Hay una prevalencia importante de la acidosis metabólica en los pacientes que padecen enfermedad renal crónica, presentándose en niveles tempranos de pérdida de filtrado glomerular. La patogénesis se basa en la falta de síntesis de bicarbonato sérico con la acumulación de ácidos de naturaleza orgánica e inorgánica, ocasionando daño tubulointersticial a través de la retención de amoniaco y el depósito de complemento, aunque esta última hipótesis se ha cuestionado en el pasado. El uso empírico de bicarbonato oral representa una opción terapéutica interesante que ha sido utilizada en estudios clínicos recientes. La disponibilidad de bicarbonato de sodio oral en sus diversas formas representa una opción barata y simple de utilizar para decelerar la progresión de la enfermedad renal, sin mencionar las mejoras en el catabolismo proteico, la osteodistrofia renal y la mortalidad.
In the chronic kidney disease population metabolic acidosis is prevalent presenting already in the early stages of renal dysfunction. The pathogenesis associates the lack of bicarbonate production with the accumulation of organic/inorganic acids and the development of tubulointerstitial damage through ammonium retention and complement deposition. The empiric use of oral sodium bicarbonate represents an interesting therapeutic option that has been documented in a few clinical trials in human subjects. The availability of oral sodium, in its diverse forms, represents an inexpensive and simple way of treating an entity that could hasten the progression of kidney disease, as well as protein catabolism, bone disease and mortality.
INTRODUCTION
Metabolic acidosis traditionally defined as a reduction in serum bicarbonate (HCO-3) concentration often associated with a reduction in blood PH, is a common accompaniment of progressive chronic kidney disease (CKD).1 It originates from the reduced capacity of the kidney to synthesize ammonia (NH3) and excrete hydrogen (H+) ions. It can have adverse consequences on protein, muscle and bone metabolism through negative nitrogen balance, increased protein degradation and decreased albumin synthesis, leading to protein energy malnutrition, loss of lean body mass and muscle weakness. It can also affect bone turnover due to its buffer effect compensating for excess acid. This action interferes with vitamin D metabolism, developing osteomalacia and renal osteodytrophy in patients with renal impairment. The mechanisms underlying these effects appear to be complex, involving several different pathways.
There is a high incidence of CKD in the general population based on a cross-sectional analysis of the most recent National Health and Nutrition Examination survey (NHANES, from 1988-94 and 1990-2004).2 There is also a correlation between decreased GFR and decrease plasma HCO3- based on the concept of nephron loss and impairment of ammoniagenesis. The question is if metabolic acidosis could be linked to the progression of kidney disease and if so can its correction delay CKD progression.3 Metabolic acidosis may be corrected by oral HCO3 supplementation in CKD and dialysis patients by increasing the HCO3 concentration in dialysis solutions. The absolute benefits of the correction of acidosis are not clearly identified and it is important to review the evidence. This evidence is very limited in pre-end-stage renal disease (ESRD) patients with lack of past or recent randomized control trials (RCT’s). Long term use of oral sodium HCO3 has not been evaluated in patients at risk for sodium overload especially nephrotic syndrome, hypertension or heart failure. Randomized control trials are needed to determine the benefits and risks of HCO3 therapy in preventing progression of CKD in pre-ESRD patients, targeting ideal HCO3 levels to initiate therapy with alkali agents. Di Iorio and colleagues in Italy will be conducting a prospective, multicenter, randomized, controlled trial looking at the correction of metabolic acidosis with oral HCO3 use and its influence in the progression of kidney disease. Randomization will involve 600 patients with CKD stages 3b and 4. Three hundred patients will receive oral sodium HCO3 or citrate.4
