The relationship between mineral metabolism disorders, bone fractures and vascular calcifications in kidney transplant recipients has not been established.
MethodWe performed a cross-sectional study in 727 stable recipients from 28 Spanish transplant clinics. Mineral metabolism parameters, the semi-quantification of vertebral fractures and abdominal aortic calcifications were determined centrally.
ResultsVitamin D deficiency (25OHD3<15ng/ml) was more common in female recipients at CKD-T stages I–III (29.6% vs 44.4%; p=0.003). The inverse and significant correlation between 25OHD3 and PTH was gender-specific and women exhibited a steeper slope than men (p=0.01). Vertebral fractures (VFx) with deformity grade ≥2 were observed in 15% of recipients. Factors related to VFx differed by gender; in males, age (OR 1.04; 95% CI 1.01–1.06) and CsA treatment (OR: 3.2; 95% CI: 1.6–6.3); in females, age (OR 1.07; 95% CI: 1.03–1.12) and PTH levels (OR per 100pg/ml increase: 1.27; 95% CI: 1.043–1.542). Abdominal aortic calcifications were common (67.2%) and related to classical risk factors but not to mineral metabolism parameters.
ConclusionsVitamin D deficiency is more common among female kidney transplant recipients at earlier CKD-T stages, and it contributes to secondary hyperparathyroidism. Prevalent vertebral fractures are only related to high serum PTH levels in female recipients.
La relación entre las alteraciones del metabolismo mineral, las fracturas óseas y las calcificaciones vasculares en receptores de un trasplante renal no han sido establecidas.
MétodoRealizamos un estudio transversal en 727 receptores estables procedentes de 28 centros de trasplante españoles. Se determinaron de manera centralizada los parámetros del metabolismo mineral; también se centralizó la semicuantificación de las fracturas vertebrales y de las calcificaciones de la aorta abdominal.
ResultadosLa deficiencia de vitamina D (25OHD3 < 15ng/ml) fue más frecuente en mujeres y en los estadios CKD-T I-III (29,6 vs. 44,4%; p=0,003). La relación inversa y significativa observada entre los niveles de 25OHD3 y PTH fue modificada por el género de tal manera que la pendiente fue mayor en las mujeres que en los hombres (p=0,01). Un 15% de los receptores mostró alguna fractura vertebral (VFx) con un grado de deformidad ≥2. Los factores relacionados con la VFx diferían en función del género: en los hombres, la edad (OR: 1,04; IC 95%: 1,01-1,06) y el tratamiento con CsA (OR: 3,2; IC 95: 1,6-6,3); en las mujeres la edad (OR: 1,07; IC 95%: 1,03-1,12) y los niveles de PTH (OR per 100pg/ml increase: 1,27; IC 95%: 1,043-1,542). Las calcificaciones de la aorta abdominal fueron comunes (67,2%) y se relacionaron con los factores de riesgo clásicos, pero no con los parámetros del metabolismo mineral.
ConclusionesLa deficiencia de vitamina D es más frecuente en las mujeres receptoras de un trasplante renal y en los estadios más tempranos de la CKD-T, y es un factor que contribuye al desarrollo de hiperparatiroidismo secundario. Las VFx prevalentes están relacionadas con unos niveles más elevados de PTH solamente en las mujeres.
Mineral and bone disorders (MBD), inherent complications of moderate and advanced chronic kidney disease, are frequent in stable kidney transplant (KT) recipients.1,2 Prevalent PTH levels are elevated at each CKD-T stage, and vitamin D insufficiency and deficiency are common.3–6 Mineral disorders have been suggested to be associated with post-transplant outcomes, such as higher serum phosphate, calcium7,8 and FGF23 levels,9 and are associated with increased mortality after KT.
Bone fractures are common after KT, and their cumulative incidence is higher than in the general and haemodialysis populations.10,11 The lower extremities are the most common locations,10,11 but the prevalence of asymptomatic vertebral deformities detected by vertebral morphometry has been described in up to 30–50% of stable KT recipients.12,13 Age, gender, and diabetes are common risk factors.10–13 However, the role of mineral alterations, including PTH and 25OHD3 levels, is unclear. Retrospective and cross-sectional studies have shown that persistent hyperparathyroidism is a risk factor for fracture after renal transplantation.13,14 However, whether gender modifies the relationship between mineral alterations and bone fractures is still unknown.
The presence of preexisting vascular calcifications of the aorta and iliac arteries at the time of KT is an independent predictor of both cardiovascular and all-cause mortality.15 Coronary and aortic calcification progression, assessed by spiral CT, is substantial within 4 years in prevalent KT recipients and is associated with several traditional and nontraditional cardiovascular risk factors.16 Higher serum phosphate and lower serum 25OHD3 levels are independent predictors of calcification progression.16
The relationship between MBD, bone fractures, and vascular calcification in the setting of KT is undetermined. The aim of this multicentre, cross-sectional study was to evaluate the mineral metabolism parameters at different stages of CKD-T and their relationship with prevalent vertebral fractures and abdominal aorta calcification scores in a large sample of stable KT recipients. Since female gender is a risk factor for bone fractures after transplantation (10–13), we especifically examined differences according to gender and whether gender modifies the relationship between mineral bone disorders and bone fractures and vascular calcifications.
MethodsDesign, setting, and participantsA cross-sectional study of 727 stable KT recipients from 28 transplant clinics across Spain (95.4% single kidney deceased donor; 1.2% double kidney deceased donor, and 3.3% living donor) was performed. At the time of the study, a blood sample and lateral X-ray of the dorsal and lumbar spines were obtained and sent to a central laboratory and X-ray diagnosis unit, respectively. Demographic and clinical data were retrospectively recorded from each participant's chart.
The vast majority of patients were Caucasian (95%). Inclusion criteria were: age>18 years, date of transplantation between January 2008 to December 2010, and post-transplantation time ≥1 year. The exclusion criteria were: pregnancy, double transplant (except double kidney), cancer (except non-melanoma skin cancer), significant liver disease, and the use of drugs possibly affecting bone and mineral metabolism other than the usual post-transplant medications.
