypertensive donors previously receiving antihypertensive drugs, subdivided into those administered one and two drugs with their respective controls. In this study, there was no significant difference in follow-up eGFR between donors with preexisting hypertension receiving antihypertensive drugs and matched controls, after a mean (range) of 51 months (12–214) in the 1 drug group, and 54 months (12–175) in the 2 drugs group after donation, supporting the current guidelines that donor candidates with controlled hypertension using 1 or 2 antihypertensive drugs may be acceptable for donation. Our findings may help increase the strength of this recommendation.
Donors with hypertension had conflicting outcomes of residual renal function. Some studies have found that donors with hypertension had reduced eGFR.17,18 Gracida et al.18 followed up living donors for 9 years and compared healthy donors with those with risk factors. Donors with hypertension had higher creatinine levels than controls (1.37 vs. 1.1mg/dL, P<0.001). However, other prospective studies reported that there was no significant difference in renal function at the end of the follow-up between residual with and without hypertension.3,11,13,19,20 Variation in follow-up duration, study participants, and definition of hypertension among these studies contributed to the discordance. Janki et al.13 reported no progressive decline of renal function in 30 donors with preexisting hypertension compared to non-hypertensive donors 5 years after donor nephrectomy. They defined “pre-existing hypertension” as SBP≥140mmHg and/or DBP≥90mmHg and/or the use of antihypertensive drugs. However, neither the number of donors on antihypertensive drugs nor the results of them were mentioned.
Previously, we reported that stage 3b CKD (eGFR 30–44mL/min/1.73m2) patients had higher risks of adverse renal outcomes than stage 3a CKD (eGFR 45–59mL/min/1.73m2) patients.21 In this study, there were only two donors with an eGFR≤45mL/min/1.73m2 in the 2 drugs group, and had no statistical differences were observed on comparison with the matched controls at final follow-up. A recent study of hypertensive kidney donors with a follow-up duration of 14.3±10.1 years has reported that hypertensive donors are more likely to have an eGFR<60 and <45mL/min/1.73m2; however, the incidence of eGFR<30mL/min/1.73m2 or ESKD was comparable in hypertensive and normotensive donors.22
In Tables 1 and 2, when we compared the blood pressures between the 1 drug group and the matched control, we observed higher SBP and DBP in the 1 drug group than in the controls at pre-donation and at final follow-up. However, no difference was noted between the 2 drugs group and the matched controls, except for the DBP at final follow-up. There results suggest that the 1 drug group might need and increased dose of an antihypertensive agent or an additional antihypertensive drug.
Previous studies assessing the change in BP of living kidney donors and the prevalence of hypertension after donation have different results. In a meta-analysis of hypertension in living kidney donors, Boudville et al.23 reviewed controlled studies involving a total of 157 donors and 128 controls in which the average follow-up duration was at least 5 years (range 6–13), and reported that the mean SBP and DBP were 6 and 4mmHg higher in kidney donors than in controls, respectively. Sanchez et al.14 reported that 26.8% of kidney donors had new-onset hypertension after a mean follow-up of 16.6 years. In recent study, Ibrahim et al.22 reported that 2319 out of 7173 donors with normal BP were observed to develop hypertension, 5.1±9.2 years after donation. However, a recent 9-year prospective study by Kasiske et al.24 followed up 205 living donors and 203 healthy controls and reported that blood pressure change and 24-h ABPM were not different between the two groups. Moreover, the prevalence of post-donation hypertension was comparable with that of controls. A recent meta-analysis with more recent and better qualified studies reported that donors had no increase in risk of hypertension.25 In our study, the follow-up SBP and DBP of hypertensive donors previously receiving 1 antihypertensive drug were higher than those of matched controls. No difference was found in the follow-up SBP between the 2 drugs group and the matched control, but the DBP at final follow-up was higher in donors in the 2 drugs group compared to the controls. In our study, the results of post-donation HTN were not consistent with the SBP or DBP of the 1 drug or 2 drugs groups, and there was a possibility that the 1 drug group might be undertreated. A shorter follow-up duration made it difficult to draw any conclusion with respect to post-donation hypertension.
In this study, multiple regression analysis showed that eGFR at pre-donation was the only independent factor that affected follow-up eGFR. Wang et al. reported that pre-donation GFR is a critical factor to assure the remaining kidney function after nephrectomy.26 Rook et al. found that pre-donation GFR was a significant predictor of post-donation GFR.27 Additionally, the study of prognostic factors associated with the compensation of renal function one year after donor nephrectomy showed that higher baseline creatinine and lower eGFR were the variables predicting renal compensation.28
This study has several limitations, with the major one being its retrospective and observational nature, which may have inherent selection bias. Second, this study had a relatively small sample size. Third, we measured eGFR using the MDRD equation instead of CKD-EPI, which has been available in our center since 2013. Fourth, the mean duration of follow-up was approximately 50 months. It would have been meaningful to follow-up with the final cohort of kidney donors for a longer duration, such as median of 10–15 years. In addition, we measured ABPM only in 35% (34/97) of hypertensive donors, which an objective parameter of hypertension before donation. Assessment of living donors should have included series of manual BP measurements on at least three separate outpatient visits as a minimum evaluation. If elevated manual BP is detected, then it may be worthwhile performing home self-BP measurements or ABPM.8 However, with limited ABPM or home BP measurement, it was difficult to exclude white-coat hypertension.
In conclusion, this study supports the current guidelines that donor candidates with controlled hypertension receiving 1 or 2 antihypertensive drugs may be acceptable for donation and may increase the strength of this recommendation.
Ethics approval and consent to participateThis study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the Ethical Guidelines for Epidemiological Research issued by the Ministry of Health and Welfare of Korea. The study was approved by an ethics review board of Asan Medical Center (2019-0249). The need for informed consent was waived owing to the retrospective design of the study.
Author's contributionResearch design; Soon Bae Kim.
Writing of the paper; Eun Hye Yang.
Performance of the research; Sung Shin, Young Hoon Kim, In Gab Jeong, Bumsik Hong.
Data analysis; Chung Hee Baek, Hyosang Kim.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Conflict of interestThe authors declare no conflicts of interest.
The authors thank to Seon Ok Kim, MS for the statistical assistance and to Editage for the English language review.