Obesity has become a global pandemic1 and is considered an independent cardiovascular risk factor (CVRF) for the development and progression of chronic kidney disease (CKD).2 Furthermore, visceral adipose tissue itself is also a CVRF. Recent studies have found that the accumulation of ectopic renal fat (ERF) in non-adipose tissue, called “fatty kidney”, is related to obesity-associated kidney disease, and has a better correlation with total and visceral fat than measurements such as waist circumference or body mass index.2,3
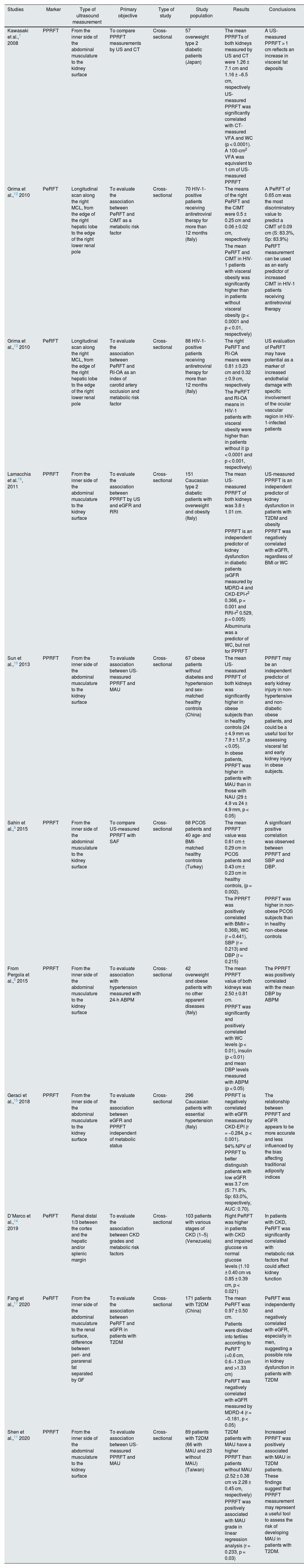
The anatomical distribution of ERF is divided into: a) renal sinus fat (RSF), adipose tissue located on the medial surface of the kidney, which shares space with vascular, nervous and lymphatic structures, major and minor calyces, renal pelvis and proximal ureter; b) perirenal fat (PeRF), which is located between the renal capsule and Gerota’s fascia (GF); c) pararenal fat (PaRF), which surrounds the kidney outside the GF; and d) renal parenchymal fat (RPF), which is the adipose tissue within the renal cortex and medulla. The impact of lipid toxicity on the kidneys arises from an accumulation of lipid droplets in the renal parenchyma (podocytes, mesangium and proximal tubular cells), contributing to kidney dysfunction through chronic inflammation mechanisms via the release of adipokines and cytokines that can exacerbate atherosclerosis and other cardiovascular pathological processes, oxidative stress, mitochondrial dysfunction and autophagy, in addition to direct mechanical compression by fat deposition resulting in hypoperfusion.4,5
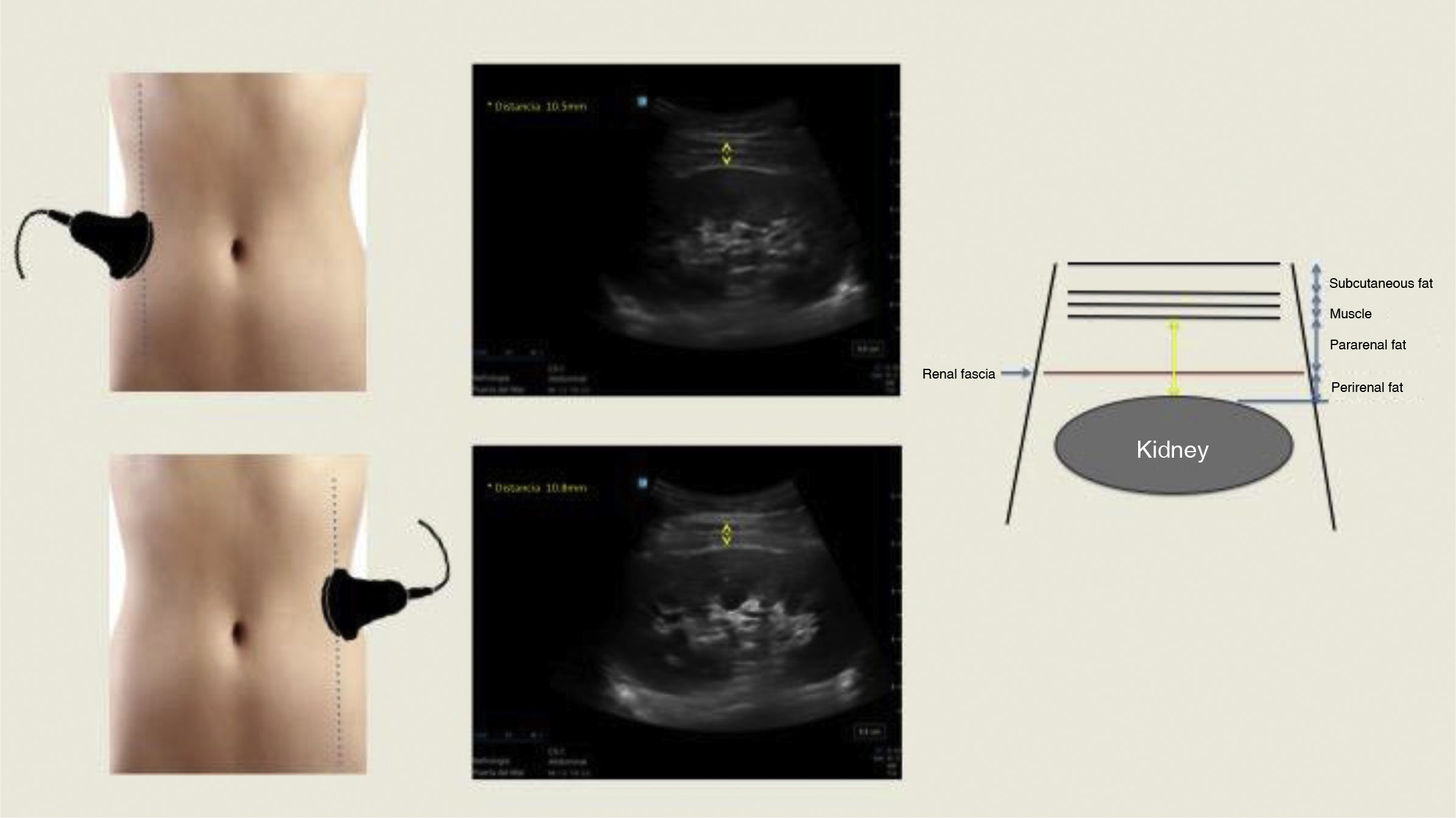
Multiple imaging techniques such as ultrasonography (US), computerised axial tomography (CT) and magnetic resonance imaging (MRI) have been used to quantify peri-pararenal fat thickness (PPRFT), RSF and RPF, respectively.4 These measurements provide a direct insight into the ectopic distribution of visceral fat. However, MRI and CT are expensive, and the latter also uses ionising radiation, making it difficult to be used on a large scale. US-measured abdominal fat thickness is a non-invasive, accessible, cost-effective and reliable imaging technique that correlates significantly with CT.6 Kawasaki et al.7 were pioneers in measuring PPRFT by US, demonstrating