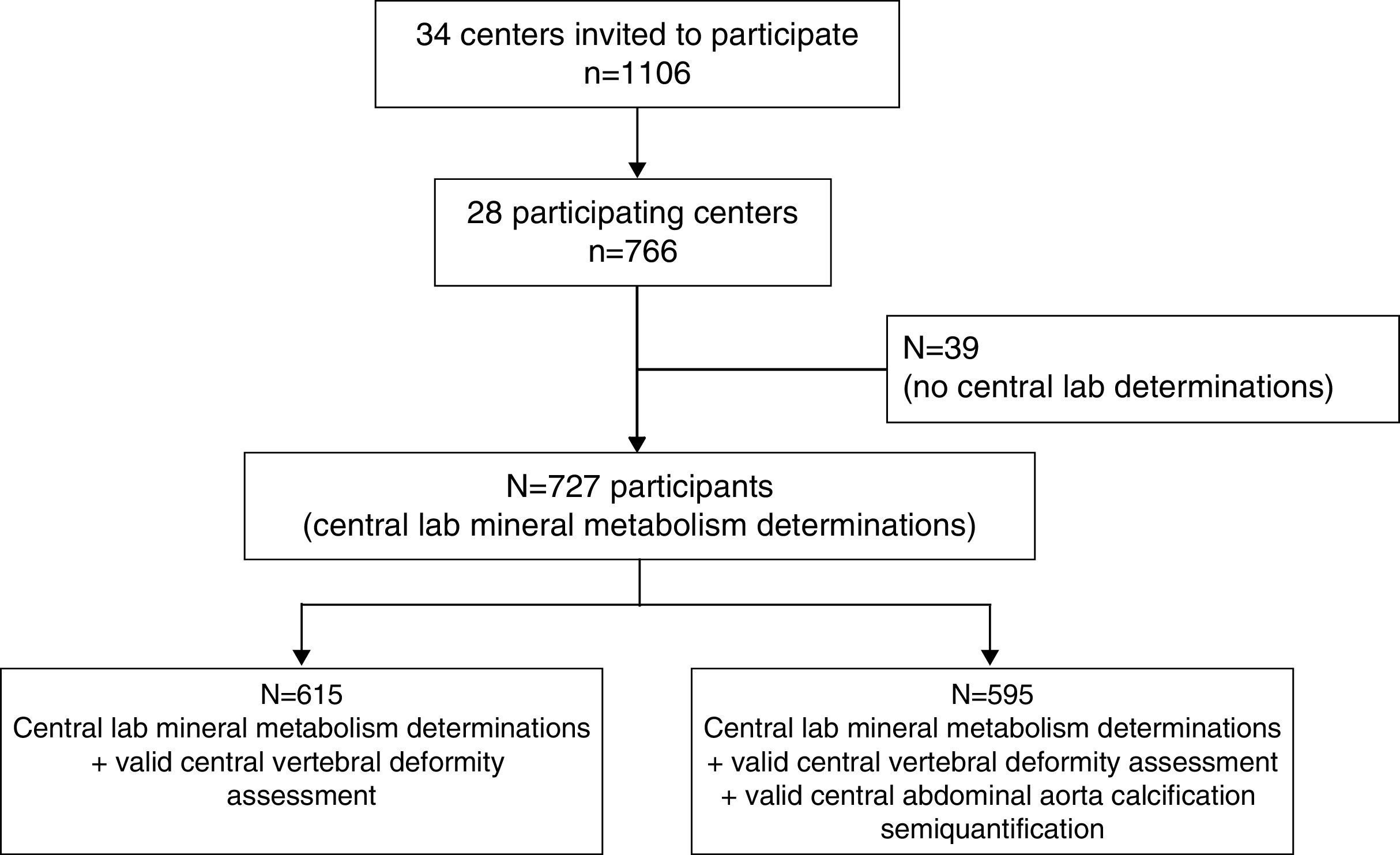
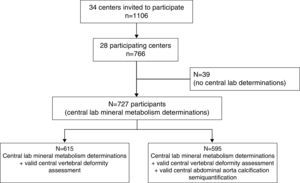
The number of patients included in each CKD-T stage was estimated according to the 95% confidence interval of the variance observed for phosphate levels in the UK Renal Registry.3 This required a total of 1106 patients to be included. Because 28 out of 34 centres participated, we were able to enrol 766 patients (68.7%). For logistical reasons, central laboratory determinations were unavailable for 39 patients. Consequently, a total of 727 patients were enrolled (Fig. 1): 512 stage 1–3T and 215 stage 4–5T. This sample size gave a precision >95% to estimate the phosphate levels of CKD-T stages I–III from the UK Registry. In order to avoid bias, the number of patients included per transplant centre was proportional to the number of kidney transplantations performed per year at that centre, ranging from 5 to 33. In addition, each centre included consecutive patients who fulfilled the inclusion criteria and attended the outpatient clinic at some point between April 2010 and February 2011.
No significant differences were observed between the 39 recruited patients who did not have a central laboratory determination and the 727 patients who were ultimately included in this study (data not shown).
MeasurementsDemographic and clinical data were recorded from each participant, including a history of clinical fractures and parathyroidectomy. Past and current immunosuppression, as well as bone and mineral medications (calcium and/or vitamin D supplements, vitamin D receptor activators, phosphate binders, calcimimetics and bisphosphonates), were also recorded. Graft function was derived from the MDRD-IV formula as the eGFR. Proteinuria in a 24-hour sample or as the protein/creatinine ratio (mg/g creatinine) in a first morning voided sample was also measured using standard methods at each participating centre.
A blood sample was obtained from each participant, and serum albumin, total calcium, phosphate, total and bone-specific alkaline phosphatase, PTH, 25OHD3, and 1,25(OH)2D3 levels were evaluated at a central laboratory. Calcium levels were corrected with serum albumin.17 PTH and 25OHD3 (Liaison®) were measured by chemiluminescence (Immulite®); 1,25(OH)2D3 was measured by RIA (LKB Counter), and bone alkaline phosphatase was measured by IRMA (LKB Counter). Serum creatinine determination at each study Centre was calibrated to be traceable to IDMS. The seasons of the year when the samples were obtained were: spring, 43.7%; summer, 37.3%; autumn, 18%; and winter, 1%. New onset diabetes was defined as a fasting glucose ≥126mg/dl in the two most recent determinations or the use of anti-diabetic drugs. Ischaemic heart disease, cerebrovascular disease, peripheral vascular disease were defined by standard clinical criteria.18
At enrolment, lateral X-rays of the dorsal and lumbar spine were obtained. Vertebral morphometry was assessed using the semi-quantitative method of Genant.19 Briefly, a decrease in the anterior, mid-body, or posterior vertebral height of 20–25% was considered mild (grade 1), 25–40% moderate (grade 2), and >40% severe (grade 3) (Supplementary Figure 1). Similarly, calcifications of the abdominal aorta were assessed with the semi-quantitative method of Kaupila20 and scored from 0 to a maximum of 24. Both vertebral morphometry and abdominal aorta calcification were centrally semi-quantified by an expert radiologist or nephrologist, respectively, who lacked knowledge of the patient's clinical and biochemistry data. Valid data for vertebral deformities were obtained in 615 cases and for aortic calcification in 595 (Fig. 1). No significant differences were found in age, gender, time after transplantation, or mineral metabolism parameters between the recipients with or without a valid assessment of vertebral deformities or aortic calcification, with the exception of 25OHD3 levels which were slightly higher in those with a valid assessment of vertebral deformity (18.2±9.9 and 21.8±13.3ng/ml; p=0.04).
The Ethics Committee of the Hospital Universitario de Canarias approved the study, and each participant signed an informed consent form.
StatisticsContinuous variables are expressed as the mean and SD or median and interquartile range, as appropriate. Absolute and relative frequencies were used to describe the categorical variables. Variables with a skewed distribution were log transformed. Univariate analysis for two groups was performed using the t test or Mann–Whitney U test, as appropriate. For more than two groups, we used ANOVA or Kruskal–Wallis one-way analysis of variance by ranks, as appropriate. Categorical data were compared using the chi-square test. Correlations between biochemical parameters were performed by univariate regression analysis after log transformation of PTH, 25OHD3 and 1,25(OH)2D3 levels. We also performed partial correlation analysis to investigate the relationship between 25OHD3 and PTH levels, as well as between 25OHD3 and 1,25(OH)2D3 levels, after adjusting for the eGFR.
A binary backward multivariable logistic regression analysis was performed to investigate the following: (a) variables independently associated with grade ≥2 vertebral deformity and (b) variables independently associated with an abdominal aorta calcification score in the third versus first tertile. Variables with p<0.2 in the univariate analysis or known to be clinically important were introduced in the multivariable analysis. We did not violate the rule of a minimum of 10 events per predictor variable to achieve the most parsimonious model. The Hosmer–Lemeshow goodness of fit was the principal criterion for selecting the final model. All analyses were performed using SPSS 18 (Chicago, IL).
