La Fibrosis Sistémica Nefrogénica (FSN) fue descrita por primera vez como entidad singular en el año 1997, y después se confirmó y documentó en la literatura médica durante el año 2000 como una enfermedad escleromixedematosa fibrosante que se presentaba en pacientes con afecciones renales. La prevención, el diagnóstico precoz y el tratamiento son esenciales para limitar su impacto, siendo el fracaso renal agudo y la enfermedad renal crónica, junto con la administración de agentes de contraste que contienen Gadolinio (GD) los factores de riesgo más importantes que pueden hacer desarrollar la enfermedad. Estos agentes causan FSN mediante la eliminación y el depósito de radicales libres de GD3 (gadolinio). Las comisiones internacionales de agencias del fármaco recomiendan la no utilización de estos agentes en pacientes con un filtrado glomerular menor de 30 ml/min/1,73 m2. Desafortunadamente, carecemos de terapias contrastadas y efectivas, y la evidencia disponible se basa en series pequeñas y grupos de casos aislados. Las recomendaciones para el uso de agentes de contraste que contengan gadolinio, en pacientes con insuficiencia renal avanzada, deberán individualizarse y tomarse en cuenta multidisciplinariamente por equipos formados por internistas, radiólogos y nefrólogos.
Nephrogenic systemic fibrosis (NSF) was first recognized as a unique entity in 1997 and subsequently defined in the literature in 2000 as a novel fibrosing disorder occurring in the setting of renal disease. Prevention, early recognition and treatment are essential to limiting its impact. The most important risk factors for developing NSF are chronic or significant acute kidney disease (especially dialysis dependent patients) and the administration of gadolinium (GD3) containing contrast agents, agents that cause NSF by releasing free gadolinium (GD3) into tissues based on their pharmacokinetics. International commissions in drug control and medicinal products recommend to avoid gadolinium based contrast agents in patients with GFR <30ml/minute/1.73m2. Unfortunately there is lack of universally effective therapy at this time and the literature is based on case reports and small case series. Recommendations to guide the use of gadolinium based contrast agents in patients with underlying kidney disease should be individualized and considered in consultation with the ordering physician, radiologist and nephrologist.
INTRODUCTION
NSF is a systemic disease that only affects patients with advanced renal failure. Cowper was the first person to describe the disease almost a decade ago in 14 patients undergoing haemodialysis who presented with hardened, thickened and darkened skin. Originally, this was defined as nephrogenic fibrosing dermopathy but was later referred to as nephrogenic systemic fibrosis. 215 cases have been described since December 2006.1 Until now, evidence has indicated that gadolinium (GD) based contrast agents; used in Nuclear Magnetic Resonance Imaging (NMRI), are responsive for this clinical entity. The literature does not mention any cases described before 1997. The medical repercussions have been significant and agencies like the FDA in the United States, the Commission on Human Medicines (CHM) in the United Kingdom and the European Committee for Human Medicinal Products (CHMP) have published recommendations on the use of GD-based products on patients with renal failure which stipulate that they must not be administered to patients with a GFR below 30ml/minute/1.73m2.2,3 However, there is a risk of applying draconian measures to patients that actually require these tests.
The clinical manifestations of NSF vary in seriousness and initial symptoms mainly affect the skin. The diagnosis is made by evaluating the patient history and the skin biopsy findings. In its most severe form, patients may present muscle an joint spasms and a reduction in joint mobility. In these cases, patients are usually confined to a wheelchair or bed because they are severely debilitated.4,5 There is an increase in mortality 24 months after the appearance of NSF skin manifestations.6 There is no evidence of effective treatments and improvement in patients has only been identified in those who have undergone transplantation.7
The disease has a number of causes. Hypercoagulation, high levels of erythropoietin and acidosis are just some of the factors involved. NSF affects patients with acute as well as chronic kidney failure, patients undergoing dialysis and kidney recipients with delayed graft function; however not all patients exposed to GD-based contrast agents develop the disease. The incidence in some studies is 4.3 cases per 1000 patients per year. The risk appears to be higher among peritoneal dialysis patients.8 Therefore, there is evidence that GD-based contrast agents may trigger the development of NSF and it may suggest that the use of NMRI as a diagnostic test in patients with kidney failure should be considered a second option, with Computerized Axial Tomography as an alternative especially in CKD patients.