that the examination was simple and technically reproducible, and that satisfactory images were obtained without interference from intestinal gas. The examination is performed with the subject in the supine position, with the convex probe placed perpendicular to the skin on the lateral surface of the abdomen. A longitudinal scan is performed and the probe is slowly moved laterally until the optimal position is found, in which the surface of the kidney is almost parallel to the skin. As little pressure as possible is exerted on the probe to avoid compression of the fat layers. PPRFT measurement is performed from the inner surface of the abdominal muscles to the surface of the kidney and the mean US measurement of the maximum thickness values in both kidneys is recorded (Fig. 1). The technique was validated by performing comparisons with CT measurements, obtaining a good degree of correlation (r = 0.760; p = 0.003).7
Several studies have shown a significant positive correlation between PPRFT separately and together (PaRF, PeRF) with traditional cardiovascular risk (CVR) markers, such as arterial hypertension (HTN),8–10 insulin resistance,9 albuminuria,10,11 metabolic syndrome9,12–15 and endothelial damage12,13 (Table 1). Furthermore, in patients with and without obesity, diabetes and different stages of CKD, US PPRFT16 and perirenal fat thickness (PeRFT)17 measurements show a negative correlation with decreased glomerular filtration rate estimated with the Chronic Kidney Disease Epidemiology Collaboration, creatinine equation-2009 (CKD-EPI) and Modification of Diet in Renal Disease-4 (MDRD-4) equations. This association is particularly relevant, since chronic inflammation and metabolic dysfunction are prominent factors that contribute to the progression of CKD and the development of CV complications.2,14 Even in obese patients without HTN or diabetes, the PPRFT shows a positive correlation with the albumin-creatinine ratio (ACR), which makes it to be considered a predictive factor of early renal injury.10 Although there are no established cut-off values for normality in US-measured PPRFT, some studies have observed values in healthy controls ranging from 4.3 ± 2.3 to 7.95 ± 1.57 mm.9,10
Studies demonstrating the association between ectopic renal fat thickness and cardiovascular risk factors.