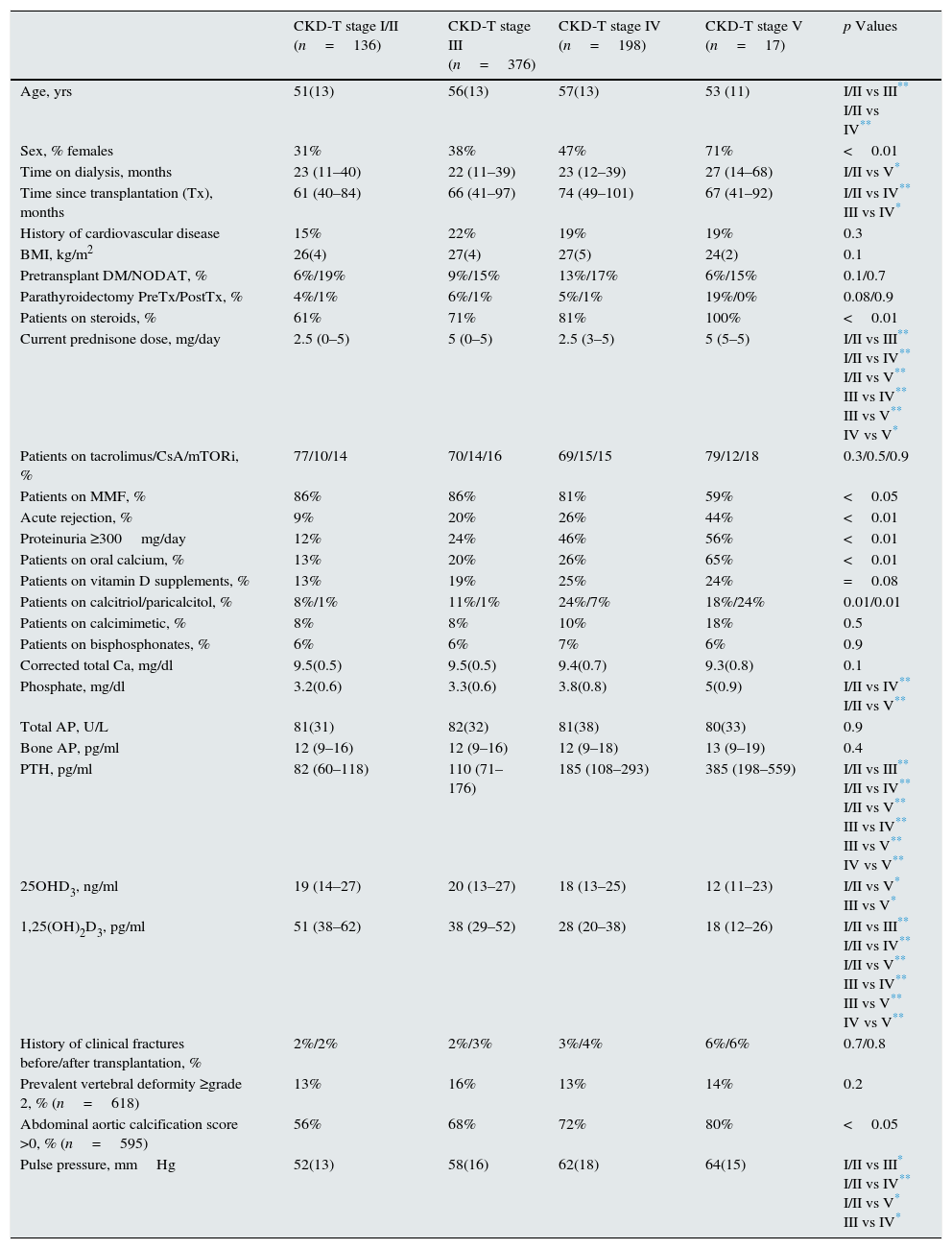
ResultsPatient characteristicsTable 1 shows the demographic, clinical, and biochemical data as a function of the CKD-T stage at the time of the study. Additionally, the percentage of patients with grade ≥2 vertebral deformity and an aortic calcification score >0 is shown. Age, female gender, time since transplantation, a history of acute rejection, patients on steroids, and proteinuria significantly increased with advancing CKD-T stage. The proportion of patients receiving oral calcium (as supplement or as a phosphate binder), as well as vitamin D receptor activators, increased with advancing CKD-T stages. Serum PO4 and PTH levels increased while serum calcitriol decreased with advancing CKD-T stages. Hypercalcemia (>10.3mg/dl) was observed in 6.5% of recipients and hypophosphatemia (<2.5mg/dl) in 6.2%. PTH levels were above the normal (>70pg/ml) in 76.9% of patients. Vitamin D insufficiency (15–30ng/ml) or deficiency (<15ng/ml) was observed in 50.8% and 32.5% of patients, respectively. Therefore, only 16.7% of patients had normal serum vitamin D levels.
Demographic, clinical, and biochemical data of 727 kidney transplant recipients from 28 transplant clinics as a function of their CKD-T stage. Mean and standard deviation or median and interquartile range as appropriate, are shown.
| CKD-T stage I/II (n=136) | CKD-T stage III (n=376) | CKD-T stage IV (n=198) | CKD-T stage V (n=17) | p Values | |
|---|---|---|---|---|---|
| Age, yrs | 51(13) | 56(13) | 57(13) | 53 (11) | I/II vs III** I/II vs IV** |
| Sex, % females | 31% | 38% | 47% | 71% | <0.01 |
| Time on dialysis, months | 23 (11–40) | 22 (11–39) | 23 (12–39) | 27 (14–68) | I/II vs V* |
| Time since transplantation (Tx), months | 61 (40–84) | 66 (41–97) | 74 (49–101) | 67 (41–92) | I/II vs IV** III vs IV* |
| History of cardiovascular disease | 15% | 22% | 19% | 19% | 0.3 |
| BMI, kg/m2 | 26(4) | 27(4) | 27(5) | 24(2) | 0.1 |
| Pretransplant DM/NODAT, % | 6%/19% | 9%/15% | 13%/17% | 6%/15% | 0.1/0.7 |
| Parathyroidectomy PreTx/PostTx, % | 4%/1% | 6%/1% | 5%/1% | 19%/0% | 0.08/0.9 |
| Patients on steroids, % | 61% | 71% | 81% | 100% | <0.01 |
| Current prednisone dose, mg/day | 2.5 (0–5) | 5 (0–5) | 2.5 (3–5) | 5 (5–5) | I/II vs III** I/II vs IV** I/II vs V** III vs IV** III vs V** IV vs V* |
| Patients on tacrolimus/CsA/mTORi, % | 77/10/14 | 70/14/16 | 69/15/15 | 79/12/18 | 0.3/0.5/0.9 |
| Patients on MMF, % | 86% | 86% | 81% | 59% | <0.05 |
| Acute rejection, % | 9% | 20% | 26% | 44% | <0.01 |
| Proteinuria ≥300mg/day | 12% | 24% | 46% | 56% | <0.01 |
| Patients on oral calcium, % | 13% | 20% | 26% | 65% | <0.01 |
| Patients on vitamin D supplements, % | 13% | 19% | 25% | 24% | =0.08 |
| Patients on calcitriol/paricalcitol, % | 8%/1% | 11%/1% | 24%/7% | 18%/24% | 0.01/0.01 |
| Patients on calcimimetic, % | 8% | 8% | 10% | 18% | 0.5 |
| Patients on bisphosphonates, % | 6% | 6% | 7% | 6% | 0.9 |
| Corrected total Ca, mg/dl | 9.5(0.5) | 9.5(0.5) | 9.4(0.7) | 9.3(0.8) | 0.1 |
| Phosphate, mg/dl | 3.2(0.6) | 3.3(0.6) | 3.8(0.8) | 5(0.9) | I/II vs IV** I/II vs V** |
| Total AP, U/L | 81(31) | 82(32) | 81(38) | 80(33) | 0.9 |
| Bone AP, pg/ml | 12 (9–16) | 12 (9–16) | 12 (9–18) | 13 (9–19) | 0.4 |
| PTH, pg/ml | 82 (60–118) | 110 (71–176) | 185 (108–293) | 385 (198–559) | I/II vs III** I/II vs IV** I/II vs V** III vs IV** III vs V** IV vs V** |
| 25OHD3, ng/ml | 19 (14–27) | 20 (13–27) | 18 (13–25) | 12 (11–23) | I/II vs V* III vs V* |
| 1,25(OH)2D3, pg/ml | 51 (38–62) | 38 (29–52) | 28 (20–38) | 18 (12–26) | I/II vs III** I/II vs IV** I/II vs V** III vs IV** III vs V** IV vs V** |
| History of clinical fractures before/after transplantation, % | 2%/2% | 2%/3% | 3%/4% | 6%/6% | 0.7/0.8 |
| Prevalent vertebral deformity ≥grade 2, % (n=618) | 13% | 16% | 13% | 14% | 0.2 |
| Abdominal aortic calcification score >0, % (n=595) | 56% | 68% | 72% | 80% | <0.05 |
| Pulse pressure, mmHg | 52(13) | 58(16) | 62(18) | 64(15) | I/II vs III* I/II vs IV** I/II vs V* III vs IV* |
CKD-T stages I >90; II 60–89; III 30–59; IV 15–29; V <15ml/mn/1.73 m2. NODAT, new onset diabetes after transplantation; AP, alkaline phosphatase.
The prevalence of compression fractures (grade ≥2) was 14.6%, which was not significantly different across CKD-T stages. Overall, 67.2% of patients had some degree of abdominal aortic calcification; however, this proportion increased at CKD-T stages III–V compared with stages I/II. Immunosuppression and the percentage of patients on statins (57%) or ACEI/ARA (56%) were not different among CKD-T stages.
