Alport syndrome (AS), first described in 1927,1 is a genetic disorder that affects the basement membranes. Its estimated prevalence is 1/5000–1/10,000.2 It is characterised by the presence of haematuria associated with sensorineural deafness, ocular lesions and alterations of the glomerular basement membrane that eventually lead to end-stage renal disease (ESRD) and it is responsible for 1–2% of patients with ESRD in western countries.3
In AS, the alteration is localised in the glomerular basement membrane, which comprises a type IV collagen network formed by α3, α4 and α5 chain trimers coded by the genes COL4A3, COL4A4 and COL4A5, respectively.4 In most cases (80–85%), AS is transmitted by X-linked inheritance (SALX) with mutations in the COL4A5 gene. Males are more severely affected since they present the mutation in hemizygosity, while women are heterozygous carriers of the altered gene with a generally less severe clinical involvement.5 The remaining 10–15% are affected by recessive autosomal inheritance with mutations of the gene COL4A3 or COL4A4.6 Traditionally, a third type of autosomal dominant inheritance was included, which today falls under “collagen type IV (α3–α4) nephropathy”.
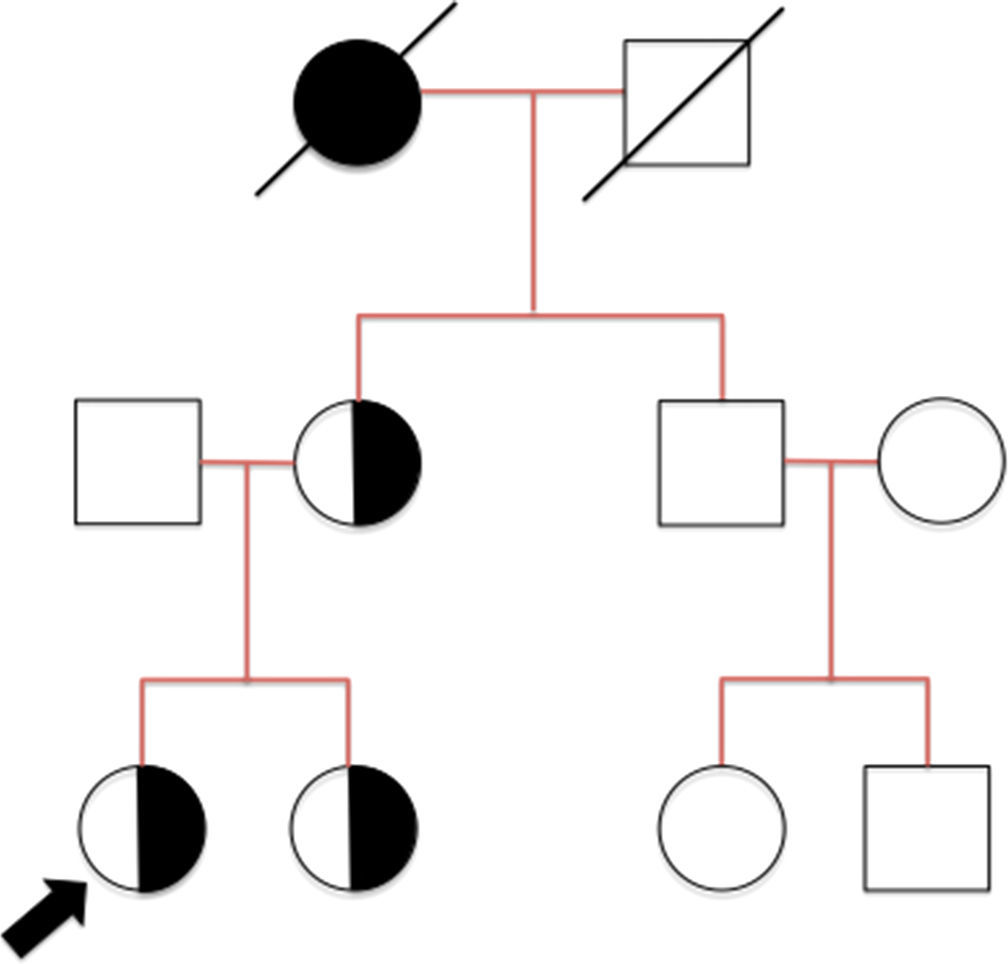
We present two cases of paediatric AS; two sisters, carriers of a new X-linked mutation in the COL4A5 gene.