RISK FACTORS
All patients affected by NSF who were exposed to GDbased contrast agents presented acute or chronic renal failure, with a Glomerular Filtration Rate (GFR) below 30ml/min/1.73m2.9 Pharmacokinetic studies have demonstrated their elimination via glomerular filtration, prolonging the half-life of the contrast agent to more than 30 hours. The risk factor in patients with higher levels of kidney function has still not been established. There is epidemiological evidence that suggests that patients undergoing peritoneal dialysis present a higher risk of suffering from the disease. NSF can also affect patients with liver failure who present with acute hepatorenal syndrome.10,11 Most NSF cases described have been linked to the use of GD-based contrast agents as mentioned previously.12 These agents were previously considered effective from a diagnostic point of view and had no side effects in terms of nephrotoxicity, however, their use is being reconsidered. GD3 makes the structure stable through binding ligands.13 The ion has a tendency to separate itself from the ligand during a process called binding-blocking, which, along with a transmetallation process, causes NSF. Of all the contrast agents, non ionic linear contrast agents are the least stable and increase the risk of transmetallation.15 The ones with a non ionic linear structure are the least stable. The NSF risk is greater in patients who tend to suffer thrombosis, especially those that receive erythropoietin and those who undergo vascular surgery.16 Metabolic acidosis affecting patients with renal failure may accelerate the transmetallation process with the release of free GD ions which increase toxicity. The use of intravenous iron during haemodialysis may increase the risk of NSF because of the effect that free iron has on metabolic acidosis, accompanied by the separation of GD from the binding agent. Hypercalcaemia may also compete with the GD molecule, thereby inducing transmetallation. Hyperphosphataemia may facilitate tissue deposition of free GD in tissues, increasing the risk of fibrosis.
CLINICAL SYMPTOMS
Lesions develop progressively and over a period of days or weeks. Presenting features are edema in hands and feet, erythema, café au lait macules, papules and nodules accompanied by itching, pain and redness.17 They are symmetrical, affecting distal and proximal portions spreading to the buttocks and chest in some cases. These symptoms may be misdiagnosed as cellulitis and treated as such by mistake. The patient has no fever. Another interesting symptom is the presence of a conjunctivitis like-picture in 76% of cases.18 There are no specific analytical parameters for NSF. The histological analysis indicates an increase in the number of fusiform cells in the dermis with deposition of collagen fibers and oedema, cells that stain positive for CD34 and procolagen I. Over time, mucin and immature collagen fill the space between the aforementioned cells and fibres as the disease progresses.19
GD CONTRASTS FOR NUCLEAR MAGNETIC RESONANCE IMAGING
Gadolinium (GD3) is an element used as a contrast agent in NMRI because of its properties. It is highly magnetic because of the structure of its electrons. This structure produces a change in the position of the protons on the surface of water molecules. There are nine contrast agents used in Magnetic Resonance Imaging (MRI) which has been approved for use in the United States and Europe. In Spain the following contrast agents are used: gadobenate dimeglumine, gadobutrol, gadodiamide, gadofosveset, gadopentetate dimeglumine, gadoterate meglumine, gadoteridol and gadoxetate disodium.
In 2006, 26.9 million procedures involving MRI were carried out and in 45% of cases these agents were used. The standard dose of GD for an MRI is 0.1mmol/kg, which is the dose authorised by the FDA in the US. In Europe up to 0.3mmol/kg is used.20 Not all GD-based agents cause NSF to the same extent.
