In acute kidney injury (AKI), a strong inflammatory component is activated in response to the renal damage, and one of the main mediators behind this process is the pro-inflammatory interleukin 6 or IL-6. Beside to this phenomenon, there are also alterations in different components of mineral metabolism, such as those dependent on fibroblast growth factor (FGF)23 and the anti-ageing cofactor klotho. The aim of this work was to explore the association between renal function and systemic levels of IL-6, as well as FGF23 and klotho in the early stages of AKI, analysing the predictive capacity of IL-6 in early mortality associated with AKI.
Material and methodsPlasma levels of IL-6, klotho and FGF23 were analysed in samples from 28 patients with AKI and related to renal function on hospital admission, and after 24 and 72 h. In addition, the predictive capacity of IL-6 on AKI-associated mortality was analysed at the three study time points. In an experimental nephrotoxic -AKI mouse model, systemic IL-6 and FGF23 values were also analysed 24 and 72 h after induction of kidney damage, as well as in mice overexpressing the anti-ageing protein, klotho.
ResultsSystemic IL-6 levels increased in AKI patients, especially in hospital admission time, and decreased in parallel with improving renal function. At the same time as IL-6 values increased, there was an increase in FGF23 and a decrease in klotho levels, with a significant and positive correlation between IL-6 and FGF23 levels. In addition, we obtained that systemic IL-6 levels were a good predictor of mortality in these patients, with an area under the curve equal to one at 72 h after AKI. In the experimental mouse AKI model, we also observed an increase in plasma levels in both IL-6 and FGF23 after 24 h of kidney damage. Nevertheless, in transgenic mice overexpressing klotho, there was no such increase in any of them.
ConclusionsThere is an association between renal damage and increased levels of IL-6 and FGF23 in patients with AKI, especially on hospital admission time. Moreover, IL-6 levels are able to predict mortality in these patients, being a promising prognostic biomarker at any study time with a strong prediction at 72 h after patient admission. Maintaining adequate klotho levels could prevent the IL-6 mediated inflammatory response and therefore also reduce the degree and severity of renal damage after AKI.
En un fracaso renal agudo (FRA) se activa un fuerte componente inflamatorio como respuesta al daño renal, y uno de los principales mediadores de este proceso es la interleuquina pro-inflamatoria 6 o IL-6. A su vez, ligado a este fenómeno también se producen alteraciones en los distintos componentes del metabolismo mineral como son aquellos dependientes del factor de crecimiento de fibroblasto (FGF)23 y el cofactor anti-envejecimiento klotho. El objetivo de este trabajo fue explorar la asociación entre la función renal y los niveles sistémicos tanto de IL-6, así como de FGF23 y klotho, en las etapas tempranas de un FRA, analizando la capacidad predictora de IL-6 en la mortalidad temprana asociada al FRA.
Materiales y métodosSe analizaron los niveles plasmáticos de IL-6, klotho y FGF23 en muestras procedentes de 28 pacientes con FRA, y se relacionaron con la función renal en el momento del ingreso hospitalario, y después de 24 y 72 horas. Además, se analizó la capacidad predictora de IL-6 sobre la mortalidad asociada al FRA en los tres tiempos del estudio. En un modelo experimental de FRA nefrotóxico en ratón, se analizaron también los valores sistémicos de IL-6 y FGF23 tras las 24 y 72 horas desde la inducción del daño renal, así como en ratones que sobreexpresan la proteína anti-envejecimiento, klotho.
ResultadosLos niveles de IL-6 aumentaron en los pacientes con FRA, especialmente en el momento del ingreso hospitalario, y fueron disminuyendo en paralelo a la mejora de la función renal. Al mismo tiempo que el aumento de IL-6, se produjo el incremento sistémico de FGF23 y el descenso de klotho, existiendo una correlación significativa y positiva entre los niveles de IL-6 y FGF23. Además, obtuvimos que los niveles sistémicos de IL-6 son un buen predictor de la mortalidad en los pacientes, con un área bajo la curva igual a uno tras las 72 horas desde el diagnóstico de FRA. En el modelo de FRA experimental en ratón, observamos también un incremento en los niveles plasmáticos tanto de IL-6 como de FGF23, tras las 24 horas del daño renal. Sin embargo, en los ratones transgénicos con sobreexpresión de klotho, no se produjo tal incremento en ninguna de ellas.
ConclusionesExiste una asociación entre el daño renal, el incremento de la IL-6 y de FGF23 en pacientes con FRA, especialmente en el momento del diagnóstico. Además, los niveles elevados de IL-6 predicen la mortalidad de estos pacientes a corto y medio plazo, considerándose un buen biomarcador pronóstico con una predicción total tras las 72 horas desde el diagnóstico del FRA. Mantener unos niveles adecuados de klotho podría prevenir la respuesta inflamatoria mediada por la IL-6 y por lo tanto también aminorar el grado y severidad del daño renal tras un FRA.
