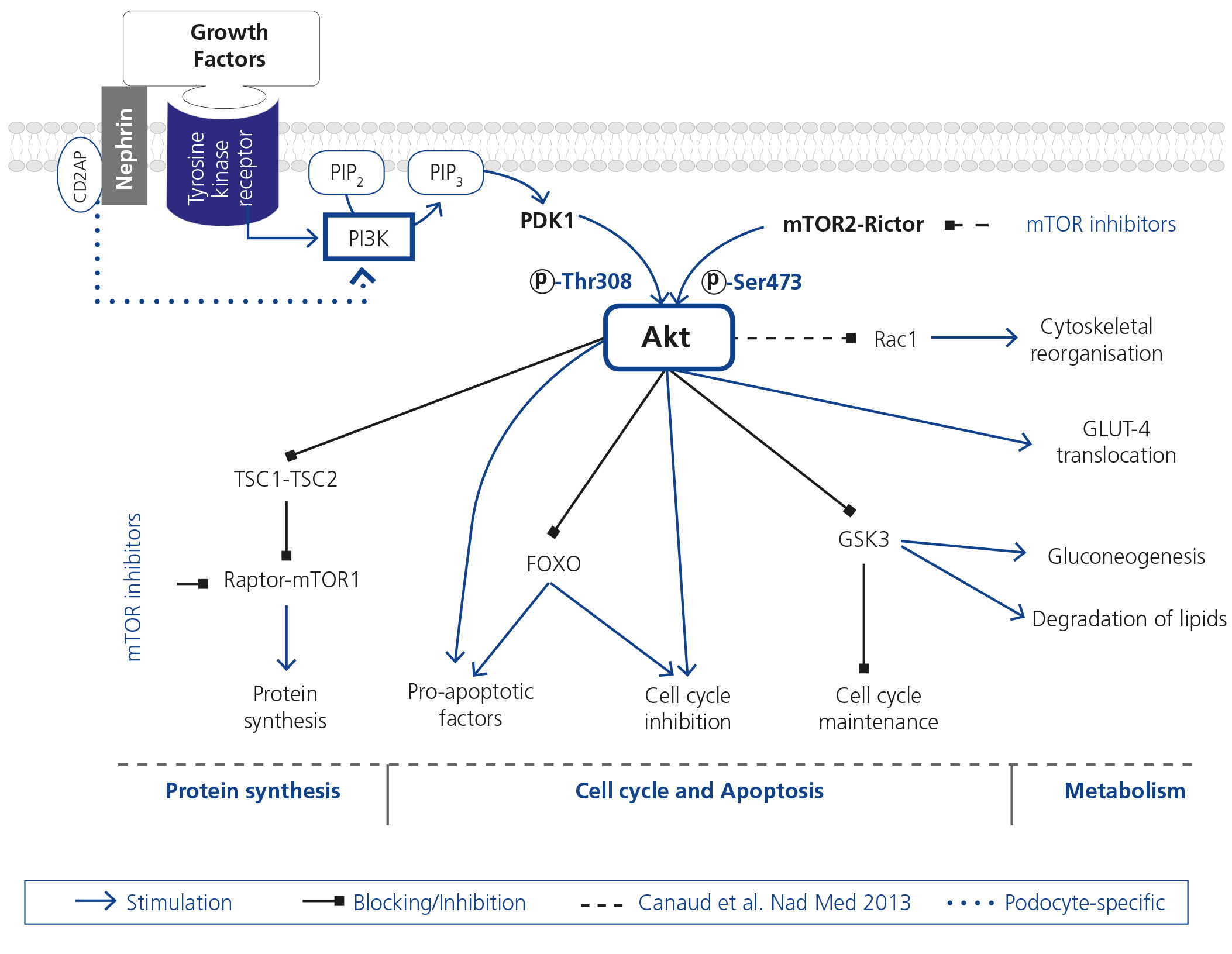
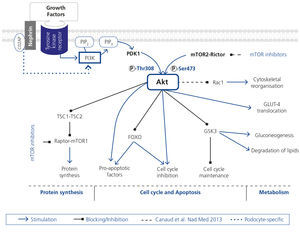
Despite up to 9 % of the population suffering from some degree of chronic kidney disease (CKD), the physiopathological pathways that participate in the disease’s progression are still unknown in detail1. Podocytes, highly specialised glomerular epithelial cells, play an essential role in maintaining the glomerular filtration barrier. Precise regulation of actin cytoskeleton is necessary for this maintenance; its reorganisation produces functional and morphological alterations that cause proteinuria. The Akt protein family are serine/threonine kinases that regulate metabolic, growth and cell survival factors (Figure 1)2. Podocytes have a protective role against apoptosis3-6. Nephrin and CD2AP, essential for maintaining cell structure and function, play a role in the regulation of cell apoptosis7 and a possible function in the regulation of cytoskeleton8, both Akt effects.
Canaud et al. have recently published the results of a study in which they demonstrate, using experimental in vivo, in vitro models and human biopsies, Akt2 activation as a protective mechanism in the podocyte against a reduction of kidney mass9. The loss of Akt2 or the decrease in one of the phosphorylations required for its activity [pAkt (Ser473)] worsens podocyte injury, causing proteinuria. A failure in its phosphorylation, blocking the mTOR2 complex by sirolimus, could explain, at least in part, the undesirable effects of this drug group observed in some transplant patients.