EPIDEMIOLOGY
Twenty six million Americans have evidence of kidney disease.2 A major determinant of serum HCO3 levels is kidney function. The exact prevalence of metabolic acidosis in patients with CKD is unknown. The Third National Health and Nutrition Examination Survey (NHANES III) analysis found a decrease in plasma HCO3 concentration with estimated glomerular filtration rate (eGFR)5 < than 20mL/min1.73m2. If hypobicarbonatemia caused by metabolic acidosis develops when eGFR is less < than 25% of normal parameters, it would be predicted that 300,000 to 400,000 individuals in the United States might have metabolic acidosis associated with CKD.6 After patients reach age 60 plasma HCO3 concentration is less than normal (12% of the population) and a great number of individuals could develop CKD related metabolic acidosis.7 Eighty percent of patients will manifest hypobicarbonatemia when GFR decreases to less than 20-25% of normal. The metabolic acidosis is mild in degree, with plasma HCO3 concentration between 12mEq/L and 22mEq/L.8 It is rare to have plasma HCO3 values < than 12mEq/L in the absence of increments in dietary acid load or superimposed tubular defect in bicarbonate absorption or generation.9 On the other hand, 10 to 20% with stage 5 CKD, many of whom have diabetes, have a plasma HCO3 concentration close to 23mEq/L.10
There could be an inverse correlation between plasma HCO3 concentration and renal function. Widmer et al., evaluated forty one patients retrospectively showing an inverse linear relationship between serum carbon dioxide (CO2) and serum creatinine, with a lower limit for serum CO2 of 11mEq/L at a serum creatinine of 12 mg/dL.11 Data from the NHANES III12 showed that HCO3-levels of 22mEq/L or less were present in 19% of those with eGFR of 15 to 29mL/min/1.73m2. However, the exact prevalence of metabolic acidosis caused solely by CKD remains to be determined. Shah et al.13 studied data from 5,422 patients with different comorbidities (diabetes 21%, HTN 41%) race (African-american 45%, Hispanics 11%) and gender (women 69%). Nine percent had GFR <60ml/min/1.73m2. The lowest quartile of serum HCO3 (≤22 mEq/L) was associated with a 54% increased hazard of progression of kidney disease. After adjusting for potential confounders, the relative hazard ratio (rHR) for progression of kidney disease was 1.54 (95% confidence interval (CI) 1.13-2.09). The study was observational making causality difficult and lack of blood gas analysis could not rule out a respiratory component.
Lower serum HCO3- levels are associated with higher all-cause mortality in patients with moderate and advanced CKD. Kovesdy et al.14 evaluated a cohort of 1240 CKD patients (eGFR % 27.5±5.5 to 45.9±19.1). Multivariable-adjusted Cox models showed a U-shaped association with the highest mortality rate with serum HCO3 <22 mmol/L (95%CI) and the lowest mortality with serum bicarbonate of 26-29mmo/L, even after controlling for the confounding effect of nutritional status and inflammation. In 1094 patients,15 from the African American Study of Kidney Disease and Hypertension (AASK) cohort trial study, each 1mmo/L increase in serum HCO3 was associated with reduced risk of death (HR 0.942). One may speculate that protein-energy malnutrition may be related to acidemia and represents a risk factor for poor outcome in renal failure. Despite different clinical scenarios and covariants there is a correlation between worsening renal function and acidosis in the pre-ESRD population.
PATHOGENESIS
An important function of the kidney is ammoniagnesis. Ammonia is produced in the proximal tubule from glutamine at a high rate. One half of renal NH3 produced leaves via renal veins and the remainder is eliminated in the urine.