Acute kidney injury (AKI) is a clinical syndrome characterised by a sudden loss of renal function, diagnosed by an increase in serum creatinine and a decrease in diuresis.1,2 This significant public health problem affects millions of people annually and its incidence has increased considerably in recent years, especially among subjects admitted to intensive care units (ICU).3 The aetiology of AKI is highly diverse, but they share a marked renal inflammatory component that perpetuates this damage.4 This phenomenon triggers an immune response in the kidney where inflammatory mediators, such as cytokines and chemokines, are released, producing a proinflammatory response, which can also be detected systemically in subjects.5,6 Relevant inflammatory mediators in AKI include tumour necrosis factor alpha (TNF-α), and interleukins 18 (IL-18) and 6 (IL-6).7 IL-6, in particular, plays a crucial role in renal damage, and it has a high predictive value on the severity of damage, cardiovascular complications8,9 and mortality in critically ill patients in the ICU.10 Despite its importance, it is not a cytokine that is routinely analysed in subjects with AKI upon admission, thereby it losses its predictive capacity in the onset of possible serious complications associated with AKI. At an experimental level, different studies have demonstrated an association between AKI and IL-6, for example in murine models of AKI induced by renal ischaemia-reperfusion, or nephrotoxic models, connecting local and systemic inflammation through this cytokine.11,12 These findings suggest that IL-6 could be a good biomarker and a potential therapeutic target for the clinical management of AKI.
On the other hand, it is well known that kidney disease is also associated with alterations in the components of bone mineral metabolism, such as phosphates, fibroblast growth factor (FGF)23 and the anti-ageing factor, klotho. Hyperphosphataemia is a common condition in kidney disease that induces increased FGF23 to promote phosphaturia. Kidney dysfunction contributes to the build-up of phosphates and FGF23 in the blood. All of this is aggravated by the decrease in the FGF23 co-receptor, αklotho. Klotho is an anti-ageing protein that is synthesised mainly in the kidneys,13 and whose renal expression as well as its plasma levels are abnormal in AKI.14 In recent years, among the various beneficial actions of klotho, it has also been attributed an anti-inflammatory action, even beyond the kidneys, where exogenous treatment with klotho has been shown to be capable of decreasing the release of proinflammatory cytokines, and of improving the function of ventricular cardiomyocytes after experimental AKI.15 Despite all this evidence, the relationship between the components of mineral metabolism and the inflammatory context associated with AKI has not been not fully defined. Therefore, the aim of this paper was to study cytokine IL-6 along with the levels of FGF23 and klotho as biomarkers of renal damage after AKI, as well as their predictive power of mortality as of hospital admission. Furthermore, we explored klotho’s nephroprotective role in the context of experimental AKI and its possible relationship with IL-6.
MethodsA pilot study with AKI patientsWe conducted a retrospective clinical study with plasma samples from 28 patients with AKI obtained from the Biobank of the Ramón y Cajal University Hospital-IRYCIS (National Biobank Registry B.0000678, Madrid), part of the ISCIII Biobanks and Biomodels platform (PT20/00045). We studied the course of AKI at the time of admission to the hospital, and after 24 and 72 h. All subjects signed an informed consent form. The inclusion criteria in the study were: 1) being over 18 years of age, and 2) having been diagnosed with AKI at the time of hospital admission. The exclusion criteria were: 1) being under 18 years of age, 2) pregnancy, and 3) not having informed consent. The type of AKI was diagnosed and classified according to the AKIN (Acute Kidney Injury Network) guidelines.16,17 Demographic and clinical data, as well as the presence of comorbidities, were collected in the first 24 h of admission to the hospital to calculate the APACHE (Acute Physiology and Chronic Health Evaluation) II score.18 The control samples corresponded to a total of 26 healthy individuals with no pathologies, also from the Ramón y Cajal University Hospital-IRYCIS Biobank. This study was approved by the 12 de Octubre University Hospital Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki.
Analysis of IL-6, FGF23 and klotho levels in patients with AKIBlood samples from subjects were collected and processed at the time of hospital admission, and 24 and 72 h after admission. Plasma samples were obtained after centrifuging the blood at 2,000 rpm for 10 minutes at 4 °C and were stored at −80 °C in the Ramón y Cajal University Hospital-IRYCIS Biobank. The Department of Biochemistry at the Ramón y Cajal University Hospital analysed the main laboratory parameters related to renal function used in routine clinical practice, such as serum creatinine (sCreat), urea and estimated glomerular filtration rate (eGFR). Correlation analyses were performed between the different renal function variables, mineral metabolism parameters and cytokine IL-6 at the three study time points. In addition, all variables were analysed based on whether the subjects survived (no death) or died (death). Plasma levels of the proinflammatory cytokine IL-6 (ProcartaPlex™ Kit, Invitrogen, Paris, France), C-terminal FGF23 (cFGF23, ELISA, Immutopics, San Clemente, CA), and soluble α-klotho (ELISA, IBL, Fujioka-Shi, Japan) were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits.