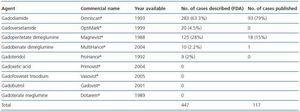
In January 2006 Grobner published a study on the relationship between GD and NSF.21 The Danish Medicines Agency published 20 cases of NSF linked with the use of gadodiamide in May 2006.22 Most of the cases described identify gadodiamide as the causing agent of NSF (93 out of 117 cases in some studies.)23 There have been 18 cases associated with gadopentetate dimeglumine.24 The relative chance of developing NSF once exposed to a GD-based contrast agent is 8.97, 22.3 and 32.5, respectively, as indicated in three different studies. High doses and greater accumulated dose increases the risk of NSF. The prevalence in patients exposed to gadodiamide is 4.0%. In some institutions no new cases of NSF have been reported because the use of gadodiamide was prohibited.25 The relative chance of developing NSF in patients exposed to gadopentetate is 14.7.26 No cases have been described involving dialysis patients who received gadoteridol.27 The incidence of cases involving other contrast agents like gadoversetamide or gadobenate dimeglumine (Multihance) has not been assessed. The incidence and prevalence described until now refer to cases published in the current literature. There is data based on cases voluntarily referred to different agencies like the FDA in the US which were not subjected to a strict evaluation carried out by the scientific community, which may affect the number of incidences. With regard to these cases, in October 2007, 283 cases associated with the use of gadodiamide, 125 with gadopentetate, 20 with gadoversetamide, 10 with gadobenate dimeglumine and 9 with gadoteridol were referred to the FDA, although in the latter two groups most patients received a combination of contrast agents. GE (General Electric) and Bayer have published 120 cases linked to gadodiamide and 99 cases associated with gadopentetate on their Websites respectively. There is currently a lack of comprehensive information about the use of agents in the market as no data has been published on this subject. The only available data comes from the Veterans Administration hospital record of purchases in the US. This data indicates that gadopentetate is used in 53.9% of cases, followed by gadodiamide in 25.6% of cases and gadoversetamide in only 6.1% of cases. This data could modify NSF incidence based on the use of the different contrast agents (table 1.)
PHYSIO-PATHOLOGICAL MECHANISMS IN THE LINK BETWEEN GD-BASED CONTRAST AGENTS AND NSF
The deposition of GD in the skin of patients with NSF has been described as part of the link between GD-based contrast agents and NSF.28 The amount of gadodiamide present in the bone fragments of patients without renal failure who received GD-based contrast agents during magnetic resonance imaging before hip surgery is four times higher compared to gadoteridol.29 This data reinforces the hypothesis that NSF is linked to a dissociation of GD (GD3) from the contrast which leads to its deposition in tissues. Free GD3 stimulates a tissue response in order to recruit circulating fibrocytes. This response creates collagen deposits and fibrosis by increasing the levels of transforming growth factor beta-1.30 The presence of kidney failure with the concomitant reduction of glomerular filtration rate contributes to the release of free GD3: by increasing the transmetallation.31 However, this hypothesis has also been criticised. A total of seven cases of NSF which did not involve the use of gandolinium-based contrast agents have been described in studies.32 Nevertheless, the hypothesis continues to be compelling.
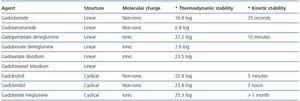
GD is not soluble in water and is highly toxic in its unbound state.33 It acts as a contrast agent by altering the stability of aqueous protons. Hydrosoluble binders were designed for the chemical composition of the contrast agent to be water soluble. There are two types of binders: cyclical and linear.
The former are water soluble and have the ability to bind to the gandolinium molecule, preventing the separation of the binder which leads to transmetallation and toxicity. Another point to be taken into consideration is the molecular charge as ionic binders are more stable. The kinetic stability of the binding process, which is defined as the time that it takes for GD to separate from the binder, is also an important property. This concept is expressed as half-life.
Linear and non-ionic binders tend to separate from the GD molecule more quickly than the cyclical and ionic binders, because of their flexible structure which allows them to break the molecular bond in sequence at different points.34 When there is a low glomerular filtration rate, GD takes longer to filtrate, which facilitates its separation and transmetallation (table 2.)
Transmetallation is a chemical reaction during which a second free metal with an affinity for the binder facilitates the separation of GD (GD3.) There are metals that compete with the GD molecule in vitro and enable its separation. Zinc is one of those metals that significantly contribute to the transmetallation process with large amounts appearing in urine, which from a clinical perspective, could be used as a diagnostic tool.35 The elimination of zinc is greater with gadodiamide, and lower with gadoteridol and gadoterate.
In short, linear and non-ionic binders tend to facilitate the separation of GD more often; gadodiamide and gadoversetamide are more effective at doing this. Gadoterate meglumine is probably the most kinetically stable of all gadolinium (GD) based contrast agents use in Nuclear Magnetic Resonance Imaging.
The majority of cases linked to NSF involved gadodiamide, which indicates that the recommendations stipulating that these contrast agents should be avoided on patients with kidney failure are completely justified.
The relationship between NSF and other gadolinium-based contrast agents like gadoversetamide is more controversial for a number of reasons, like the fact that is not used as frequently as gadodiamide. However, regulatory agencies for medicines like the one in the United Kingdom have also banned its use, especially for patients with glomerular filtration rate below 30ml/minute/1.73m2.
