Intravenous iron therapy is increasingly being used worldwide to treat anemia in chronic kidney disease and more recently iron deficiency in heart failure. Promising results were obtained in randomized clinical trials in the latter, showing symptomatic and functional capacity improvement with intravenous iron therapy. Meanwhile, confirmation of clinical benefit in hard-endpoints such as mortality and hospitalization is expected in large clinical trials that are already taking place. In chronic kidney disease, concern about iron overload is being substituted by claims of direct cardiovascular benefit of iron supplementation, as suggested by preliminary studies in heart failure.
We discuss the pitfalls of present studies and gaps in knowledge, stressing the known differences between iron metabolism in heart and renal failure. Systemic and cellular iron handling and the role of hepcidin are reviewed, as well as the role of iron in atherosclerosis, especially in view of its relevance to patients undergoing dialysis. We summarize the evidence available concerning iron overload, availability and toxicity in CKD, that should be taken into account before embracing aggressive intravenous iron supplementation.
La suplementación con hierro intravenoso es cada vez más frecuente a nivel mundial en el tratamiento de la enfermedad renal crónica (ERC) y, más recientemente, se ha utilizado para tratar el déficit de hierro en la insuficiencia cardiaca (IC). En esta última, se obtuvieron resultados alentadores en estudios clínicos randomizados, demostrando una mejoría sintomática y en la capacidad funcional de la IC. Sin embargo, aún se aguarda la confirmación de beneficio clínico en los objetivos principales como mortalidad y hospitalización en los estudios clínicos amplios que actualmente están en curso.
En la presente revisión se discuten las dudas que se presentan en los estudios actuales y las lagunas en el conocimiento en relación con las diferencias entre la ERC y la IC sobre el metabolismo del hierro. Se ofrece una revisión sobre el manejo celular y sistémico del hierro, así como sobre el papel de la hepcidina. Adicionalmente, se explica el papel del hierro en la ateroesclerosis y su importancia principalmente en los pacientes en diálisis. Por otra parte, en esta revisión se incluye un resumen actualizado sobre lo que existe de evidencia en relación con la sobrecarga de hierro, su disponibilidad y toxicidad en la ERC, lo cual se debe tener en cuenta antes de decidir realizar una terapia intravenosa agresiva con hierro.
Intravenous (IV) iron supplementation is nearly universal in hemodialysis and is widely used in non-dialysis chronic kidney disease (CKD).1,2
More recently, IV iron has been increasingly used in chronic heart failure (HF), as new evidence has suggested clinical benefit of iron-repletion.
CKD and HF are commonly coexistent conditions and both share common risk factors and comorbidities.3 However, there are some important differences worth noting.
Iron in heart failureMyocardial iron content has been shown to be lower in HF, measured by magnetic resonance imaging (MRI)4 as well as in samples of left ventricle of the human heart.5 The role of iron in keeping the normal activity of key enzymes in the mitochondria has been invoked as a potential, although speculative explanation for the symptomatic benefit of iron administration in this setting.5,6
Administration of intravenous (IV) iron in patients with HF is becoming a common clinical practice as recent studies have been published showing promising results with this approach.7–9 Indeed, the idea of treating anemia in these patients to improve outcomes is not new. Anemia and HF share common symptoms, such as fatigue, breathlessness and reduced functional capacity. The coexistence of both conditions exacerbates disability and contributes to poor clinical prognosis.10
The first trials addressing anemia treatment in HF were designed with erythropoietin stimulating agents (ESA's).11–14 Significant improvements in hemoglobin concentration, left ventricular ejection fraction and New York Heart Association (NYHA) functional classes were found. When hard endpoints, such as mortality and hospitalizations were analyzed in a large randomized, double-blind trial with darbepoetin-alfa (RED-HF), it ended in disappointment, as it did not show any benefit, but increased the risk of thromboembolic events and ischemic stroke.15,16
Similar results had been found in randomized clinical trials (RCT's) with the use of ESA's in CKD.17–19
However, as the first trials in cardiac failure used concomitant IV iron and ESA's, the idea that iron could play a beneficial role in HF has been reconsidered.