Experimental model of AKI in wild-type and klotho-overexpressing miceFor the experimental study there were used, adult male mice C57BL/6J (wild-type strain, WT) from Charles River International Inc. (Wilmington, MA) aged 12–14 weeks. AKI was induced by a single intraperitoneal injection of folic acid (FA, 250 mg/kg, Sigma-Aldrich, Sant Louis) dissolved in 0.3 M NaHCO3; control mice were given 0.3 M NaHCO3 as a vehicle.19 Plasma samples were extracted from the different experimental groups 24 and 72 h after the FA injection. Transgenic mice overexpressing the klotho protein (Tg-Kl mice; EFmKl46), provided by Dr Kuro-O (Jichi Medical University, Japan), were also used. These mice are able to overexpress the transmembrane form of klotho ubiquitously by inserting the human elongation factor 1-α promoter (EFmKl).20Tg-Kl mice underwent the same AKI induction experimental procedure as WT mice and plasma samples were collected at the same time points. Animal studies were conducted in accordance with the European Union Guidelines for the Ethical Care of Experimental Animals (2010/63/EU) and were approved by the Autonomous University of Madrid Bioethics Committee and by the Directorate General of Agriculture and Environment of the Community of Madrid’s Regional Ministry of the Environment (PROEX 186.5/20).
Analysis of IL-6 and FGF23 in the AKI experimental modelPlasma levels of cFGF23 and IL-6 were analysed using ELISA kits following the manufacturers’ instructions. For the quantification of cFGF23, the ELISA kit from Immunotopics, Inc. (San Clemente, CA) was used, and IL-6 levels were analysed with the ProcartaPlex™ kit (Invitrogen, Paris, France).
Statistical analysisTo determine whether the data follow a normal distribution, the Kolmogorov-Smirnov test was performed. Differences between parametric variables were analysed using Student’s t-test or one-way ANOVA with the Newman-Keuls test. The probability of mortality in subjects with AKI based on cytokine IL-6 plasma concentration was calculated by calculating the area under the curve (AUC) using the ROC (receiver operating characteristic curve) analysis. The optimal cut-off value from which the IL-6 plasma concentration discriminates between subjects who survived versus those who did not was obtained by calculating the Youden index.21 The different correlations between the variables were calculated with the Pearson correlation coefficient. For statistical analysis of the data, GraphPad Prism v8.0 software (GraphPad Software Inc., San Diego, CA) and SPSS Statistics v22 (IBM, Armonk, NY) were used. Data are represented as mean ± standard deviation in the clinical study, and mean ± standard error in the experimental study. A p-value <0.05 was considered statistically significant.
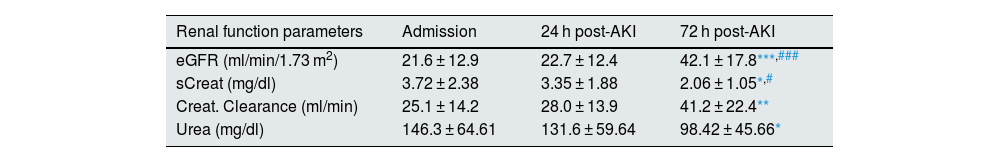
ResultsAssessment of renal function and mineral metabolism parameters in patients with AKIOf the cohort of 28 subjects with AKI, 75% (21) were males with a mean age of 64.3 ± 16.1 years. According to the AKIN criteria for diagnosis and classification of AKI stages, of the total number of subjects, 8.7% were in stage 1, 17.4% in stage 2 and 73.9% in stage 3. The causes of AKI were as follows: acute tubular necrosis (19), prerenal (7), acute glomerulonephritis (1) and acute tubulointerstitial nephritis (1). Of the total number of subjects, six died (21.4%). Table 1 shows the results of the biochemical parameters by renal function, the concentration of biomarkers related to mineral metabolism in patients with AKI at the three study time points, as well as the values obtained in the control group. All subjects, regardless of the time point, had an eGFR lower than 60 ml/min/1.73 m2 after AKI, and the values of eGFR increased progressively and significantly after 72 h post-AKI as compared to the values at hospital admission and with the 24 h post-AKI (p < 0.001). The highest values for both serum creatinine (sCreat) and urea were recorded at hospital admission, decreasing significantly at 72 h (p < 0.05 vs admission, p < 0.05 vs 24 h post-AKI for serum creatinine). An improvement in creatinine clearance was also observed 72 h after admission compared to admission (p < 0.01). Supplementary Fig. S1 in Appendix A shows the results for the renal function parameters analysed, separating subjects who survived from those who did not. We found that serum creatinine decreased significantly between admission and 72 h in subjects who survived, while no changes were observed in those who died. Regarding urea, a significant decrease occurred in the survivor group after 72 h. In contrast, in the non-survivor group, urea levels remained significantly high compared to the survivor group after 72 h. Finally, eGFR increased after 72 h in survivors, while there were no changes in non-survivors. Regarding the markers of mineral metabolism, it was observed that as compared with the reference values of the control group, there was a significant elevation of cFGF23 at the three study time points (p < 0.001 vs admission, p < 0.01 vs 24 h post-AKI and p < 0.05 vs 72 h post-AKI), as well as a significant decrease in klotho (p < 0.001 vs admission, 24 h post-AKI and 72 h post-AKI). However, among AKI patients no significant differences were found in cFGF23 and klotho plasma levels between the three study time points. These results indicate the improvement in renal function after 72 h, was not associated with changes in cFGF23 and klotho marker levels at admission and at 24 and 72 h post-AKI.