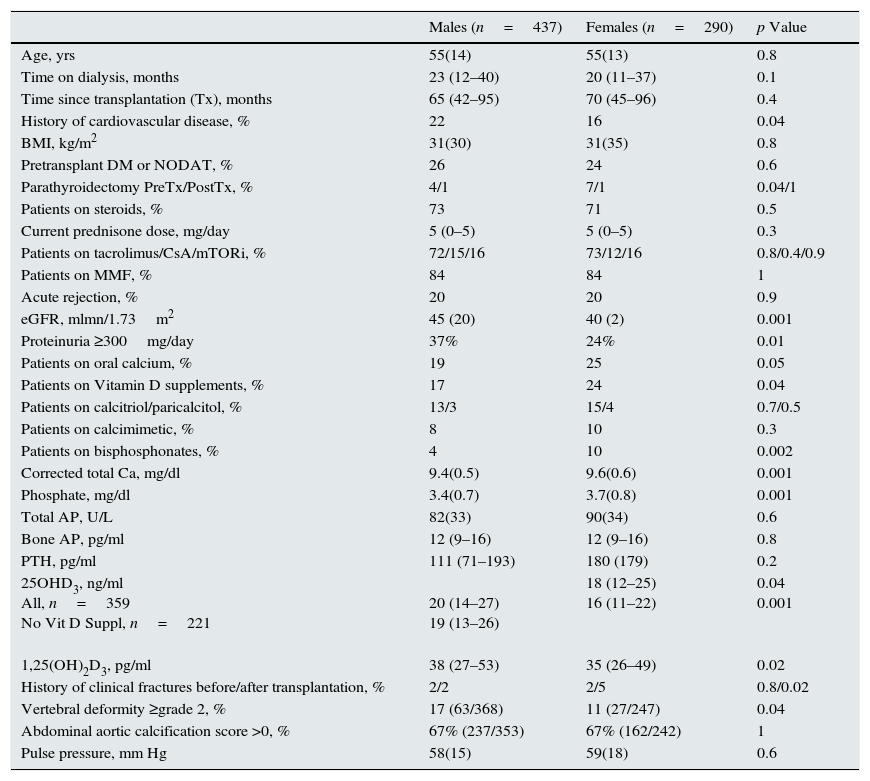
Gender differencesTable 2 shows a comparison of male vs. female recipients. Estimated GFR, 25OHD3 and 1,25(OH)2D3 levels were significantly lower in female recipients. Serum calcium and phosphate levels were significantly higher in female recipients, and no significant differences were observed in the circulating levels of PTH or bone alkaline phosphatase. A significantly higher proportion of women were receiving oral calcium (as a supplement or as a binder), vitamin D supplements, and bisphosphonates. Although a history of clinical fractures after transplantation was more common in female recipients (5.9 vs. 1.2%, respectively), prevalent vertebral fractures with vertebral deformity grade ≥2at the time of study were less common in women (10.9% vs. 17.1%).
Demographic, clinical, and biochemical data of 721 kidney transplant recipients from 28 transplant clinics as a function of gender. Mean and standard deviation or median and interquartile range as appropriate, are shown.
| Males (n=437) | Females (n=290) | p Value | |
|---|---|---|---|
| Age, yrs | 55(14) | 55(13) | 0.8 |
| Time on dialysis, months | 23 (12–40) | 20 (11–37) | 0.1 |
| Time since transplantation (Tx), months | 65 (42–95) | 70 (45–96) | 0.4 |
| History of cardiovascular disease, % | 22 | 16 | 0.04 |
| BMI, kg/m2 | 31(30) | 31(35) | 0.8 |
| Pretransplant DM or NODAT, % | 26 | 24 | 0.6 |
| Parathyroidectomy PreTx/PostTx, % | 4/1 | 7/1 | 0.04/1 |
| Patients on steroids, % | 73 | 71 | 0.5 |
| Current prednisone dose, mg/day | 5 (0–5) | 5 (0–5) | 0.3 |
| Patients on tacrolimus/CsA/mTORi, % | 72/15/16 | 73/12/16 | 0.8/0.4/0.9 |
| Patients on MMF, % | 84 | 84 | 1 |
| Acute rejection, % | 20 | 20 | 0.9 |
| eGFR, mlmn/1.73m2 | 45 (20) | 40 (2) | 0.001 |
| Proteinuria ≥300mg/day | 37% | 24% | 0.01 |
| Patients on oral calcium, % | 19 | 25 | 0.05 |
| Patients on Vitamin D supplements, % | 17 | 24 | 0.04 |
| Patients on calcitriol/paricalcitol, % | 13/3 | 15/4 | 0.7/0.5 |
| Patients on calcimimetic, % | 8 | 10 | 0.3 |
| Patients on bisphosphonates, % | 4 | 10 | 0.002 |
| Corrected total Ca, mg/dl | 9.4(0.5) | 9.6(0.6) | 0.001 |
| Phosphate, mg/dl | 3.4(0.7) | 3.7(0.8) | 0.001 |
| Total AP, U/L | 82(33) | 90(34) | 0.6 |
| Bone AP, pg/ml | 12 (9–16) | 12 (9–16) | 0.8 |
| PTH, pg/ml | 111 (71–193) | 180 (179) | 0.2 |
| 25OHD3, ng/ml All, n=359 No Vit D Suppl, n=221 | 20 (14–27) 19 (13–26) | 18 (12–25) 16 (11–22) | 0.04 0.001 |
| 1,25(OH)2D3, pg/ml | 38 (27–53) | 35 (26–49) | 0.02 |
| History of clinical fractures before/after transplantation, % | 2/2 | 2/5 | 0.8/0.02 |
| Vertebral deformity ≥grade 2, % | 17 (63/368) | 11 (27/247) | 0.04 |
| Abdominal aortic calcification score >0, % | 67% (237/353) | 67% (162/242) | 1 |
| Pulse pressure, mm Hg | 58(15) | 59(18) | 0.6 |
NODAT, new onset diabetes after transplantation; AP, alkaline phosphatase.
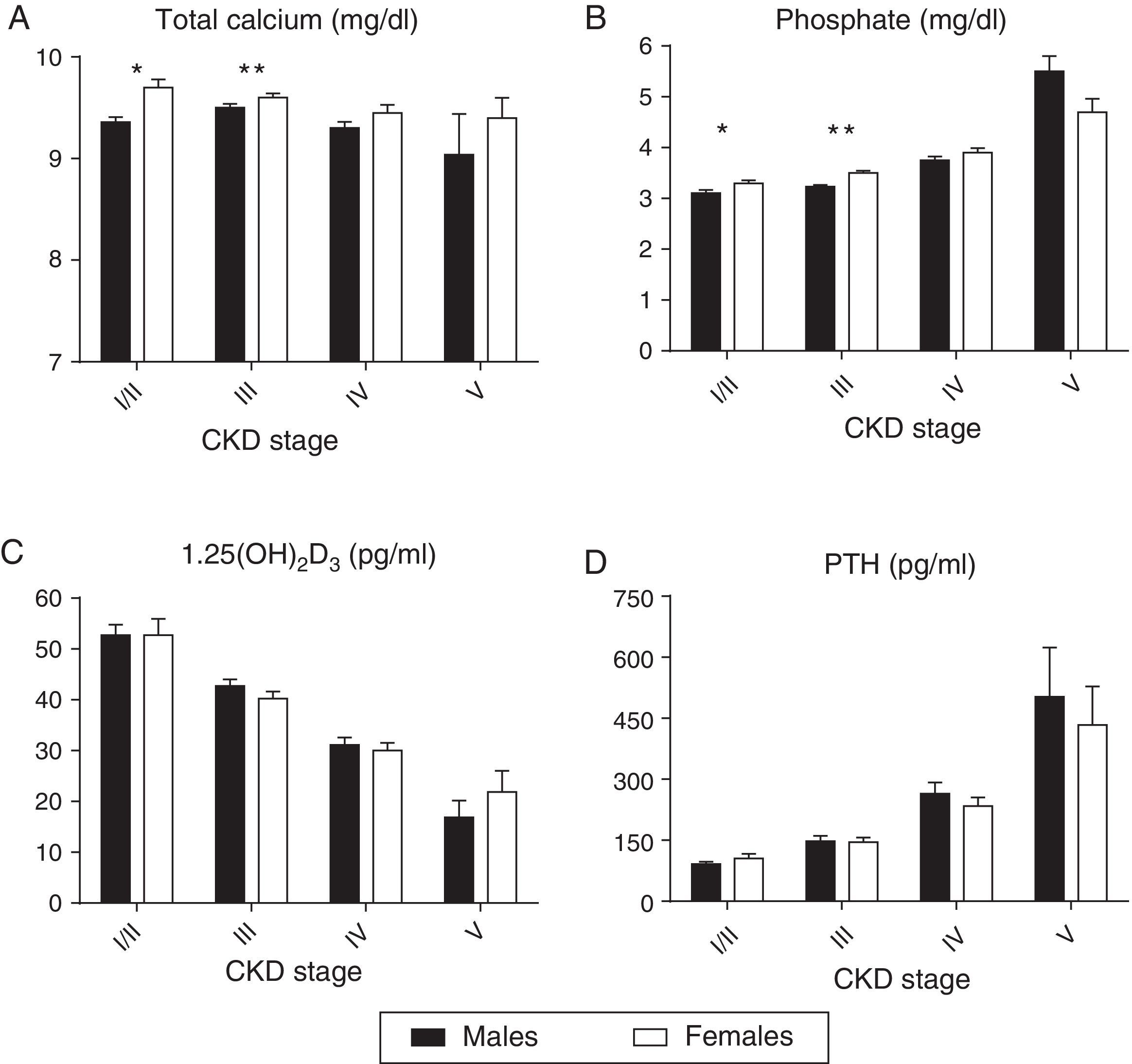
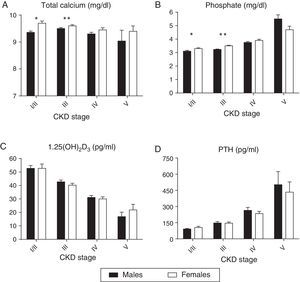
To avoid the confounding effect of eGFR differences, a comparison of the mineral metabolism parameters by gender in each CKD-T stage is provided in Supplementary Table 1. The eGFR was similar in each CKD-T stage. As shown in Fig. 2a, serum calcium was significantly higher in women in CKD-T I/II. As a result, more women were receiving a calcimimetic in this stage (16.7% vs. 4.3%; p=0.03). Serum calcium and phosphate levels were significantly higher in CKD-T stage III women (Fig. 2a and b), and a higher proportion of women were treated with vitamin D derivatives (35.9% vs. 26%; p=0.045) and oral calcium supplements (24.6% vs. 16.5%; p=0.06) compared with men. Serum PTH and 1,25(OH)2D3 levels were similar between men and women at each CKD-T stage (Fig. 2c and d).