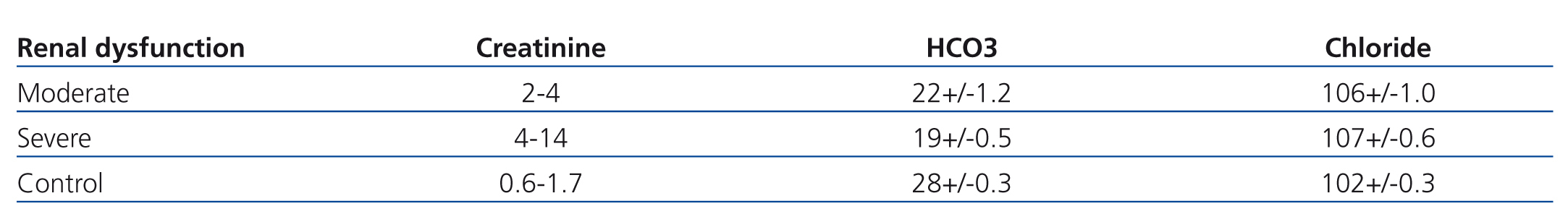
Patients with CKD present with normal and increased anion-gap metabolic acidosis at early and late stages respectively.9 The mechanism is the impaired renal bicarbonate generation with and without concomitant decreased bicarbonate absorption16 and retention of H+ ions.14 Total ammonium (NH4+) excretion begins to fall when GFR <40 to 50mL/min. Widmer et al.,11 evaluated 41 patients in two different creatinine value groups in an ambulatory setting and 39 patients as controls (Table 1). The renal failure group presented with serum creatinine between 2, 4mg/dL (moderate renal failure subgroup), and 14.4mg/dl (severe renal failure subgroup) respectively. The control group’s serum creatinine ranged from 0.6 to 1.7mg/dl. Serum HCO3 concentration was significantly lower in the moderate and severe groups in comparison with controls. Decrements in CO2 were proportional to the increment in serum creatinine and associated with increments in unmeasured anions, especially in the group with serum creatinine above 4mg/dl. There was no increment of unmeasured anions in the control group. In the other hand, serum Chloride (Cl‾ ) remained unchanged. In a retrospective analysis of 911 patients, Hakim et al.8 noted mixed normal and high anion gap metabolic acidosis, even early in the course of CKD. Dietary differences or abnormalities in gastrointestinal or renal absorption of organic/inorganic anions could account for the lower than expected anion gap in the early stages of renal failure. In fact serum anion gap could be altered by the accumulation of cations (calcium, magnesium or paraproteins).17 Kidney disease associated with more severe tubulointerstitial damage can be accompanied by more severe acidosis in the early stages of renal failure.18
Metabolic acidosis develops due to reduced renal mass and inability of the remaining nephrons to excrete the daily acid load through ammoniagenesis. The renal tubular production of NH3 is stimulated by intracellular acidosis. When the systemic acid load is modestly increased, balance is maintained by increase in NH4+ production and excretion. Failure to excrete sufficient NH4+ leads to the net retention of H+ ions and the development of metabolic acidosis. Defects in the ability to secrete NH4+ (proximal tubule) or H+ ions (distal tubule, type A intercalated cells), will translate into tubular acidosis through a pH dependent mechanism. Hyperkalemia, on the other hand, can induce intracellular alkalosis and also competes with potassium in the Na+/K+/2Cl‾ pump located in the thick ascending loop of Henle, diminishing NH4+ formation and preventing excretion of the acid load in the collecting tubules. As stated before single-nephron ammoniagenesis increases as compensation for decreased functioning nephrons.19 Generation of NH3 per nephron is increased but global ammoniagenesis is reduced. Decrease in urine NH4+ excretion is a reflection of decreased proximal renal tubular glutamine uptake and subsequent generation of NH4+ and bicarbonate from α-ketoglutarate.20 Decreased in HCO3 generation from glutamine metabolism leaves the kidney dependent on bicarbonate generation from titratable acid excretion, which is later reduced in later stages of renal failure. Other mechanisms generating metabolic acidosis are: HCO3 wasting due to decrease absorption, with bicarbonate excretion ranging from 4.25% to 17.65% in uremic patients,21 hyporeninemic hypoaldosteronism, and impaired distal renal acidification.22