Parameters of renal function and mineral metabolism in patients with AKI at admission, and 24 and 72 h after hospital admission.
| Renal function parameters | Admission | 24 h post-AKI | 72 h post-AKI |
|---|---|---|---|
| eGFR (ml/min/1.73 m2) | 21.6 ± 12.9 | 22.7 ± 12.4 | 42.1 ± 17.8***,### |
| sCreat (mg/dl) | 3.72 ± 2.38 | 3.35 ± 1.88 | 2.06 ± 1.05*,# |
| Creat. Clearance (ml/min) | 25.1 ± 14.2 | 28.0 ± 13.9 | 41.2 ± 22.4** |
| Urea (mg/dl) | 146.3 ± 64.61 | 131.6 ± 59.64 | 98.42 ± 45.66* |
| Mineral metabolism parameters | Controls | Admission | 24 h post-AKI | 72 h post-AKI |
|---|---|---|---|---|
| FGF23 (RU/ml) | 67.0 ± 34.1 | 631.0 ± 546.9*** | 477.7 ± 450.7** | 371.2 ± 408.6* |
| Klotho (pg/mL) | 822.1 ± 355.0 | 533.0 ± 273.6** | 460.0 ± 225.2*** | 410.0 ± 217.4*** |
Data are expressed as mean ± SD.
AKI: acute kidney injury; eGFR: estimated glomerular filtration rate; FGF23: fibroblast growth factor 23; sCreat: serum creatinine.
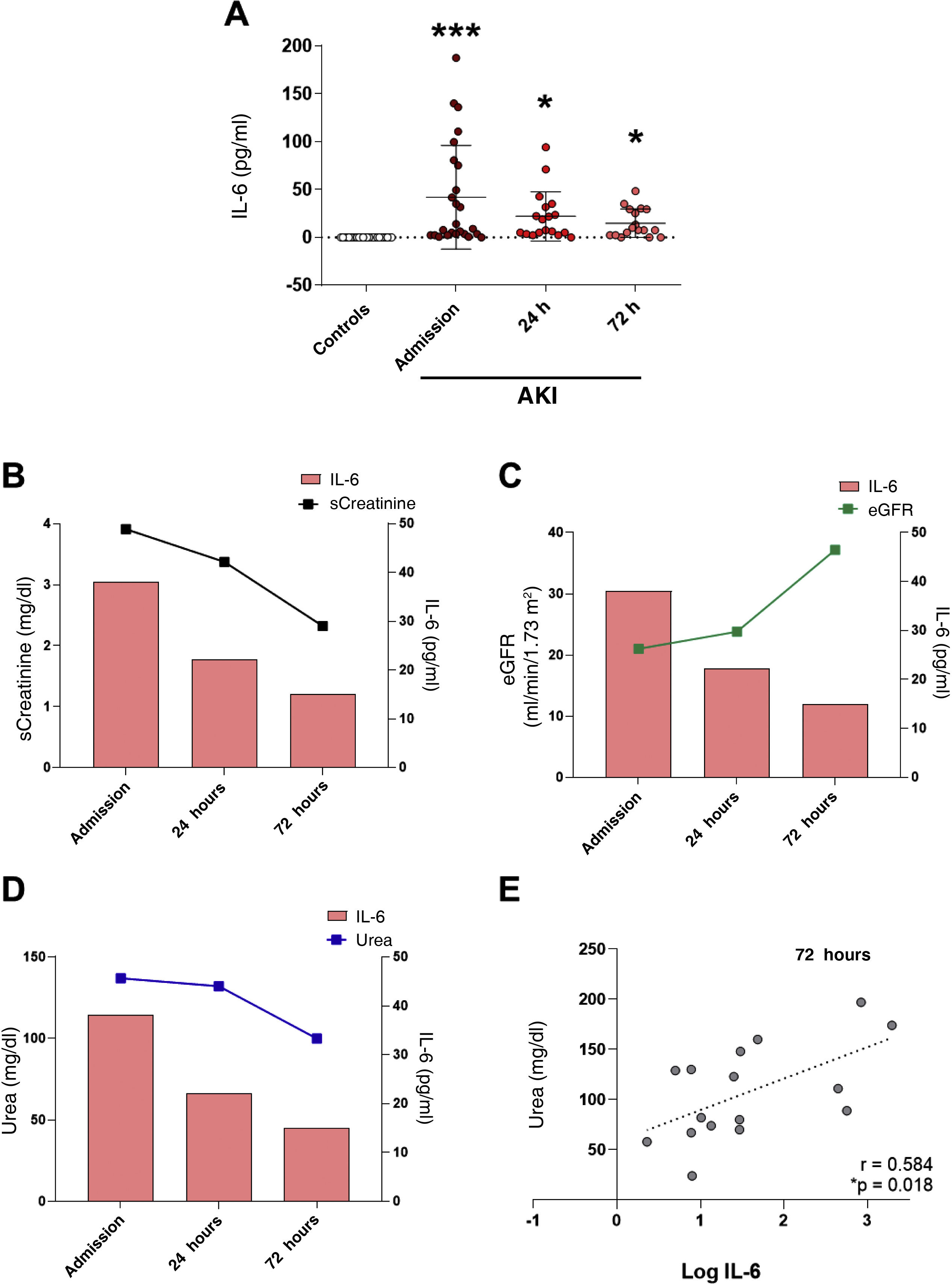
Plasma IL-6 concentration was significantly higher in patients with AKI as compared with the control group at the three study time points, with the highest circulating IL-6 concentration at hospital admission (Fig. 1A, p < 0.001 vs control group). IL-6 levels decreased but remained significantly elevated compared to the reference control group 24 and 72 h after admission (Fig. 1A, p < 0.05 vs control group). A comparison of systemic IL-6 levels and the change over time of the different renal function parameters measured at the post-hospital admission time points (sCreat, urea and eGFR) revealed that, as renal function improved with an increase in eGFR (Fig. 1C), a decrease in sCreat (Fig. 1B) and a decrease in urea (Fig. 