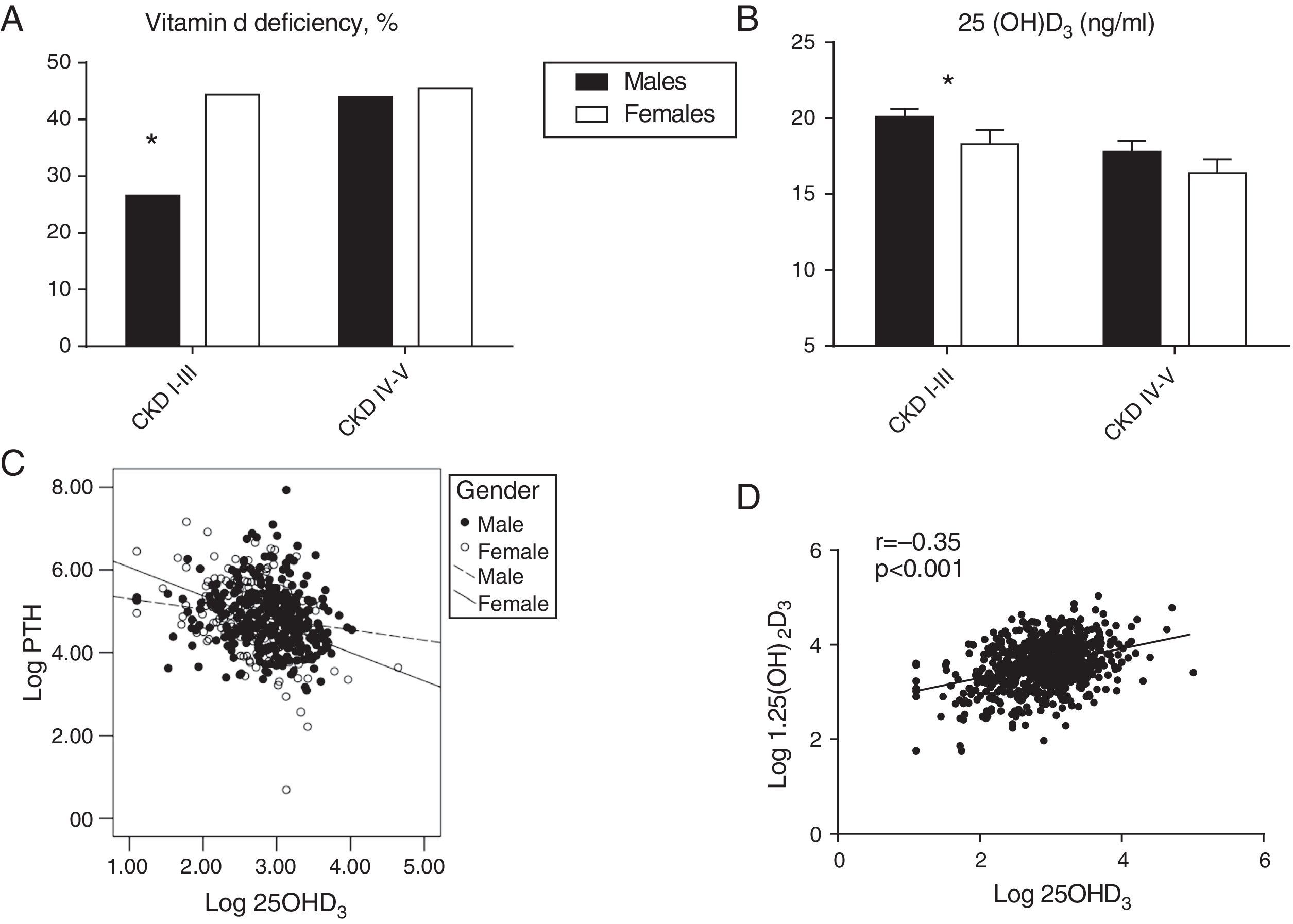
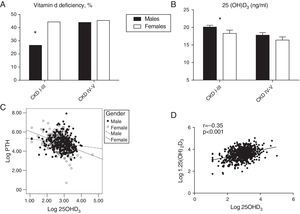
Overall, 25OHD3 levels were not significantly different between genders at each CKD-T stage; however, the proportion of women with vitamin D deficiency (<15ng/ml) was higher than in men in CKD-T stages I–III (36.8% vs. 26.9%; p=0.02) but not in CKD-T stages IV (36.6% vs. 35.2%) or V (50% vs. 60%). After excluding patients receiving vitamin D supplementation, gender differences in vitamin D deficiency at CKD-T I–III increased (44.4% vs. 29.6%; p=0.003) (Fig. 3a); in addition, serum 25OHD3 levels were significantly lower in women compared with men at CKD-T stages I–III (Supplementary Table 1; Fig. 3b, p=0.003), which was not the case for stages IV and V. These differences in vitamin D status by gender were not explained by the season of the year when the blood samples were collected (males: 52.8% in summer or autumn, 46% in spring, and 1.1% in winter; females: 59% in summer or autumn, 40.3% in spring, and 0.7% in winter).
Prevalence of Vitamin D deficiency (25OHD3 <15ng/ml) by the CKT-T stage as a function of gender (a). For those recipients who were not treated with vitamin D, 25OHD3 by CKD-T stage serum levels (b) and correlation of log transformed 25OHD3 with log transformed PTH levels are shown as a function of gender (slope was steeper in women; p=0.01). For all recipients, the correlation between the log transformed 25OHD3 and log transformed 1,25(OH)2D3 is shown (r=−0.35; p<0.001) (d). (*) p=0.003 for the comparison of males vs females in CKD-T Stages I through III; for CKD-T stages IV–V no significant differences between males vs. females.