Several factors have been implicated in the progression of renal failure, in particular intraglomerular hypertension.23 Experimental studies in Wistar rats, subject to 5/6 nephrectomies, indicated that metabolic acidosis of CKD could have a role in exacerbating proteinuria, tubulointerstitial injury, and worsening renal failure.24 This data has not been confirmed in other animal studies where the presence of metabolic acidosis delayed the progression of renal failure through prevention of calcium phosphate deposits and increased renal clearance of phosphate and lesser degree of hyperparathyroidism.25
The levels of NH3 in vascular and cortical tissues are increased when maximally produced by the renal tubule. The factors which influence the production of NH3- in the kidney are angiotensin II, potassium and aldosterone, whose levels are increased in entities like renovascular hypertension.26 Increased concentration of angiotensin II stimulates ammoniagenesis as well as gluconeogenesis. Potassium depletion and administration of aldosterone may also increase ammoniagenesis.27
Four mechanisms have been suggested to explain acidosis induced renal injury:
1) Increased in NH3- production28 and activation of the alternative complement pathway with generation of inflammatory mediators as well as alkalinization of the interstitium.29 Increased intrarenal ammonia reacts with the thioester bond in C3 and then induces C3b-like properties (form C3 covertase). Subsequent activation of the alternative pathway results in peritubular deposition of C3 and the membrane attack complex C5b-9 generating chemoattractants of tissue injury.30 On the other hand, there is lack of inflammation in the area of the renal medulla where there is increase NH3 concentration. High urea concentration can dissipate the cytotoxic effect of complement activated my NH3.
2) H+ retention induces endothelin and aldosterone mediated GFR decline even before metabolic acidosis, as demonstrated in 2/3 nephrectomized rat models. Dietary alkali better preserved GFR than both endothelin and aldosterone receptor antagonist.31
3) Acidosis induced aminoacid degradation through ubiquitin-proteasome with increased renal excretion of NH3.32
4) Ammoniagenesis may cause renal injury by stimulating the hypertrophy of renal tubular cells.27
Renal damage through cell mediated immunity, involving the interaction of cross reacting antigens and antibodies with Tamm-Horsfall glycoproteins in the tubule has been described in patients with renal tubular acidosis with a concomitant urinary tract infection or autoimmune liver disease.33