1D), IL-6 levels also decreased, indicating a clear relationship between acute kidney injury and the increase in this proinflammatory cytokine at hospital admission. Correlations between the different renal function parameters and IL-6 levels at the three study time points were analysed, without finding significant correlation between them (data not shown), except for a positive, significant correlation between urea and IL-6 levels after 72 h (Fig. 1E).
Analysis of systemic levels of the proinflammatory cytokine IL-6 in subjects with AKI and its relationship with renal function. (A) Plasma IL-6 levels and the relationship with serum creatinine (sCreat) (B), estimated glomerular filtration rate (eGFR) (C) and urea (D) values in subjects with AKI at hospital admission, and 24 and 72 h after admission. (E) Correlation between systemic IL-6 levels and urea 72 h post-AKI. *p < 0.05, ***p < 0.001 vs control.
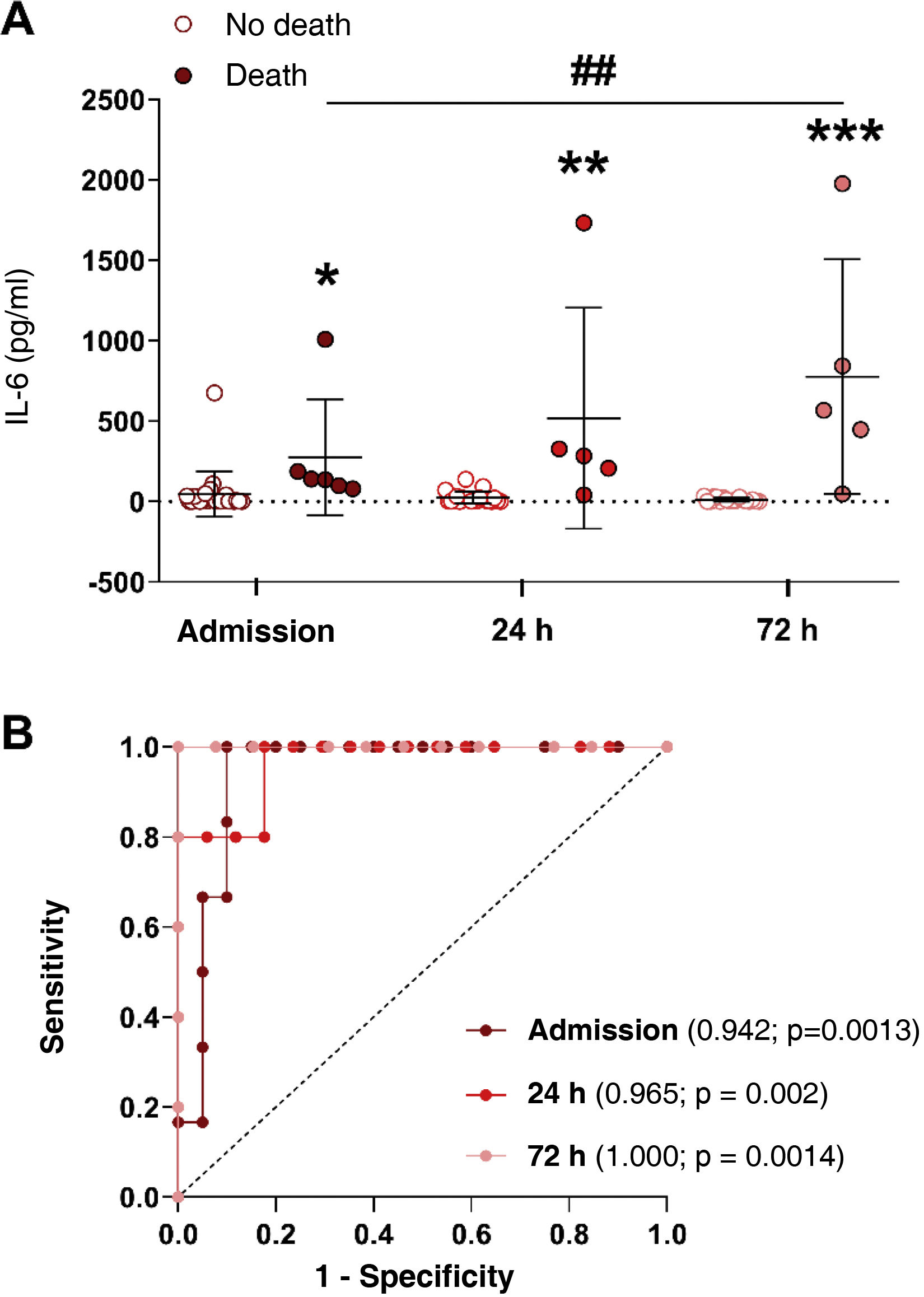
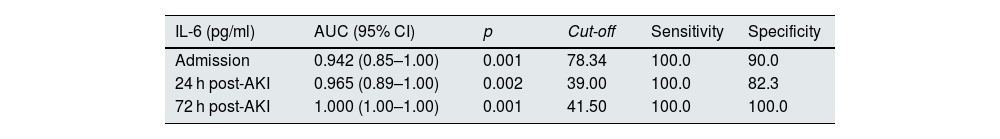
It was analysed whether plasma IL-6 levels varied in AKI subjects who survived (no death) versus those who ultimately died (death) at the three study time points. Fig. 2A shows that in surviving patients, the concentration of IL-6 decreased from admission to 72 h post-admission, while patients who died had higher IL-6 values compared to those who survived at all time points, particularly at 72 h post-admission (Fig. 2A, p < 0.05, p < 0.01 and p < 0.001 vs survivors after admission, 24 and 72 h, respectively). Furthermore, IL-6 levels after 72 h were significantly higher than admission in those subjects who died (Fig. 2A, p < 0.001 vs admission). For this reason, we analysed whether IL-6 could be a good biomarker for