Initial randomized trials with a small number of HF patients20,21 indicated clinical improvement in isolated iron sucrose administration. Subsequent larger trials used ferric carboxymaltose. These have shown significant symptomatic improvement in the treated group, measured by Patient Global Assessment (PGA) score or exercise capacity (six-minute walk test (6MWT), peak oxygen consumption7,9) ultimately leading to improvement in the NYHA functional scale. Taking FAIR-HF (Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency),8 a placebo double-blind randomized trial with 459 patients, roughly 50% patients in the treated group showed improvement (PGA score, NYHA class and 6MWT) compared to 30% in the placebo group (who received saline). These results were replicated in other clinical trials, such as CONFIRM-HF (Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency)7 and EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise. Capacity in Patients With Iron Deficiency and Chronic Heart Failure).9
The magnitude of the placebo cannot be overstated, as one third of participants reported improvement without iron therapy, also depicting the limitations of symptom-based HF classifications and management.22 Although merely speculative, one may suspect that on the treatment arm, out of the 50% of patients that improved, only 20% really had any benefit.
These findings were limited to symptomatic improvement, however, two meta-analysis of randomized trials23,24 suggested benefit of IV iron in HF in reducing hospitalizations and mortality.
As testing this hypothesis requires a larger sample and longer follow-up, there are already many ongoing large trials of IV Iron in Heart Failure.25
Nonetheless, the American College of Cardiology/American Heart Association/Heart Failure Society of America26 issued a focused update of the 2013 Guideline for the Management of HF, including a new recommendation that intravenous repletion of iron may improve exercise capacity and quality of life and consideration of ferric carboxymaltose administration. IV iron is also already recommended in the European Society of Cardiology Heart Failure 2016 Guidelines for the treatment of symptomatic patients with systolic chronic heart failure and iron deficiency.27
The above mentioned RCT's with ferric carboxymaltose7–9 included patients with absolute or functional iron deficiency, independently of the presence of anemia, however none was powered to separately evaluate the effects of the correction of iron deficiency in anemic and non-anemic groups.
The long-term safety of iron therapy in HF is presently unknown.
In medical history, many were the promising drugs that have fallen from grace.
Recent history in cardiology shows the examples of HDL-raising drugs, an apparent good idea to reduce a risk factor for atherosclerotic disease and lidocaine to prevent arrhythmias in the acute coronary syndrome, that did not show any benefit when hard endpoints were tested.28,29
Hepcidin's roleIt is important to note that human iron metabolism is tightly regulated, in order to protect against iron deprivation and overloading.
Approximately, only 1–2mg is absorbed in the diet each day, matching the same amount that is lost every day.
The main defense mechanism against excess iron is the capture and storage in the core of the hollow globular structure of the ferritin molecule. Iron loading, when severe, may overcome the sequestrating capacity of cellular ferritin and result in the generation of toxic reactive oxygen species (ROS), which are cytotoxic.30,31
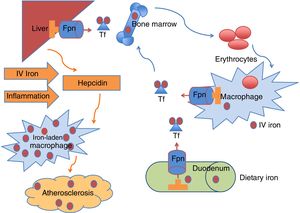
The delicate balance between what is absorbed and used is mastered by hepcidin (Fig. 1), a 25 amino-acid hormone synthesized by the liver.32 This hormone dictates the expression of ferroportin, a transporter that lets iron cross the cell membrane in enterocytes and macrophages,33 making it available to transferrin, which delivers iron to tissues. Recently another player was discovered: erythroferrone, a suppressor of hepcidin secreted by erythroblasts in the marrow and extramedullary sites that facilitates iron delivery during stress erythropoiesis but also contributes to iron overload in anemias with ineffective erythropoiesis.34
Iron circulates in the bloodstream bound to transferrin (Tf). The majority of Tf-bound iron is delivered to the bone marrow for erythrocyte production, and the excess transported to the liver for storage. Iron homeostasis is maintained predominantly by recycling iron from senescent erythrocytes via reticuloendothelial macrophages. A smaller amount of iron is provided by dietary absorption in the duodenum, which is matched by an unregulated loss of iron through desquamation and blood loss. The hormone hepcidin is mainly produced in the liver and regulates plasma iron concentrations by controlling ferroportin (Fpn) expression on duodenal enterocytes, recycling macrophages of the spleen and liver, and hepatocytes. Increased hepcidin limits iron availability through induced degradation of Fpn, supressing iron export from macrophages, that become iron-laden, a proatherogenic phenotype. Known stimulus to hepcidin synthesis are IV (intravenous) iron administration, iron excess and inflammation.