THE USE OF DIALYSIS TECHNIQUES FOR PREVENTING NEPHROGENIC SYSTEMIC FIBROSIS AFTER RECEIVING GADOLINIUM-BASED CONTRAST AGENTS
GD-based contrast agents are excreted via the kidney. The half-life of these contrast agents (T1/2) is longer in patients with advanced kidney disease and acute kidney failure.36 As mentioned before, this means that recommendations for their use have been issued by the different governing medical organisations. Unfortunately, they still must be used in some situations from a diagnostic point of view. However, these agents can be eliminated using renal replacement therapy techniques because of their chemical properties and this has lead to the use of dialysis techniques immediately after receiving the agents. GD binders have a molecular weight which varies between 500 and 1000 Daltons, they do not bind to plasma proteins and they are not lipophilic, which means that after they are administered intravenously they distribute and reach equilibrium within the extracellular space, not the intracellular space. The distribution volume is 0.26-0.28L/kg. As a result the GD binders have good glomerular filtration capacity. Renal clearance is similar to the glomerular filtration rate and since they are not secreted or reabsorbed into the renal tubule, over 95% of injected GD is eliminated from the body within 24 hours.37 The T1/2 of GD increases exponentially as creatinine clearance decreases and is 5.6 hours in patients with stage 3 CKD, 9.2 hours in patients with stage 4 CKD and 34.3 in patients with stage 5 CKD who are not undergoing dialysis.38,39
The fact that GD-based contrasts can be filtered through the glomerular basement membrane means that they are ideal for elimination using haemodialysis.40 Joffe et al. evaluated nine patients with chronic renal failure who were on dialysis and had a glomerular filtration rate of between 0.6 and 13.5ml/minute/1.73m2. After administering a bolus of 0.1mmol/kg gadodiamide and undergoing four hours of dialysis using low permeability membranes and blood flow of 250ml/minute, they demonstrated that the clearance rate of the contrast agents was 70ml/min/1.73m2, with an average T1/2 of 2.6 hours (1.5 hours in patients with normal kidney function), while it was 34.3 hours for patients with the same renal function but who were not undergoing dialysis. As a result, 68% of the injected contrast agent was eliminated in a single dialysis session.
Okada et al. administered 0.1mmol/kg of gadopentetate intravenously to 11 patients on dialysis. The treatment sessions were four hours long. The average amount of contrast agent removed was 78.2% in the first dialysis session, followed by 95.6, 98.7 and 99.5% in the following three sessions.
Saitoh et al. used 0.1mmol/kg of gadodiamide in thirteen patients undergoing haemodialysis. The total duration of treatment was three hours per sessions for 10 patients and four hours for three patients. During the first session 73.8% of the dose administered was eliminated; during the second and third dialysis session 92.4 and 98.9% of the dose administered was eliminated.41 Lackner et al. Obtained similar results.
Ueda et al. used two types of membranes of different thicknesses (one made of cellulose diacetate with a diameter of 38 Amstrongs, and the other of cellulose triacetate with a diameter of 70 Amstrongs), and three different contrast agents (gadodiamide, gadoteridol and gadopentetate.) 50% more binder was eliminated using the high flow membranes with a greater diameter when any of the aforementioned contrasts were used. The only problem with this study is that it was based on in vitro models and not on patients like the other studies.42
As mentioned before, some GD binders are ionic and some are non-ionic. Gadopentetate and gadoteridol were used in some studies with membranes that had different potentials, which demonstrated that there was no difference in clearance when using gadoteridol, however the clearance was reduced when membranes with negative potential were used with gadopentetate.43
The data referring to the elimination of GD-based contrast agents using peritoneal dialysis is not as impressive as that associated with haemodialysis. Joffe et al. evaluated peritoneal clearance of GD in nine patients on peritoneal dialysis who received 0.1mmol/kg gadodiamide. Peritoneal clearance of GD was 3.8ml/minute/1.73m2 with a T1/2 of 52.7 hours, data that is not surprising given the characteristics of the peritoneal membrane clearance. 75% of the administered dose was removed using peritoneal dialysis in five days. It seems that other factors contributed to the lack of similarity between the results regarding elimination of contrast agents using peritoneal dialysis and haemodialysis. Factors like the volume of distribution of the gadolinium-based contrast agents, which was higher in patients undergoing peritoneal dialysis and could reach up to 40%. This data has been confirmed in studies carried out using iodized contrasts. In any case, the data suggests that peritoneal dialysis is not efficient in eliminating these contrast agents.