predicting mortality in our cohort of AKI patients by calculating the area under the curve (AUC) and analysing the ROC curves at the three study time points. Whether the AUC of a biomarker is one, the ability to predict an outcome is very high yielding a cut-off value from which the biomarker would indicate a high probability of mortality. Fig. 2B shows the AUCs of IL-6 at the three study time points in AKI patients, and Table 2 details the AUC values, confidence intervals (CI), cut-off points, sensitivity, specificity and p-values of IL-6 at admission and at 24 and 72 h post-admission. As can be observed, IL-6 is able to significantly discriminate between AKI subjects who will survive from those who will not at the three study time points, already achieving good discrimination at the subject’s admission to the hospital (AUC = 0.942; p = 0.0013; Fig. 2B), and reaching a total prediction with an AUC value of one 72 h post-hospital admission (Fig. 2B, Table 2, AUC = 1.000, 95% CI = 1.00–1.00, p = 0.001). The cut-off point for plasma IL-6 concentration values that predict mortality is 78.34 pg/ml at hospital admission and 41.50 pg/ml at 72 h post-admission (Table 2).
IL-6 levels in subjects who survived AKI versus those who did not and their capacity to predict for mortality. (A) Plasma levels of cytokine IL-6 in subjects with AKI who survived (no death) versus those who died (death) at hospital admission, and 24 and 72 h after AKI diagnosis. (B) ROC curves to determine the predictive value of mortality of cytokine IL-6 at hospital admission (maroon line), 24 (red line) and 72 h after AKI diagnosis (pink line) (B). *p < 0.05, **p < 0.01, ***p < 0.001 vs their respective no death group by study time point.