This study stands out methodologically for the use of genetically engineered animals. Its authors created knockout (KO) animals for Akt2 and podocyte-specific KO, enabling the evaluation of these cells’ significance in the evolution of renal lesions. These animals, together with their respective and suitable controls, are subjected to a reduction of kidney mass through subtotal nephrectomy or assessed at 13 months old as an ageing model. Similarly, they develop a KO-specific mouse for Rictor, an essential component of the mTOR2 complex.
An increase in Akt2 protein level, primarily present at a podocyte level, is observed in the reduction of kidney mass. Its increase seems to be a protective mechanism against damage, since, in relation to their respective controls, the KO animals for Akt2 show podocyte injury with pedicel effacement associated with an increase in Rac1, increase in apoptosis at a glomerular level, higher degree of glomerular lesion and as a result, higher albumin level. Similar alterations are found in KO mice for Akt 2 specifically in the podocytes, which confirms the significance of podocyte Akt2 in renal function maintenance. However, not only the absence of Akt2 causes renal alterations: phosphorylation deficiency, as that observed in KO mice for Rictor, causes equivalent alterations. The in vitro results reinforce the in vivo findings. KO podocytes for Akt2 or treated with sirolimus show alteration of its cytoskeleton with redistribution of actin fibres and appearance of adhesion foci.
The studies carried out on human biopsies show, in the same way as in the animal model, that Akt2 is mainly expressed in podocytes. An increase of pAkt (Ser473) is also observed at a glomerular level in patients with various pathologies, mainly of vascular origin.
The study of transplant patients with different degrees of renal dysfunction shows that only patients with severe nephron reduction present proteinuria when undergoing treatment with sirolimus. Patients with worse renal function show intense stains for pAkt (Ser473) and Rictor; this is not the case for patients with worse renal function being treated with sirolimus. This group shows greater cell apoptosis at a glomerular level and, clinically, the presence of proteinuria. One of the most noteworthy findings is the correlation in time between sirolimus withdrawal, increase of pAkt (Ser473) in renal biopsy and reduction of proteinuria.
With an excellent design in the in vivo and in vitro studies, this study identifies Akt2 as the central element in the pathophysiology of podocyte injury in CKD. Severe nephron reduction models were used, making the findings potentially applicable to CKD of any aetiology. It was recently demonstrated that the activation of Rac1 causes alterations of podocyte cytoskeleton, leading to pedicel effacement10. Canaud et al. showed that Akt2 reduction activates Rac1 causing cytoskeletal alterations.
In this way, previous studies on podocytes and on other experimental models which show that mTOR inhibitors do not only block mTOR1, but also mTOR2, are reaffirmed11,12. In addition, from the study by Canaud et al., it can be concluded that there is another kinase, aside from mTOR2, that phosphorylates Akt, since pAkt (Ser473) is not totally absent in the KO model for Rictor. Akt analysis is carried out through studies of total Akt, its isoforms and pAkt (Ser473); however no study was undertaken on the status of specifically Akt2 nor pAkt (Thr308) phosphorylation, which, although not dependent on the mTOR2 complex, would supply relevant information for the complete evaluation of the functional status of Akt13. In addition, the valuation of Rictor-mTOR2 in the various animal models could contribute to clarifying its role and regulation. Surprisingly, in presence of sirolimus, modifications in molecules phosphorylated by the mTOR1 complex, not specifically studied, were not detected.
One of the most remarkable findings is that the defect in Akt phosphorylation seems to explain, at least in part, the development of proteinuria observed in transplant patients with poor renal function when an mTOR inhibitor is introduced14. The patients were grouped into high and low estimated glomerular filtration rate; renal function and proteinuria prior to sirolimus introduction or duration of treatment with sirolimus, data which would help to establish the possible predictive role of Akt on renal function following the onset of treatment, were not explained. Studies on human renal biopsies show an increase in glomerular pAkt (Ser473) in various pathologies, but the Akt2 isoform was not specifically studied in any of the groups, which would obviously be of interest in the context of the present study.
Future research, in both animal and cell models, should be directed at determining which are the stimuli causing the elevation of Akt2, which could be specific to different pathologies. This aforementioned study focused on established renal damage models, but the study of this pathway in models with early damage would be significant. The prevalence of diabetic nephropathy and the role of the PI3K/Akt pathway in its pathophysiology, including podocyte injury3-6, make it a clear objective for furthering analysis of the role of Akt2. In addition, in-depth research on apoptosis mechanisms, regulation of the cell cycle (especially in the podocyte, terminally differentiated cell) and modifications of cytoskeleton, all regulated by Akt, is necessary to understand the pathophysiological consequences of the changes in this molecule.
In-depth research in these different fields would facilitate the ability to define possible therapeutic targets which would lead to the design of new drugs or the use of current drugs to stop, or even prevent, the development of established renal damage.
Finally, findings in human biopsies open the door to new clinical decision-making tools in the management of immunosuppression. With specifically designed studies, it would be possible to confirm the probable prognostic value of total Akt, its phosphorylations and its isoforms when assessing post-transplant biopsies as a step prior to converting to mTOR inhibitors. The study of possible non-invasive markers associated with the results of this study would result in an improvement in routine clinical practice.
Figure 1. Schematic and simplified view of Akt action pathways