When iron is administered intravenously, the first step is phagocytosis by macrophages, to degrade the carbohydrate shell inside lysosomes. After that, it will be released to transferrin to be transported to cells, if hepcidin levels are low. Otherwise, it will remain inside ferritin granules in the cytoplasm of macrophages and other reticuloendothelial cells, such as Kupffer cells in the liver.
Hepcidin is increased in inflammation (e.g. chronic disease) and in the presence of iron itself, in a negative feedback, counteracting the release of stored iron and causing macrophages to be iron-replete.
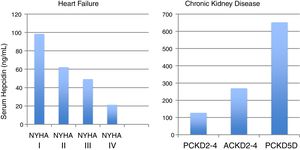
Hepcidin levels take opposite directions in CKD and HF (Fig. 2).35,36 In CKD, considered a chronic inflammatory condition, hepcidin significantly increases according to the CKD stage,35,37 while in Heart Failure (HF), the exact opposite occurs, as hepcidin paradoxically declines as HF progresses.36 Moreover, low hepcidin independently relates to unfavorable outcomes in HF.36
Median serum hepcidin levels across heart failure stages (NYHA) and chronic kidney disease (CKD) stages, in adult (A) and pediatric (P) patients.35,36
The decline of hepcidin in cardiac failure is thought to be a consequence of chronic hypoxia due to lung congestion, that increases the expression of hypoxia inducible factors.6 Also, many HF patients have significant insensible blood loss due to anti-aggregation and/or hypocoagulation causing real iron deficiency and a consequent suppression of hepcidin.25,36
Oral or IV ironLow hepcidin levels in HF patients would predict oral iron to be efficacious, as its absorption in the enterocyte through ferroportin is not likely to be blocked.
IRONOUT-HF (Iron Repletion Effects on Oxygen Uptake in Heart Failure) trial,38 a placebo controlled randomized trial with 225 participants, has shown that apparently this is not the case in HF. Among iron-deficient participants with HF, high-dose oral iron produced minimal improvement in iron stores, and did not improve exercise capacity over a period of 16 weeks. The authors mentioned that hepcidin levels in the patients included in this study were unexpectedly high, and that this could have contributed to the negative results. Others have suggested that intestinal wall edema caused by congestion in HF, could be to blame for the lack of efficacy of oral iron.6
In non-dialysis CKD patients (CKD-ND), the subject of oral efficacy was tested in two large clinical trials that showed conflicting results.39,40
REVOKE40 randomly assigned patients with stage 3 and 4 CKD to either oral iron or IV iron sucrose. The primary outcome was the between group difference in slope of measured glomerular filtration rate (mGFR) change over two years. Although both IV and oral iron proved to have similar efficacy and mGFR decline, the trial was terminated early due to higher risk of infection and cardiovascular complications in the IV iron treatment group.
FIND-CKD39 found that IV ferric carboxymaltose (Ferrinject®) targeting ferritin 400–600mg/L was more effective than oral iron, while no difference was found in adverse events.