Areas under the curve (AUC) and cut-off values of IL-6 for the prediction of mortality in patients with acute kidney injury (AKI) at admission, and 24 and 72 h after hospital admission.
| IL-6 (pg/ml) | AUC (95% CI) | p | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Admission | 0.942 (0.85–1.00) | 0.001 | 78.34 | 100.0 | 90.0 |
| 24 h post-AKI | 0.965 (0.89–1.00) | 0.002 | 39.00 | 100.0 | 82.3 |
| 72 h post-AKI | 1.000 (1.00–1.00) | 0.001 | 41.50 | 100.0 | 100.0 |
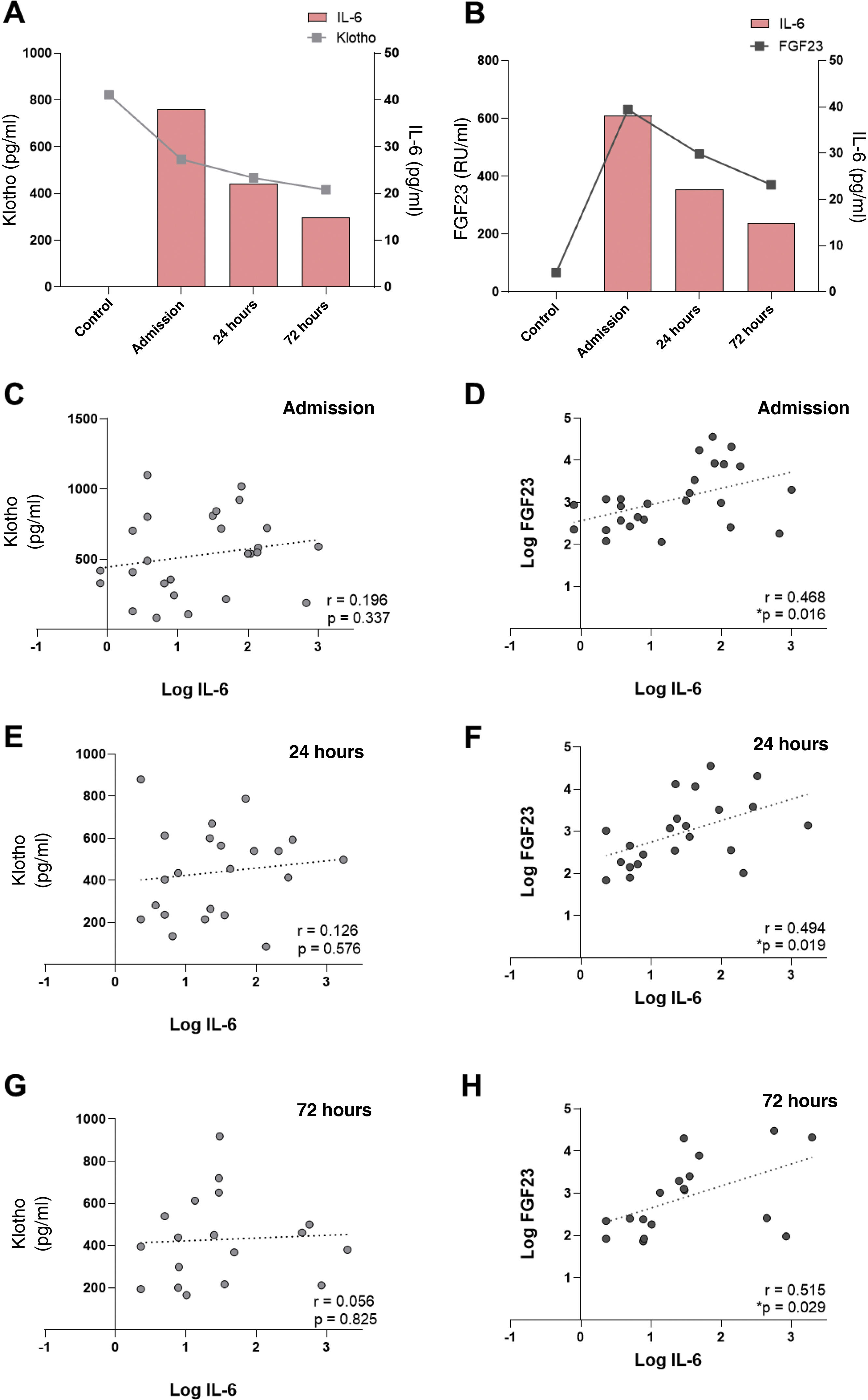
In our cohort of patients, we previously observed (Table 1) a decrease in systemic levels of klotho after the AKI subject’s admission to hospital. For this reason, we studied its relationship with IL-6 levels at each time point analyzed. It was observed that there was a relevant decrease in the plasma klotho concentration compared to the group of healthy individuals at hospital admission, which coincided with the highest concentration of IL-6 observed in AKI subjects (Fig. 3A). In contrast, circulating levels of cFGF23 increased, peaking at admission, coinciding with the maximum values observed for IL-6. Both biomarkers decreased at 24 and 72 h post-FRA (Fig. 3B). The correlations between IL-6 and the markers of mineral metabolism, klotho and cFGF23, measured at the three study time points were analyzed. There was no correlation between IL-6 and klotho levels (Fig. 3C, E and G). However, there was a significant, positive correlation between the concentration of cFGF23 and IL-6 at all time points analysed: admission, and after 24 and 72 h (Fig. 3D, F and H).