The log transformed serum PTH levels correlated inversely with the eGFR (r=−0.33; p<0.001), albumin corrected serum calcium (r=−0.14; p<0.001) and log transformed 25OHD3 levels (r=−0.23; p<0.001). After adjusting for eGFR by partial correlation analysis, the inverse correlation between PTH and 25OHD3 levels remained significant (r=−0.22; p<0.001). In patients who were not treated with vitamin D supplements, the slope of the regression line between 25OHD3 and PTH levels was steeper in women than in men (−0.25±0.08 vs. −0.62±0.13; p=0.01; Fig. 3c). The log transformed serum calcitriol levels correlated directly with eGFR (r=0.44; p<0.001) and, as shown, the log transformed 25OHD3 levels (r=−0.35; p<0.001) (Fig. 3d). The correlation between 25OHD3 and calcitriol levels remained significant after adjusting for eGFR (r=−0.36; p<0.001).
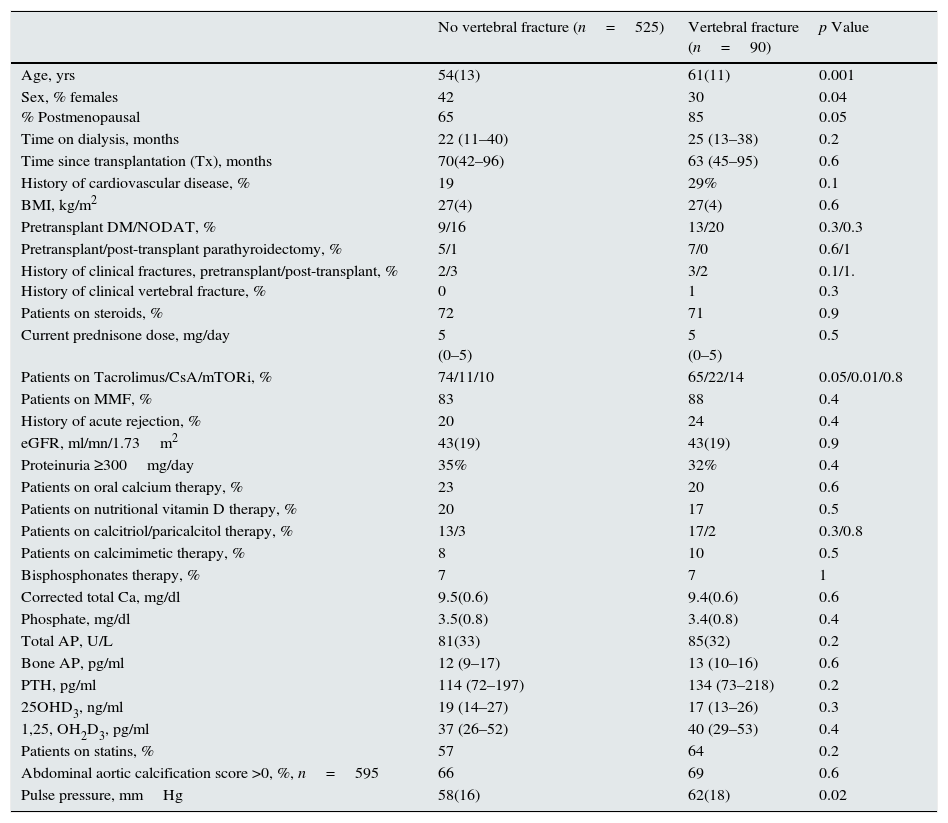
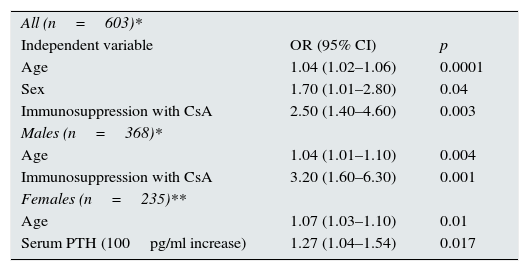
Factors related with prevalent vertebral fracturesThe prevalence of asymptomatic vertebral deformity grade ≥1 was 26%. Compression fractures that were grade 2 (deformity >25%) or higher were found in 15% of the patients. Table 3 shows the univariate comparison of patients with and without a prevalent asymptomatic vertebral fracture, defined as a deformity ≥grade 2. Patients with a prevalent vertebral fracture were older, and vertebral fractures were more common in men than in women. A postmenopausal status was more prevalent among women with vertebral fractures. A history of previous cardiovascular disease was more common among patients with vertebral fractures. Immunosuppression was different between the groups, and CsA use was more common among patients with vertebral fractures than those without a vertebral fracture (22.2% vs. 10.9%, respectively; p=0.006). Indeed, 26% of patients treated with CsA and 13% of those treated with tacrolimus or mTOR inhibitors had a vertebral fracture (p=0.005). Using backward conditional logistic regression analysis, we investigated the factors independently related to prevalent vertebral fractures. We included age, gender, BMI, time since transplantation, corticosteroid treatment (dichotomic), CsA treatment (dichotomic), eGFR, serum 25OHD3, and PTH levels as independent variables. Age, gender, and treatment with CsA were independently associated with prevalent vertebral fractures (Table 4). We also explored the interactions between gender and different mineral metabolism variables. The only significant interaction was gender*PTH (p=0.016). Therefore, it was logical to proceed with separate analyses of men (n=368) and women (n=235).
Univariate comparison of the patients with and without a prevalent vertebral fracture, defined as a ≥2 grade deformity. Mean and standard deviation or median and interquartile range as appropriate, are shown.
| No vertebral fracture (n=525) | Vertebral fracture (n=90) | p Value | |
|---|---|---|---|
| Age, yrs | 54(13) | 61(11) | 0.001 |
| Sex, % females % Postmenopausal | 42 65 | 30 85 | 0.04 0.05 |
| Time on dialysis, months | 22 (11–40) | 25 (13–38) | 0.2 |
| Time since transplantation (Tx), months | 70(42–96) | 63 (45–95) | 0.6 |
| History of cardiovascular disease, % | 19 | 29% | 0.1 |
| BMI, kg/m2 | 27(4) | 27(4) | 0.6 |
| Pretransplant DM/NODAT, % | 9/16 | 13/20 | 0.3/0.3 |
| Pretransplant/post-transplant parathyroidectomy, % | 5/1 | 7/0 | 0.6/1 |
| History of clinical fractures, pretransplant/post-transplant, % History of clinical vertebral fracture, % | 2/3 0 | 3/2 1 | 0.1/1. 0.3 |
| Patients on steroids, % | 72 | 71 | 0.9 |
| Current prednisone dose, mg/day | 5 (0–5) | 5 (0–5) | 0.5 |
| Patients on Tacrolimus/CsA/mTORi, % | 74/11/10 | 65/22/14 | 0.05/0.01/0.8 |
| Patients on MMF, % | 83 | 88 | 0.4 |
| History of acute rejection, % | 20 | 24 | 0.4 |
| eGFR, ml/mn/1.73m2 | 43(19) | 43(19) | 0.9 |
| Proteinuria ≥300mg/day | 35% | 32% | 0.4 |
| Patients on oral calcium therapy, % | 23 | 20 | 0.6 |
| Patients on nutritional vitamin D therapy, % | 20 | 17 | 0.5 |
| Patients on calcitriol/paricalcitol therapy, % | 13/3 | 17/2 | 0.3/0.8 |
| Patients on calcimimetic therapy, % | 8 | 10 | 0.5 |
| Bisphosphonates therapy, % | 7 | 7 | 1 |
| Corrected total Ca, mg/dl | 9.5(0.6) | 9.4(0.6) | 0.6 |
| Phosphate, mg/dl | 3.5(0.8) | 3.4(0.8) | 0.4 |
| Total AP, U/L | 81(33) | 85(32) | 0.2 |
| Bone AP, pg/ml | 12 (9–17) | 13 (10–16) | 0.6 |
| PTH, pg/ml | 114 (72–197) | 134 (73–218) | 0.2 |
| 25OHD3, ng/ml | 19 (14–27) | 17 (13–26) | 0.3 |
| 1,25, OH2D3, pg/ml | 37 (26–52) | 40 (29–53) | 0.4 |
| Patients on statins, % | 57 | 64 | 0.2 |
| Abdominal aortic calcification score >0, %, n=595 | 66 | 69 | 0.6 |
| Pulse pressure, mmHg | 58(16) | 62(18) | 0.02 |
NODAT, new onset diabetes after transplantation; AP, alkaline phosphatase.