A 13-year-old girl, monitored since age 1 due to micro-haematuria and non-nephrotic proteinuria. Family history (Fig. 1): 45-year-old mother has presented proteinuria and haematuria since age 11, currently with normal glomerular filtration, requiring hearing aids since age 37. Maternal grandmother diagnosed with nephrotic syndrome at age 14, deceased on haemodialysis programme due to a non-specific nephropathy. No history of kidney disease in the paternal family.
Due to clinical and family history, a kidney biopsy was performed at age 5, with an optical microscopy revealing a focal segmental glomerulosclerosis. Electron microscopy showed alterations in the basement membrane of the capillaries with a difference in thickness and a parallel rupture, creating grooves with disappearance at certain points of the lamina densa. All of this pointed to a kidney disorder as part of AS. Hearing test was normal.
Due to the family's history, her sister (three years younger) was studied from age 2, detecting non-nephrotic proteinuria and micro-haematuria with no hearing alterations.
No kidney biopsy was performed since she had a similar clinical/analytical profile to her sister. At present, both maintain a normal glomerular filtration rate and are under treatment with angiotensin II receptor antagonists.
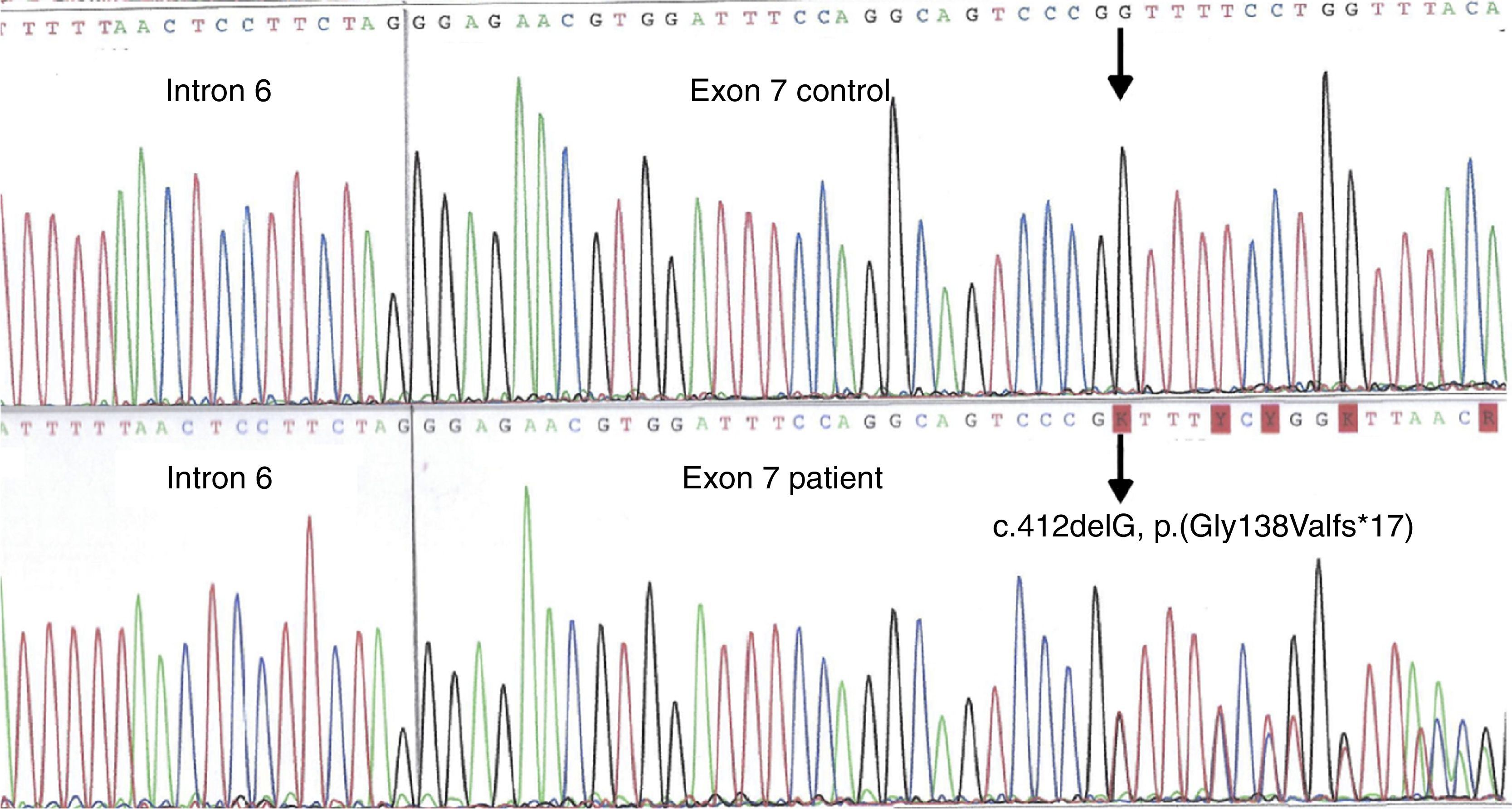
A genetic study was performed on the mother and on both sisters, with all three being heterozygous carriers of the mutation COL4A5 c.412delG, p.(Gly138Valfs*17), undescribed to this moment, confirming the clinical suspicion of SALX (Fig. 2). This is a frameshift mutation that involves changing the reading pattern on glycine amino acid 138 resulting in the generation of a premature translation termination codon at position 184. This mutation is expected to produce a protein of an anomalous size, with only 183 amino acids rather than the 1685 found in the normal protein (α5 chain of typeIV collagen, protein coded by the COL4A5 gene). This mutation has yet to be described in the literature and it does not appear in COL4A5 gene mutation databases. Since it is a truncating mutation, it can definitely be considered pathogenic and, therefore, the cause of the nephropathy in this family.