There are no studies on the use of extrarenal techniques, like continuous haemofiltration or continuous veno-venous haemodiafiltration for eliminating contrast agents. At the moment there are no clear indications for their use except in cases of compromised cardiac function, which is when these techniques could be used with the aim of obtaining higher urea clearance rates in order to subsequently eliminate gadolinium-based contrast agent.44
What recommendations can be given regarding the use of these extrarenal techniques for patients who are at risk of suffering NSF? As we are already aware, one session of haemodialysis can eliminate 70% of the contrast agent administered, two sessions can eliminate 95% and three 98%.45 Nevertheless, it is unclear how much GD-based contrast agent is needed to cause NSF. The European Society of Urogenital Radiology recommends three haemodialysis sessions lasting nine hours in total. The first session should be carried out after receiving the contrast agent and after the radiology exam has taken place. Once again, it is important to state that there is no data to support the fact that the use of these recommendations reduces the risk of developing NSF after receiving gadolinium-based contrast agents. Randomized studies using different dialysis techniques have not been carried out until now because of the seriousness of this condition and the ethical implications associated with this kind of study.
In study by Broome et al., three of the patients that developed NSF did so despite receiving daily dialysis for three consecutive days.46
There seems to be a directly proportional relationship between the dose of GD-based contrast agent and the risk of developing NSF, especially with doses of 0.2-0.3mmol/kg. As previously mentioned, cases of NSF are limited to patients with stages 4 and 5 CKD. What is recommended for patients with stage 5 CKD who are not undergoing dialysis treatment and receive 0.1mmol/kg of contrast agent? The risk for this kind of patients is lower than that of anuric patients who receive doses of up to 0.3mmol/kg. There is no consensus regarding interventions for patients undergoing peritoneal dialysis. The only guarantee would be to avoid using GD-based contrast agents and consider other studies involving high risk patients such as the one described. Another unresolved point is the use of haemodialysis after receiving cyclical GD binders. Some contrast agents like gadofosveset bond more with serum albumin and stay in the intravascular space for a longer period of time (18 hours on average), which makes them difficult to eliminate during haemodialysis. As a result, a set of practical guidelines must be established in order to help the doctor manage these high risk renal patients.
THERAPEUTIC OPTIONS FOR NEPHROGENIC SYSTEMIC FIBROSIS
The relationship between NSF in patients with deteriorated kidney function and GD-based contrast agents is well established; however, there is no effective treatment and most therapeutic options are anecdotal and based on isolated cases with little scientific evidence to support them, since there are few randomized clinical trials on this subject. Treatment will become more effective as we begin to understand the disease mechanisms.
There are several therapeutic options that focus on the relief of symptoms.
Rehabilitation helps to prevent or slow down the progression of joint spasms. Swimming and massage can also improve symptoms. Pain management is another aspect that should be taken into consideration when rehabilitating these patients.
Extracorporeal photopheresis is a treatment used in systemic sclerosis. It is an immunomodulatory treatment that involves the irradiation of leukocytes in plasma using UVA light and a photosensitive agent given to the patient called 8-Methoxypsoralen (MOP), which increases the sensitivity of the lymphocytes to the apoptotic effect of UVA light. The immunomodulatory mechanism is made up of an antigen-specific response against the T cells which could slow down the synthesis and deposition of collagen in NSF. This treatment is carried out in two or three times a week cycles and is repeated three or four weeks later.
Sodium thiosulphate is a substance with antioxidant and binding properties. It has been used to stop the toxic effects of carboplatin and cisplatin, as well as to treat cyanide ingestion, although it is used more often in treating calciphylaxis.47 This agent blocks the reaction started by transforming growth factor beta, reducing the subsequent deposition of collagen and fibrosis. Similarly, it can act as a binding agent for free GD, maintaining its stability and preventing deposition in tissues. Unfortunately, like the aforementioned therapies, its use has been confined to anecdotal cases.