As patient approaches ESRD plasma bicarbonate concentration tend to stabilize between 12-20mEq/L. A small number of this patients have a normal plasma HCO3 concentration and anion gap maybe due to decreased protein intake (decreased sulfate generation) and/or increased fruit intake (citrate).34
TREATMENT
Bicarbonate supplementation preserves renal function in experimental CKD. Administration of either citrate or sodium bicarbonate to rats with CKD decreased the severity of tubulointerstitial disease and or the decline in GFR compared with controls receiving sodium chloride.28 The chronic administration of base to Han.S-PRD rats (an animal model of polycystic kidney disease) decreased cyst enlargement and prevented development of interstitial inflammation, chronic fibrosis, and severe renal failure.35 The mechanisms underlying the decline in GFR with metabolic acidosis was examined in rats by Nath et al.28 These investigators provided evidence for a link between acidosis induced stimulation of renal NH3 production by the kidney and progressive tubulointerstital injury, an effect initiated by activation of the complement cascade. Others have suggested that the stimulation of new HCO3 production in the kidney alkalinizes the interstitium encouraging precipitation of calcium in the kidney and renal injury.29 Finally, studies in rats using the remnant kidney model of CKD indicated that the decline in GFR was mediated in part by the actions of excess aldosterone and endothelin, the latter acting via activation of endothelin A receptors.36 The above mentioned studies suggest that metabolic acidosis when present can contribute to progression of CKD, and therefore base (alkali) treatment is warranted. Few studies have examined the effects of amelioration of metabolic acidosis on renal function in humans with CKD, not on renal replacement therapy (only three randomized underpowered controlled trials in ESRD).37 In short term studies, oral sodium HCO3 given to patients with moderate renal failure led to reduced protein catabolism, NH3 production, and tubular damage.38
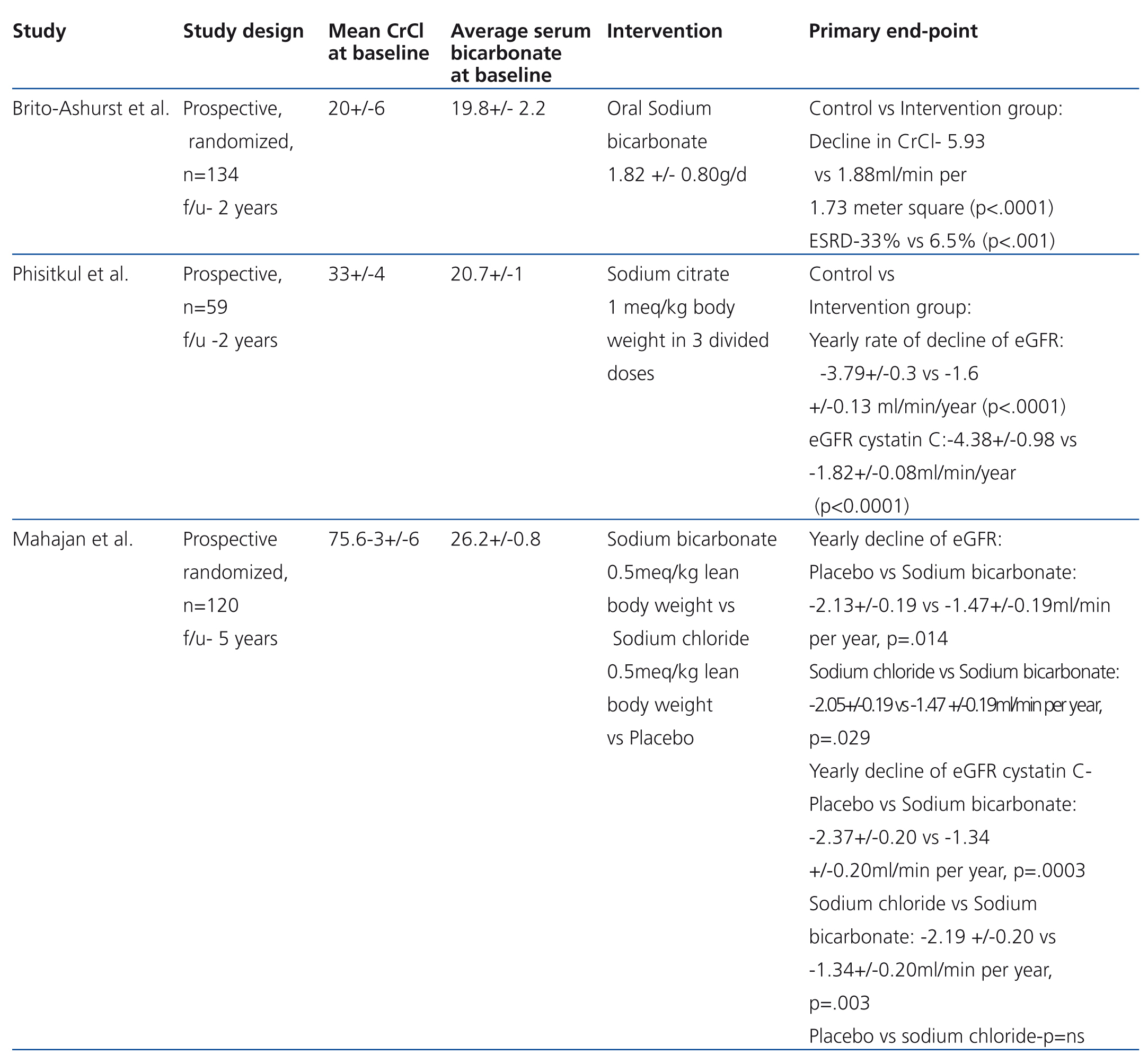
Three recently published studies tried to address this hypothesis (Table 2). Brito-Ashurst et al.,39 randomized 134 patients with creatinine clearance (CrCl) between 15-30ml/min/1.73m2 and serum HCO3 16 to 20mmol/L to either oral sodium HCO3- (600mg three times a week) or no treatment. Average age 54.7 years, mostly diabetics and hypertensives, 50% on an angiotensin converting inhibitor or angiotensin receptor blocker. Baseline blood pressure 124/75.5 in both groups, urinary protein g/24h 1.8-1.7 grams, in the control and HCO3 groups respectively. Two year follow up. Primary end point was rate of CrCl decline (>3 ml/min/1.73m2) and ESRD (CrCl<10 ml/min/1.73m2). Secondary end point was dietary protein intake. Decline in GFR was slower with HCO3 supplementation (CrCl 5.93 vs 1.88ml/min/1.73m2; P<.0001). Fewer patients in the treated group developed ESRD (6.5% vs 33%). There was no mayor difference in the incidence of edema in both groups (30 and 39%). More patients in the supplementation group required hypertensive therapy adjustments (61 vs 48%); this can introduce a bias element since blood pressure elevation could alter glomerular filtration. Bicarbonate supplementation was also associated with increased dietary intake, and decreased protein catabolism and increased lean body mass. Lack of a placebo controlled group, a double blind-design and single center, difficults reproducibility.