The above-mentioned studies had different patient inclusion and exclusion criteria, treatment modalities, study-drug exposure time, adverse event reporting and funding, as REVOKE was funded by the National institutes for Health (NIH), while FIND-CKD was funded by the manufacturer of Ferrinject®.41
The question of whether adult CKD patients with iron-deficient anemia should get initial treatment with oral or intravenous iron is still open.42
Nowadays KDIGO guidelines recommend iron supplementation for anemia treatment in adult CKD-ND as long as TSAT is<30% and ferritin is <500ng/ml.42
In fact, IV iron use increased in CKD-ND patients43–45 as the use of ESA's decreased due to safety concerns, as a result of FDA warnings after the publication of TREAT's study results (A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease).17
Changes in reimbursement policies were also responsible, at least in part, for the continued increase in IV iron use in CKD patients.2,46 It is recognized that IV iron use has an ESA's dose-sparing effect, reducing the overall cost of anemia treatment, which is welcome worldwide, as CKD's-related costs pose a major challenge to healthcare systems.
Many patients with CKD do progress to stage 5, some needing dialysis for the rest of their lives. This means lifelong treatment for anemia with regular administration of IV iron for the majority of these patients.
Elevated hepcidin seen in many hemodialysis patients is the probable cause of the poor absorption of oral iron,32 leaving the IV route as the most widely used. There is no physiological way to eliminate excess iron. Blood losses are difficult to estimate and may be very different from one patient to the next. Moreover, ferritin and TSAT, the two most widely available tests for assessing iron status, lack sensitivity and specificity to adequately guide treatment.42 Indeed, while low ferritin levels are predictive of iron deficiency, high ferritin may reflect inflammation instead of overload.44 In hemodialysis patients, iron use increased in most countries in recent years, with notable increases in ferritin but not TSAT levels.47
Iron overload in CKDIn 2012, a MRI study of liver iron stores in a cohort of 119 fit hemodialysis patients receiving IV iron and ESA therapy found that 84% of patients had hepatic iron overload, and that 30% of patients had severe iron overload.48 Other MRI studies with smaller number of patients had also showed increased liver iron content (LIC) in hemodialysis patients.49–51
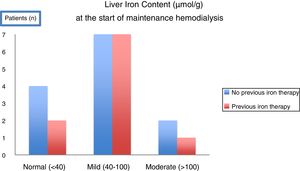
With the intent of knowing the scenario in our clinical practice, a prospective observational study using MRI to evaluate liver iron deposits was made in 23 consecutive CKD patients at the time of initiation of maintenance hemodialysis.52 Unexpectedly, at the start of hemodialysis, only 26% of patients had normal LIC and the remaining already had mild to moderate iron overload. Moreover, iron overload was observed even in patients who had not received significant amounts of iron or any iron at all (Fig. 3). After 12 months in maintenance hemodialysis under anemia treatment including IV iron therapy according to current guidelines, 7 patients repeated MRI. LIC increased significantly,52 matching the levels measured in the cohort of chronic hemodialysis patients by Rostoker et al.48 These results added new information on the high frequency of iron overload at the start of dialysis, even in patients who had not received iron before, and showed how fast is the aggravation in patients under regular IV iron in hemodialysis.
In a T2* MRI study evaluating Liver Iron Content (LIC) in 23 consecutive patients CKD5 initiating maintenance hemodialysis, the majority of patients (17/23) had already mild to moderate (>40–200μmol/g) hepatic overload. Overload was observed even in patients who had not been submitted to any previous oral or intravenous iron therapy52.