Multivariate logistic regression analysis. Dependent variable: prevalent vertebral fracture (grade ≥2 deformity).
| All (n=603)* | ||
| Independent variable | OR (95% CI) | p |
| Age | 1.04 (1.02–1.06) | 0.0001 |
| Sex | 1.70 (1.01–2.80) | 0.04 |
| Immunosuppression with CsA | 2.50 (1.40–4.60) | 0.003 |
| Males (n=368)* | ||
| Age | 1.04 (1.01–1.10) | 0.004 |
| Immunosuppression with CsA | 3.20 (1.60–6.30) | 0.001 |
| Females (n=235)** | ||
| Age | 1.07 (1.03–1.10) | 0.01 |
| Serum PTH (100pg/ml increase) | 1.27 (1.04–1.54) | 0.017 |
* Adjusted by time after transplantation, BMI, eGFR, corticosteroid use, and serum levels of 25OHD3 and PTH. ** Adjusted by eGFR.
Men with vertebral fractures were older (60±12 vs. 54±14 years; p=0.001) and more commonly received CsA as the maintenance immunosuppressant (27% vs. 12.5%; p=0.006) than those without fractures. No other significant difference was observed between the two groups (data not shown). Using backward conditional logistic regression analysis, we investigated the factors independently related to prevalent vertebral fractures in men. We included age, BMI, time since transplantation, corticosteroid treatment, CsA treatment, eGFR, serum 25OHD3, and PTH levels as independent variables. Age and CsA treatment, but not time since transplantation, were independent factors associated with vertebral fractures (Table 4). Additionally, time since transplantation was not statistically significant when excluding immunosuppression therapy from the model (p=0.7).
Women with vertebral fractures were older (63.4±9 vs. 54.2±13 years; p<0.001) and had higher serum PTH [216 (92–412) vs. 114 (71.8–189.8) pg/ml, p=0.007] and lower 25OHD3 levels [14.8 (10.4–20.9) vs. 18.4 (12.3–26.3) ng/ml; p=0.049) than women without fractures (no other significant difference was observed between the groups; data not shown). By backward logistic regression analysis, age and serum PTH levels were significantly related to vertebral fractures after adjusting for the eGFR and 25OHD3 level. Interestingly, and unlike male recipients, immunosuppression was not related to vertebral fractures in women.
Compared to patients receiving tacrolimus or mTORi at the time of the study, those who were treated with CsA had a longer post-transplantation time. This difference was similar in men (85±26.6 vs. 66.1±30.5 months; p<0.001) and women (93.9±26.3 vs. 67.7±30.7 months; p<0.001). However, the prevalence rates of vertebral fractures in patients who were treated with CsA differed by gender, 31.5% vs. 14.6% (p=0.005) in men and 10.7% vs. 13% (p=0.8) in women.
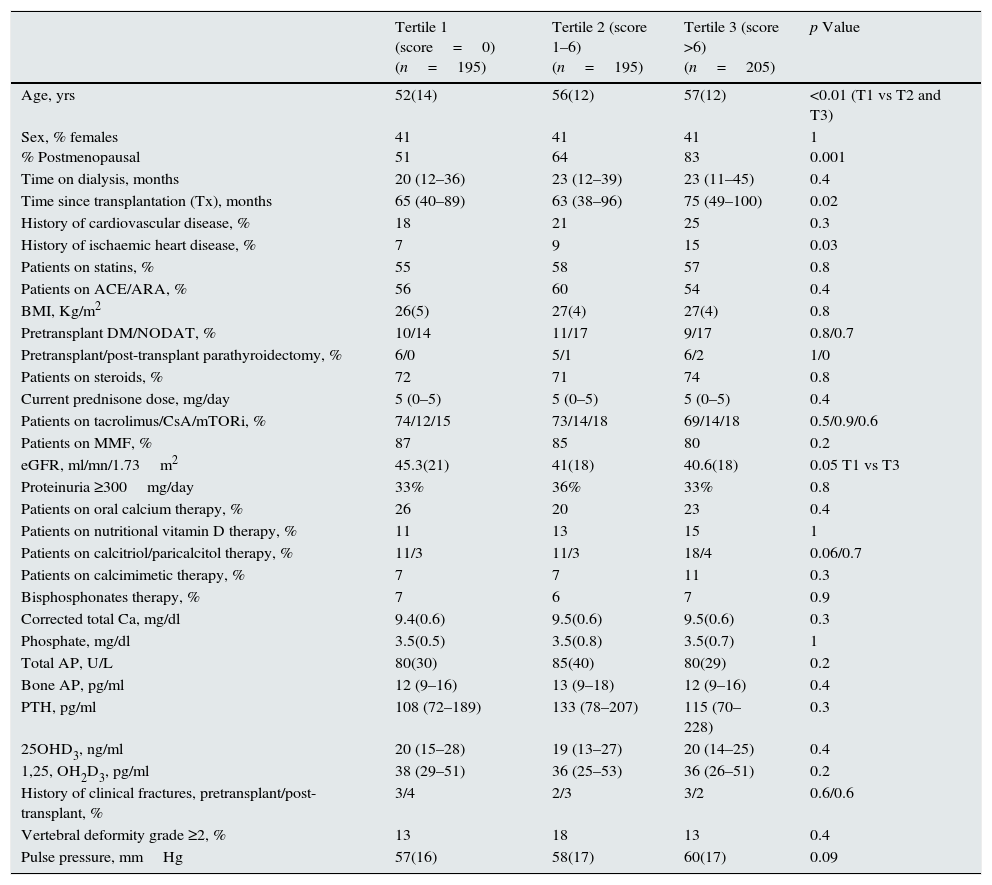
Factors related to aortic calcificationA Kaupila's score ≥1 was observed in 67.2% of the patients. Table 5 shows the univariate comparison of patients as a function of their tertile of calcification score. Age, time since transplantation, pulse pressure, and proportion of patients with prior ischaemic heart disease increased with the severity of abdominal aorta calcification (Table 5). Conversely, eGFR decreased with increasing calcification. Mineral metabolism parameters were not significantly different among the calcification tertiles.
Comparison of abdominal aortic calcification by the tertiles of Kaupila's score. Mean and standard deviation or median and interquartile range as appropriate are shown.
| Tertile 1 (score=0) (n=195) | Tertile 2 (score 1–6) (n=195) | Tertile 3 (score >6) (n=205) | p Value | |
|---|---|---|---|---|
| Age, yrs | 52(14) | 56(12) | 57(12) | <0.01 (T1 vs T2 and T3) |
| Sex, % females % Postmenopausal | 41 51 | 41 64 | 41 83 | 1 0.001 |
| Time on dialysis, months | 20 (12–36) | 23 (12–39) | 23 (11–45) | 0.4 |
| Time since transplantation (Tx), months | 65 (40–89) | 63 (38–96) | 75 (49–100) | 0.02 |
| History of cardiovascular disease, % | 18 | 21 | 25 | 0.3 |
| History of ischaemic heart disease, % | 7 | 9 | 15 | 0.03 |
| Patients on statins, % | 55 | 58 | 57 | 0.8 |
| Patients on ACE/ARA, % | 56 | 60 | 54 | 0.4 |
| BMI, Kg/m2 | 26(5) | 27(4) | 27(4) | 0.8 |
| Pretransplant DM/NODAT, % | 10/14 | 11/17 | 9/17 | 0.8/0.7 |
| Pretransplant/post-transplant parathyroidectomy, % | 6/0 | 5/1 | 6/2 | 1/0 |
| Patients on steroids, % | 72 | 71 | 74 | 0.8 |
| Current prednisone dose, mg/day | 5 (0–5) | 5 (0–5) | 5 (0–5) | 0.4 |
| Patients on tacrolimus/CsA/mTORi, % | 74/12/15 | 73/14/18 | 69/14/18 | 0.5/0.9/0.6 |
| Patients on MMF, % | 87 | 85 | 80 | 0.2 |
| eGFR, ml/mn/1.73m2 | 45.3(21) | 41(18) | 40.6(18) | 0.05 T1 vs T3 |
| Proteinuria ≥300mg/day | 33% | 36% | 33% | 0.8 |
| Patients on oral calcium therapy, % | 26 | 20 | 23 | 0.4 |
| Patients on nutritional vitamin D therapy, % | 11 | 13 | 15 | 1 |
| Patients on calcitriol/paricalcitol therapy, % | 11/3 | 11/3 | 18/4 | 0.06/0.7 |
| Patients on calcimimetic therapy, % | 7 | 7 | 11 | 0.3 |
| Bisphosphonates therapy, % | 7 | 6 | 7 | 0.9 |
| Corrected total Ca, mg/dl | 9.4(0.6) | 9.5(0.6) | 9.5(0.6) | 0.3 |
| Phosphate, mg/dl | 3.5(0.5) | 3.5(0.8) | 3.5(0.7) | 1 |
| Total AP, U/L | 80(30) | 85(40) | 80(29) | 0.2 |
| Bone AP, pg/ml | 12 (9–16) | 13 (9–18) | 12 (9–16) | 0.4 |
| PTH, pg/ml | 108 (72–189) | 133 (78–207) | 115 (70–228) | 0.3 |
| 25OHD3, ng/ml | 20 (15–28) | 19 (13–27) | 20 (14–25) | 0.4 |
| 1,25, OH2D3, pg/ml | 38 (29–51) | 36 (25–53) | 36 (26–51) | 0.2 |
| History of clinical fractures, pretransplant/post-transplant, % | 3/4 | 2/3 | 3/2 | 0.6/0.6 |
| Vertebral deformity grade ≥2, % | 13 | 18 | 13 | 0.4 |
| Pulse pressure, mmHg | 57(16) | 58(17) | 60(17) | 0.09 |
ACE/ARA, angiotensin converting enzyme inhibitors/angiotensin receptor antagonists; NODAT, new onset diabetes after transplantation; AP, alkaline phosphatase.