Phisitkul et al.40 evaluated 59 patients with hypertensive nephropathy and metabolic acidosis in a prospective interventional study. His group randomized 59 patients to sodium (Na+) citrate (30) and no therapy (29). The hypothesis was that oral Na+citrate (1mEq of bicarbonate/kg body weight per day in three divided doses) reduces endothelin production and tubulointerstitial injury in subjects with low GFR. Primary outcome was urine endothelin excretion (ET-1), secondary outcomes were changes in GFR by MDRD (modification in diet in renal disease) formula and by cystatin C derived GFR (GFRcys) (using the CKD-EPI equation). Urine N-acetyl-β-D-glucosaminidase excretion was measured as a marker of tubulointerstitial injury. Baseline GFR was 33.4±8.4 and 33.0±8.5 in both groups. Average systolic blood pressure at 6 months was 132.1±6.3 and 132.4±6.2 in controls and treated patients respectively. At 30 months GFR MDRD and GFRcys declined in both groups but was lower in controls (GFR MDRD ml/min: 24.9±9.7 vs 29.5±8.8 and GFRcys ml/min: 23.0±6.05 vs 27.8±7.4 p-value <.0001). Urine endothelin excretion was lower in the Na+ citrate group than in controls (4.83±1.47 vs 6.92±1.67 ng/g Cr P<.0001 at 30 months). No differences was noted in tubulointerstitial injury, as measured by urine N-acetyl-β-D-glucosaminidase excretion (8.±2.92 vs 9.01±92.07U/g Cr). The study suggested that treatment of metabolic acidosis associated with low GFR due to hypertensive nephropathy ameliorates progressive kidney injury. Limitations were the lack of randomization of Na+ citrate and the small population studied.
The same group41 randomized 120 patients to placebo, sodium chloride (NaCl) and sodium bicarbonate (NaHCO3) in a prospective placebo controlled blinded interventional 5 year study. Approximate average GFR was 75.5±6.3ml/min, values that were lower in cysGFR determination. Systolic blood pressure ranged between 152.6±14.7 to 155.3±12.6 ml/min.
The GFR rate of change in the NaHCO3 group was lower than the placebo and NaCl groups (-1.47±0.19 ml/min per year, -2.13±0.19 ml/min per year, P=.014, -2.05±0.19 ml/min, P=.029, respectively). GFR was statistically higher in the NaHCO3 group as compared with placebo but not with the NaCl group. By contrast, cysGFR was higher in the NaHCO3 group overall. Of interest, the net acid urinary excretion was lower in the NaHCO3 group in comparison with the placebo and NaCl groups respectively (19.2±5.4, 24.4±5.6, 24.9±5.9) which can explain that the HCO3- treated group had decreased intracellular acid generation, endothelin production and tubulointertitial injury. Limitations were again small groups and alkali effect on kidney creatinine and/or cystatin C handling, illustrating the need for a large scale study.