| Studies | Marker | Type of ultrasound measurement | Primary objective | Type of study | Study population | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Kawasaki et al.,7 2008 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To compare PPRFT measurements by US and CT | Cross-sectional | 57 overweight type 2 diabetic patients (Japan) | The mean PPRFTs of both kidneys measured by US and CT were 1.26 ± 7.1 cm and 1.16 ± −6.5 cm, respectively | A US-measured PPRFT > 1 cm reflects an increase in visceral fat deposits |
| US-measured PPRFT was significantly correlated with CT-measured VFA and WC (p < 0.0001). A 100-cm2 VFA was equivalent to 1 cm of US-measured PPRFT | |||||||
| Grima et al.,12 2010 | PeRFT | Longitudinal scan along the right MCL, from the edge of the right hepatic lobe to the edge of the right lower renal pole | To evaluate the association between PeRFT and CIMT as a metabolic risk factor | Cross-sectional | 70 HIV-1-positive patients receiving antiretroviral therapy for more than 12 months (Italy) | The means of the right PeRFT and the CIMT were 0.5 ± 0.25 cm and 0.06 ± 0.02 cm, respectively | A PeRFT of 0.65 cm was the most discriminatory value to predict a CIMT of 0.09 cm (S: 83.3%, Sp: 83.9%) |
| The mean PeRFT and CIMT in HIV-1 patients with visceral obesity was significantly higher than in patients without visceral obesity (p < 0.0001 and p < 0.01, respectively) | PeRFT measurement can be used as an early predictor of increased CIMT in HIV-1 patients receiving antiretroviral therapy | ||||||
| Grima et al.,13 2010 | PeRFT | Longitudinal scan along the right MCL, from the edge of the right hepatic lobe to the edge of the right lower renal pole | To evaluate the association between PeRFT and RI-OA as an index of carotid artery occlusion and metabolic risk factor | Cross-sectional | 88 HIV-1-positive patients receiving antiretroviral therapy for more than 12 months (Italy) | The right PeRFT and RI-OA means were 0.81 ± 0.23 cm and 0.32 ± 0.9 cm, respectively | US evaluation of PeRFT may have potential as a marker of increased endothelial damage with specific involvement of the ocular vascular region in HIV-1-infected patients |
| The PeRFT and RI-OA means in HIV-1 patients with visceral obesity were higher than in patients without it (p < 0.0001 and p < 0.001, respectively) | |||||||
| Lamacchia et al.16, 2011 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To evaluate the association between PPRFT by US and eGFR and RRI | Cross-sectional | 151 Caucasian type 2 diabetic patients with overweight and obesity (Italy) | The mean US-measured PPRFT of both kidneys was 3.8 ± 1.01 cm. | US-measured PPRFT is an independent predictor of kidney dysfunction in patients with T2DM and obesity |
| PPRFT is an independent predictor of kidney dysfunction in diabetic patients (eGFR measured by MDRD-4 and CKD-EPI-r2 0.366, p = 0.001 and RRI-r2 0.529, p = 0.005) | PPRFT was negatively correlated with eGFR, regardless of BMI or WC | ||||||
| Albuminuria was a predictor of WC, but not for PPRFT | |||||||
| Sun et al.,10 2013 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To evaluate association between US-measured PPRFT and MAU | Cross-sectional | 67 obese patients without diabetes and hypertension and sex-matched healthy controls (China) | The mean US-measured PPRFT of both kidneys was significantly higher in obese subjects than in healthy controls (24 ± 4.9 mm vs 7.9 ± 1.57, p < 0.05). | PPRFT may be an independent predictor of early kidney injury in non-hypertensive and non-diabetic obese patients, and could be a useful tool for assessing visceral fat and early kidney injury in obese subjects. |
| In obese patients, PPRFT was higher in patients with MAU than in those with NAU (29 ± 4.9 vs 24 ± 4.9 mm, p < 0.05) | |||||||
| Sahin et al.,9 2015 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To compare US-measured PPRFT with SAF | Cross-sectional | 68 PCOS patients and 40 age- and BMI-matched healthy controls (Turkey) | The mean PPRFT value was 0.61 cm ± 0.29 cm in PCOS patients and 0.43 cm ± 0.23 cm in healthy controls, (p = 0.002). | A significant positive correlation was observed between PPRFT and SBP and DBP. |
| The PPRFT was positively correlated with BMI/r = 0.368), WC (r = 0.441), SBP (r = 0.213) and DBP (r = 0.215) | PPRFT was higher in non-obese PCOS subjects than in healthy non-obese controls | ||||||