More than 1000 COL4A5 gene mutations in patients and families with SALX have been published or reported in mutational databases (The Human Gene Mutation Database [http://www.hgmd.cf.ac.uk/ac/index.php]; Leiden Open Variation Database [LOVD]; ARUP [http://www.arup.utah.edu/database/]). These mutations include large rearrangements (7%), small deletions (14%, as is the case with our patients) and insertions/duplications (6%), missense mutations (43%), nonsense mutations (6%) and splicing mutations (23%).7,8
The COL4A5 genotype has a significant predictive effect on the course of kidney disease in males, determining the likelihood of progression to ESRD in the second decade of life. Truncating mutations generally have a worse prognosis than missense mutations (90% in large deletions, nonsense, translocations; 70% in splicing mutations; 50% in missense mutations).5,9 The COL4A5 genotype also has an influence on sensorineural deafness, with deafness occurring at age 30 in 90% of males with deletions, nonsense and splicing mutations compared to 60% with missense mutations.5,9 Likewise, patients with truncating or splicing mutations will develop visual alterations 2–4 times more often than those with missense mutations.9,10
This genotype–phenotype correlation is not as apparent in females, probably due to the inactivation phenomena of the altered X chromosome. However, it is equally influential given that (for example) women with SALX and renal damage tend to have more frequent truncating mutations.
Genetic diagnosis is therefore an essential tool both for confirming the diagnosis and for ascertaining the mechanism of inheritance with a view to providing genetic counselling. The existing genetic mutation may even determine the evolutionary path of the disease. As such, we see genetic study as essential for an early diagnosis and to establish an individualised treatment plan that prevents deterioration of the glomerular basement membrane and the consequent renal damage.
FundingNo funding was received for this work.
Please cite this article as: Butragueño Laiseca L, Ars E, Martínez López AB, Álvarez Blanco O, Tejado Balsera JJ, Luque de Pablos A. Alteración genética no descrita previamente en 2 pacientes pediátricas con síndrome de Alport. Influencia pronóstica del genotipo. Nefrologia. 2017;37:352–354.