The magnitude of the hypobicarbonatemia present in patients with CKD is variable and, as stated previously, some patients can actually have normal serum HCO3- even in the face of severe renal failure.34 Therefore, the threshold for initiation of base therapy in patients with CKD is important to establish. To address this point Wesson et al.31 examined the effect of alkali on the progression of renal failure in rats with 2/3 nephrectomy who had CKD without significant hypobicarbonatemia. Alkali therapy slowed the decline in GFR, an effect which was related to increased endothelin and aldosterone production. Renal H+ content in these rats was greater than that of Sham-operated controls, observations consistent with tissue acid retention despite the absence of a reduced serum HCO3. As mentioned before41 subsequent studies have corroborated the benefitial effects of sodium bicarbonate in the preservation of GFR. Wesson and collegues42 again examined weather human subjects with stage 2 CKD (GFR 60-90ml/min) with macroalbuminuria but not hypobicarbonatemia had evidence of H+ retention and increased levels of endothelin-1 and aldosterone compared with those with a GFR of >90 ml/min. Levels of these hormones were reduced after 30 days of bicarbonate therapy. They were not able to assess the acid content of the kidney but they indirectly evaluated acid content of tissues by the impact of a HCO3 bolus on serum HCO3 and urinary net acid excretion. These results suggest that tissue acid retention was greater in the CKD 2 group. Certain assumptions were made including similar total body buffering capacity in both groups. However based on their previous studies it is not unreasonable to expect H+ retention in humans as renal function declines even if serum bicarbonate is in normal values. It appears that in both animals and humans as renal function and the ability to eliminate the daily acid load decline, acid retention occurs, which secondarily stimulates endothelin and aldosterone production that contributes to a further decline in GFR.43 The effects of excess endothelin and aldosterone on the kidney might not be the only mechanism by which metabolic acidosis contributes to a decline in GFR. Provision of dietary alkali better preserves GFR than administration of endothelin and aldosterone receptor antagonists.31
The trials mentioned above are the only human studies to date in patients with pre-ESRD that showed oral HCO3 as a therapeutic option with minimal side effects, inexpensive and with potential benefit in delaying progression of CKD.
CONCLUSION
There is a high incidence of CKD in the general population.2 Metabolic acidosis presents early in kidney disease and could be associated with worsening renal function due to perhaps an immunological process, and increased mortality due to protein catabolism. Bicarbonate supplementation represents a therapeutic option easy to apply, economical, and almost devoided of side effects.
Alkali therapy could protect against the progression of CKD, especially in early stages with normal serum bicarbonate levels. The mechanisms by which NaHCO3 therapy ameliorates nephropathy progression in unclear but nephrectomized animal models with reduced GFR provided insight to the hypothesis. Animals with reduced nephron mass and low GFR have acid retention compared with those with intact nephron mass despite no differences in serum acid-base parameters.44 Acid retention induces GFR decline mediated by tubulointerstitial injury through endothelin receptors.45
The paucity of well designed clinical trials in human subjects is counteracted by the good results obtained in these studies. The optimal therapeutic target as well as its efficacy and safety needs to be determined. The current situation, forces us to use the most practical resources in our armamentarium to try to delay, not to prevent unfortunately, the progression of kidney disease.
If confirmed by follow-up studies, implementation of this inexpensive and well tolerated therapy in at risk subjects could yield overall population and health system benefits by delaying the onset of kidney failure with its devastating effects on patient’s wellbeing and health care costs.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 2. Randomized prospective trials in the use of oral bicarbonate in patients with CKD
Table 1. Correlation of serum bicarbonate to changes in serum creatinine