Although direct liver dysfunction is not seen, the unexpected findings of iron overload in the majority of incident and prevalent hemodialysis patients raise concern and should prompt the search for its causes, as iron homeostasis in CKD is far from being well studied. It also reinforces the need to search for long-term implications of IV iron administration.53
Iron and atherosclerosisCKD patients are afflicted with premature ageing and aggressive atherosclerotic disease, with mortality being primarily due to cardiovascular events.54 Intravenous administration of iron bypasses essential regulatory mechanisms and can cause endothelial damage via the production of reactive oxygen species, through non-transferrin bound iron (NTBI).55
The immunological phenotype of patients with CKD has similarities to that of elderly individuals, including reduced numbers of naive lymphocytes, and accumulation of pro-inflammatory CD28-T cells.56 Indeed in CKD, an increase in the neutrophil to lymphocyte and platelet-to lymphocyte ratios is reported to be associated with cardiovascular events and mortality.57 Iron is known to be cytotoxic to lymphocytes, probably contributing to this phenomenon.44,58–60
The so-called iron hypothesis, first proposed by Sullivan55 suggested the difference between genders in atherosclerosis was due to iron deprivation in women (due to regular menstrual blood loss) and not to estrogen protection against atherosclerotic disease.
In fact, ferritin levels associate with carotid-intimal thickness, a surrogate marker of atherosclerosis.61,62 Following the discovery of hepcidin, another view prevailed, putting the iron-laden macrophages, an aggressive phenotype for the atherosclerotic process in the center of the link between iron and atherosclerosis.55,63 According to this view, high hepcidin levels may act as a potential iron-dependent risk factor for atherosclerosis by regulating macrophage iron accumulation and the progression of atherosclerotic plaque (Fig. 1). Although further studies are needed to fully elucidate the impact of macrophage-stored iron, hepcidin is increasingly seen as a damaging uremic toxin,64 contributing to atherosclerosis. In addition to hepcidin's inevitable accumulation in CKD, anemia's present-day therapeutic options with high doses of IV iron contribute to stimulate its elevation, so, for the time being, elevated chronic hepcidin in CKD is the elephant in the room that nobody wants to see. The prevalence and the contribution of atherosclerosis for poor cardiovascular outcomes in CKD cannot be overstated, where it is well known that the excess risk of cardiovascular disease in patients with CKD is only partially explained by the presence of traditional risk factors.65,66 It is possible that the use of novel treatments for renal anemia that inhibit hepcidin via various pathways may allow the release of iron from its storage in macrophages as well as improvement of oral iron absorption.32,67,68
Iron and adverse prognosisHowever, the question that remains is ‘does iron contribute to worse outcomes in CKD?’ It is known that high ferritin levels are associated with mortality.69 When data from 18261 patients from DOPPS 2009–201570 were analyzed, median ferritin was distinct in different regions: 718ng/mL in the USA, 405 in Europe and 83 in Japan. Interestingly, high ferritin levels were associated with elevated mortality (relative to region-specific medians) in all three regions. Although these differences between median ferritin levels may relate to different genetic and environmental backgrounds, they may as well as be partly explained by different medical practices, including IV iron administration, these being highest in the USA, followed by Europe and notably lower in Japan.70 It is worth noting that in this prospective cohort crude all-cause mortality rate during the 1-year follow-up period was much lower in Japan 0.051/year (137 deaths) than in Europe 0.139/year (757 deaths) and the USA 0.146/year (962 deaths). Japan is indeed the country with the greatest longevity of its hemodialysis population,71 and its practices should be analyzed in order to understand what could be reproduced in other countries to improve outcomes.
In fact, mean ferritin values have increased in hemodialysis patients worldwide, from 1997 to 2011, in keeping with increasing iron administration.1 However ferritin is not just an indicator of iron stores, but is also a marker of inflammation. While high ferritin levels are strongly associated with mortality, this association is almost eliminated after adjustment for patient malnutrition-inflammation complex syndrome, as seen in a historical cohort of 58,058 maintenance HD patients in the USA, where the association between serum ferritin levels>800ng/ml and mortality seemed mostly to be due to the confounding effects of malnutrition, inflammation and cachexia syndrome.72 When practices of intravenous iron administration were studied, iron doses>400mg/month tended to be associated with higher death rates, while moderate doses of administered intravenous iron were associated with improved survival.72
In another observational study analyzing DOPPS cohorts,47 iron doses above 300mg/month were associated with higher mortality and hospitalization risk.