| From Pergola et al.,8 2015 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To evaluate association with hypertension measured with 24-h ABPM | Cross-sectional | 42 overweight and obese patients with no other apparent diseases (Italy) | The mean PPRFT value of both kidneys was 2.50 ± 0.81 cm. | The PPRFT was positively correlated with the mean DBP by ABPM |
| PPRFT was significantly and positively correlated with WC levels (p < 0.01), insulin (p < 0.01) and mean DBP levels measured with ABPM (p < 0.05) | |||||||
| Geraci et al.,15 2018 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To evaluate the association between eGFR and PPRFT independent of metabolic status | Cross-sectional | 296 Caucasian patients with essential hypertension (Italy) | PPRFT is negatively correlated with eGFR measured by CKD-EPI (r = −0.284, p < 0.001). | The relationship between PPRFT and eGFR appears to be more accurate and less influenced by the bias affecting traditional adiposity indices |
| 94% NPV of PPRFT to better distinguish patients with low eGFR was 3.7 cm (S: 71.8%, Sp: 63.0%, respectively, AUC: 0.70). | |||||||
| D’Marco et al.,14 2019 | PeRFT | Renal distal 1/3 between the cortex and the hepatic and/or splenic margin | To evaluate the association between CKD grades and metabolic risk factors | Cross-sectional | 103 patients with various stages of CKD (1–5) (Venezuela) | Right PeRFT was higher in patients with CKD and impaired glucose vs normal glucose levels (1.10 ± 0.40 cm vs 0.85 ± 0.39 cm, p < 0.021) | In patients with CKD, PeRFT was significantly correlated with metabolic risk factors that could affect kidney function |
| Fang et al.,17 2020 | PeRFT | From the inner side of the abdominal musculature to the renal surface, difference between peri- and pararenal fat separated by GF | To evaluate the association between PeRFT and eGFR in patients with T2DM | Cross-sectional | 171 patients with T2DM (China) | The mean PeRFT was 0.97 ± 0.50 cm. | PeRFT was independently and negatively correlated with eGFR, especially in men, suggesting a possible role in kidney dysfunction in patients with T2DM |
| Patients were divided into tertiles according to PeRFT (<0.6 cm, 0.6−1.33 cm and >1.33 cm) | |||||||
| PeRFT was negatively correlated with eGFR measured by MDRD-4 (r = −0.181, p < 0.05) | |||||||
| Shen et al.,11 2020 | PPRFT | From the inner side of the abdominal musculature to the kidney surface | To evaluate association between US-measured PPRFT and MAU | Cross-sectional | 89 patients with T2DM (66 with MAU and 23 without MAU) (Taiwan) | T2DM patients with MAU have a higher PPRFT than patients without MAU (2.52 ± 0.38 cm vs 2.28 ± 0.45 cm, respectively) | Increased PPRFT was positively associated with MAU in T2DM patients. These findings suggest that PPRFT measurement may represent a useful tool to assess the risk of developing MAU in patients with T2DM. |
| PPRFT was positively associated with MAU grade in linear regression analysis (r = 0.233, p = 0.03) |
ABPM: Ambulatory Blood Pressure Monitoring; AUC: area under the curve; BMI: body mass index; CIMT: carotid intima-media thickness; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CT: computerised axial tomography; CVRF: cardiovascular risk factor; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; GF: Gerota’s fascia; HIV-1: human immunodeficiency virus type 1; HTN: arterial hypertension; MAU: microalbuminuria; MCL: midclavicular line; MDRD-4: Modification of Diet in Renal Disease; NAU: normal albuminuria; NPV: negative predictive value; PCOS: polycystic ovary syndrome; PPRFT: para-perirenal fat thickness; PeRFT: perirenal fat thickness; RI-OA: resistive index of ophthalmic artery; RRI: renal resistive index; S: sensitivity; SAF: subcutaneous abdominal fat; SBP: systolic blood pressure; Sp: specificity; T2DM: diabetes mellitus type 2; US: ultrasonography; VFA: visceral fat area; WC: waist circumference.
In conclusion, the use of US to measure PPRFT is a promising tool to identify obese patients with risk of developing CKD secondary to lipotoxicity, and can be easily incorporated into daily clinical practice. However it is important, to mention that further research is needed to establish standardised clinical protocols and population cut-off points, and to fully understand the biological mechanisms underlying this association.
Ethical responsibilitiesThe study complied with the principles set out in the Declaration of Helsinki. No experiments were performed on humans or animals for this study.
Declaration of Generative AI and AI-assisted technologies in the writing processThe authors declare that they have not used AI or AI-assisted technologies to write this manuscript.
FundingNo funding was received for this paper.