Pentoxyphyllin has an immunomodulatory effect and is used o treat fibrosis. Doses of up to 1200 milligrams have been used, although only two cases have been described by Grobner et al.48
Intravenous and oral glucocorticoids have been used to treat NSF because of their antifibrotic properties and because they act on transforming growth factor beta.49 However, the cases described are difficult to interpret because of lack of information regarding the doses used and the use of combination therapies. In some cases steroids did not obtain a positive therapeutic response.50
Plasmapheresis is another therapeutic option for NSF that focuses on reducing levels of transforming growth factor beta.51 Baron et al. used this technique on kidney transplant patients who developed the disease and an improvement in skin lesions and joint mobility was identified. Two of the patients experienced an improvement in kidney function before or during plasmapheresis which makes the results more difficult to interpret.
Intravenous immunoglobulin is an immunomodulatory agent that is used for treating immunological conditions like idiopathic thrombocytopenic purpura, myasthenia gravis and psoriasis. This treatment is based on the induction of anti-inflammatory cytokines.52 Unfortunately, few cases have been described and the results have been suboptimal. Cyclophosphamide and Thalidomide have been used with limited success.53,54 Similar results were obtained with interferon alpha and synthetic products derived from vitamin D3.
In short, physical therapy and pain management should be considered for these cases at all times. Extracorporeal photopheresis, sodium thiosulphate and pentoxyphyllin should also be taken into account. Future studies may explore the use of the binding treatment used in lead and aluminium poisoning and its implications for the binding of GD-based contrast agents. Specific cytokine modulators may also be the subject of further studies. Finally, the use of safer contrast agents should be explored and an effort should be made to prevent patients with kidney failure from being exposed to contrast agents. With the arrival of new compounds like ferumoxytol, which is a crystallized compound coated with synthetic carbohydrate which acts as an iron oxide nanoparticle and means that it can be used as a contrast agent in magnetic resonance imaging as well as in the treatment of iron deficiency. The controversy surrounding the use of GD-based contrast agents will soon be a thing of the past.
PRACTICAL GUIDE TO PREVENT NEPHROGENIC SYSTEMIC FIBROSIS WHEN USING GADOLINIUMENHANCED MAGNETIC RESONANCE IMAGING
1. Clinically identify patients before exposing them to more GD contrast agents.
2. Identify patients with a higher risk of developing NSF once they have received the contrast agent:
- Patients undergoing haemodialysis or peritoneal dialysis (higher risk);
- Patients with stage 4 and 5 chronic renal failure;
- Patients with acute kidney failure, especially associated with liver diseases;
- Patients with kidney and liver allografts and renal failure.
3. Once high risk patients have been identified, explore other radiology options in order to avoid any contact with the contrast agent. Obviously, this requires the collaboration of the doctor, nephrologist and radiologist working on the case.
4. If the use of a gadolinium-based contrast cannot be avoided the patient will be informed of the risks, benefits and any available alternatives.
5. Avoid high doses of GD-based contrast agents, especially doses above 0.2mmol/kg.
6. Avoid the use of gadolinium in high risk patients since most of the cases published make some reference to its use.
7. Non-linear binding agents are associated with a higher risk than ionic binding agents although there is little evidence to support this. Gadoteridol may be the safest contrast agent.
8. Avoid frequent exposure to GD-based contrast agents by establishing one week of separation between studies.
9. Avoid the use of GD in patients with acute renal failure.
10. Patients with chronic renal failure on haemodialysis should undergo dialysis treatment less than three hours after receiving gadolinium, although there is no clear evidence to suggest that this is necessary or beneficial. The increase in dialysis time increases the clearance, as does the frequency of dialysis sessions.
11. Patients with chronic renal failure undergoing peritoneal dialysis and without residual renal function are a difficult group to treat since the elimination of contrast agents using this technique is very limited. To improve clearance of the contrast agent, make sure the abdomen is not empty and make frequent exchanges, or increase the cycles of continuous ambulatory peritoneal dialysis for at least the first 48 hours after administering gadolinium.
However, the use of haemodialysis instead of peritoneal dialysis is recommended for high risk patients who receive high doses of contrast agents.
12. Patients with stage 4 and 5 chronic kidney failure that receive GD and are not undergoing dialysis, as well as those patients who suffer from acute renal failure are a difficult group to assess. An evaluation of the pros and cons of using dialysis in these cases should be carried out, taking into consideration that temporary catheters will need to be used, and the complications associated with it. In addition to this, there is no clear evidence in the literature that supports renal replacement therapies in this group of patients. Each case should be judged individually.
Table 1. Gadolinium-based contrast agents: cases associated with nephrogenic systemic fibrosis
Table 2. Structure and stability of gadolinium-based contrast agents