As observational data only reveal association not causality, in part due to selection bias by indication, many have called for well-powered clinical trials to evaluate the safety of different IV iron-dosing strategies in HD patients.
However, until now, only one study has tried to address this issue. PIVOTAL study,73 a prospective clinical trial, randomly assigned 2141 adults undergoing hemodialysis to a high-dose intravenous iron regimen administered proactively or to a low-dose regimen administered reactively. The results of this non-inferiority trial showed that the use of high-dose IV iron was not associated with higher risks of death, major adverse cardiovascular events, or infection. A primary end-point event occurred in 320 patients (29.3%) in the high-dose group, as compared with 338 (32.3%) in the low-dose group (hazard ratio, 0.85; 95% CI, 0.73–1.00; P<0.001 for noninferiority; P=0.04 for superiority). The authors speculate that the dose-sparing effect of IV high-dose iron on ESA's might have contributed to the cardiovascular safety profile, and also raise the possibility that direct cardiovascular benefits were due to replacement of iron, in line with the positive results found in HF. The study was restricted to a single country (UK), limiting the generalizability of the findings to other dialysis populations worldwide. Critics pointed to the substantial amount of iron and ESA spent in both arms of the study,74 not reflecting the usual practice in other countries. Indeed, insufficient rinsing of the extracorporeal circuit of dialysis apparatus may cause true iron deficiency, partly explaining the observed asymmetry of iron and ESA needs between hemodialysis facilities.75 The median follow-up was 2.1 years, a long period considering logistical and financial issues, however short for providing responses to long-term safety issues. Atherosclerosis progression and critical events take longer to reveal, and are probably not amenable to be tested in short-term RCT's. Remember the Japanese longevity experience!
In view of the findings of this single study, some authors already advocate that keeping low doses of iron administration is harmful for patients in hemodialysis,76,77 stating that high IV iron doses are safe, refuting previous iron toxicity concerns.
Iron research biasIt is worth pointing out that there is considerable financial pressure that can influence the design and running of clinical trials, causing biases that need to be taken into account.
One may note, for example, the lack of diversity of the names of investigators studying the use of IV iron to treat renal failure and HF. A similar problem may well exist in the lists of experts producing guidelines. It also needs to be remembered that most clinical trials are funded by industry.
These problems may apply to almost every RCT undertaken today in all fields of medicine. Independent researchers cannot afford the costs required for clinical trials. Perhaps IV iron is a good example of what is at fault with medical research worldwide.
ConclusionAtherosclerosis and heart failure are distinct entities (although they do frequently coexist) that may benefit from different approaches regarding iron repletion.
The results of ongoing large clinical trials on hard-endpoints are expected to clarify whether there is benefit with the use of iron in HF.
There are reasons to be prudent with the use of iron in CKD, mainly in the context of the known biology, with catalytic redox potential and the contribution of iron disturbing metabolism in atherosclerosis. The prolonged nature of iron supply to hemodialysis patients must also be taken into consideration.
The need to find the minimum dose of iron to obtain clinical benefit while at the same time assuring the safety of the patient cannot be understated.
CKD patients are already iron-replete, as demonstrated in magnetic resonance studies, only lacking available iron due to hepcidin's blockade to deliver stored iron to cells. This group of patients is currently overwhelmed with IV iron administration worldwide.
While long-term toxicity of aggressive iron repletion is unknown, new treatment strategies are urgently needed.
Conflict of interestsThe author has received lecture fees from Zambon.
The author would like to thank Luis Inchausteguí, Karina Soto and Simon Davis for their valuable help with language editing.