Using backward logistic regression analysis, age [OR 1.03 (1.02–1.05)], time since transplantation [OR 1.01 (1.001–1.02)], and history of ischaemic heart disease [2.4 (1.07–5.7)] were independent predictors of the highest calcification score (third tertile) compared with patients who lacked calcification (first tertile). No mineral metabolism parameters were related to aortic calcification.
DiscussionThis cross-sectional study included a large sample of stable KT recipients (>1 year after transplantation) with different CKD-T stages, which are representative of current clinical practice across Spain. We observed that secondary hyperparathyroidism was common among stable KT recipients, and a low 25OHD3 level was a contributing factor. Vitamin D deficiency was more common in female participants at earlier CKD-T stages; in addition, the relationship between 25OHD3 and PTH levels was modified by gender. Prevalent vertebral fractures were more common in males and related to age and CsA treatment as well as to age and PTH level in females. Abdominal aortic calcification was common and mainly related to time since transplantation and traditional risk factors.
Serum phosphate and PTH levels increased and calcitriol levels decreased with increasing CKD-T stage (Table 1), as reported in non-transplanted CKD patients.21,22 The prevalence of vitamin D deficiency or insufficiency was high in our study (83%), in agreement with other studies.4,5,23,24 An increased conversion of calcidiol to calcitriol, owing to high PTH levels, could be a contributing factor because 1,25(OH)2D3 levels correlated directly with 25OHD3 levels (Fig. 2D). We also report a higher prevalence of vitamin D deficiency (25OHD3<15ng/ml) among female recipients, which was only observed at earlier stages of CKD-T (eGFR >30ml/mn). Interestingly, vitamin D deficiency has also been reported more frequently in women within the first 30 days after KT.25 There may not be gender differences in the rate of conversion of 25OHD3 to 1,25(OH)2D3 because the slopes of the regression lines were similar in males and females. Additionally, most blood collections at enrolment were performed during the spring or summer, and the natural seasonal changes in 25OHD3 levels did not affect our results. Therefore, further research is required to explain the gender differences in the prevalence of vitamin D deficiency among KT recipients.
An inverse correlation between 25OHD3 and PTH levels was found in this and other studies.4,5,23,24 Thus, the KDIGO guidelines for KT recipients suggest that vitamin D deficiency and insufficiency should be corrected in patients with CKD stages 1–5T using treatment strategies that are recommended for the general population.25 Our data suggest this is especially relevant among female recipients because vitamin D deficiency is not only more common in women, but in addition, for a given decrease in 25OHD3 level the corresponding increase in the PTH level is also higher than in male recipients (Fig. 2C). Treatment with nutritional vitamin D significantly increases the 25OHD3 level and decreases the PTH level in KT recipients.26-28
Asymptomatic vertebral compression fractures (VFx) of any grade were observed in 26% of our patients. This rate is lower than the 32–57% prevalence reported in other studies that recruited fewer recipients.12,13 However, minor deformities of the midthoracic spine (especially in women) and thoracolumbar junction (especially in men) have been described in normal individuals.19 Thus, we only considered grade 2 or 3 vertebral compression as VFx. Remarkably, the 15% prevalence found in our study is higher than the 10% prevalence observed in Spanish postmenopausal women older than 50 years of age,29 which still contrasts with the less than 2% found in transplant recipients when searching according to the medical records (Table 3).30 Regardless of the definition, the importance of detecting prevalent VFx lies in the fact that they are risk factors for subsequent fractures.19,31 As expected, asymptomatic VFx increased with age, but surprisingly, they were more common in men than women (Table 3). However, a higher proportion of female recipients had a previous clinical fracture and, consequently, were more commonly treated with bisphosphonates at the time of the study (9.7 vs. 3.9%; p=0.002). Although asymptomatic vertebral fractures in the elderly may be more frequent in men than in women,31 the role of gender in the epidemiology of fractures after kidney transplantation may be different in the central versus peripheral location.
Compared with tacrolimus or mTORi, the use of CsA was a risk factor for VFx in males, but not in females, independently of post-transplantation time, history of acute rejection, or current corticosteroid use (Tables 3 and 4). Although the effect of CsA on bone metabolism in humans is controversial,32 it induces high turnover osteopenia in rats, more pronounced with CsA than with tacrolimus.33 Interestingly, the effect of CsA on bone resorption has been described in male but not female rats.34 However, given the retrospective and cross-sectional nature of the present study, the specific negative effect of CsA on VFx in males has to be interpreted with caution and merits further investigation and validation in other studies.
In a previous cross-sectional study of 102 KT recipients, high PTH levels were associated with an increased risk of VFx.13 In the present study, including 368 men and 247 women with a valid vertebral morphometry, there was a gender-related association, and women, but not men, with VFx exhibited higher PTH levels. This suggests that high PTH levels in females may explain the increased and unbalanced bone turnover after renal transplantation, leading to bone loss and fractures. Indeed, a differential bone protective effect of the bisphosphonate risedronate was recently observed in female but not male kidney transplant recipients.35 Future studies are needed to determine whether the use of paricalcitol or cinacalcet to control persistent post-transplant hyperparathyroidism, as previously reported,36–38 impacts the fracture incidence of female recipients.
Older age, prior ischaemic heart disease, and a longer time since transplantation, but not gender, were risk factors for abdominal aortic calcification. Interestingly, these factors were predictors of the progression of coronary and thoracic aorta calcification in a recent cohort study of prevalent KT recipients.16 In that study, low 25OHD3 and high serum phosphate levels at baseline were related to progression of coronary and thoracic aorta calcification, respectively.16 The fact that alterations of mineral metabolism were not related to abdominal aorta calcification in our study suggests that calcification at this location is mainly related to the common risk factors for atherosclerosis. In fact, a recent study performed in non-dialysis CKD patients (OSERCE-2 study) showed that the Adragao score, which includes the evaluation of hand arteries, but not the Kauppila score, was related to phosphorus levels.39
Our study has limitations. First, this was a cross-sectional study; therefore, a cause–effect relationship cannot be inferred. Tobacco exposure and lipid levels were not estimated as risk factors for abdominal aortic calcification; however, a history of smoking was not a determinant of vascular calcification progression in a recent cohort study.16 With these limitations, our study reflects the variability of different clinical practices across 28 transplant centres, making the observed associations more robust for planning preventive interventions.
In conclusion, vitamin D deficiency is more common among female KT recipients at earlier CKD-T stages and is a contributing factor to secondary hyperparathyroidism. In addition, gender modifies the relationship between 25OHD3 and PTH levels, and women have a steeper slope. Finally, prevalent vertebral fractures are not more common in females, but they are only related to high serum PTH levels in female recipients.
Conflict of interest statementNo conflict of interest that might bias this work.