Relationship between IL-6 levels with mineral metabolism markers cFGF23 and klotho in subjects with AKI. Relationship between plasma cytokine IL-6 concentration and systemic klotho concentration (A) and cFGF23 (B). Correlations between systemic IL-6 levels with klotho at admission (C), 24 h (E) and 72 h after AKI (G), and with cFGF23 at admission (D), 24 h (F) and 72 h after AKI (H).
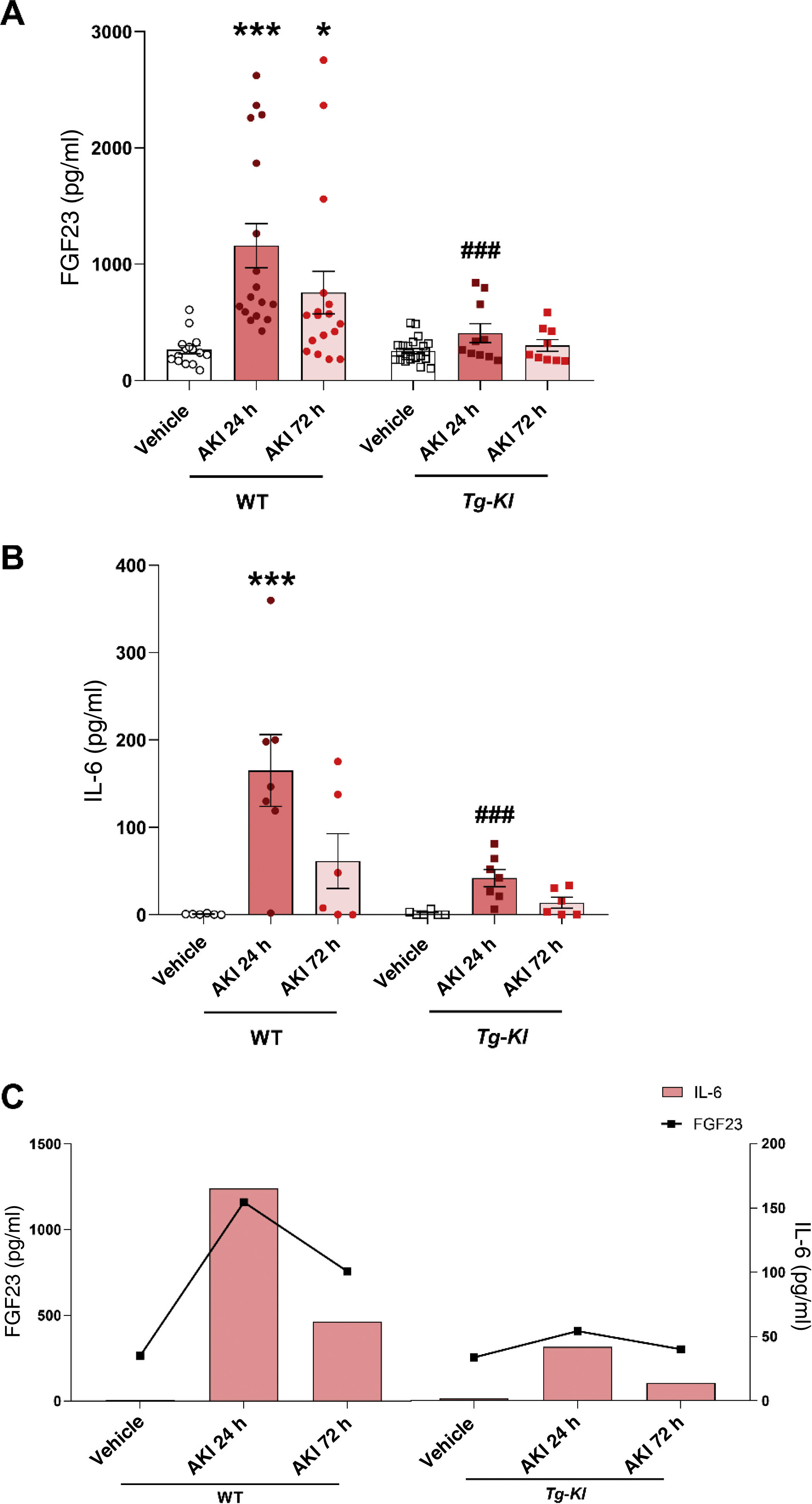
Since it was observed a decrease in plasma klotho levels in patients with AKI as compared to the control group, it was decided to study, in an experimental murine model of nephrotoxic AKI, induced by the injection of folic acid, whether klotho overexpression could prevent the increase in IL-6. First, it was analyzed the plasma IL-6 concentration in both WT mice and the klotho-overexpressing transgenic mouse strain (Tg-Kl), at 24 and 72 h after the induction of AKI. Since there was a positive and significant correlation between cFGF23 and IL-6 levels in AKI patients at hospital admission (Fig. 3D), as well as after 24 and 72 h (Fig. 3F and H), we analysed the cFGF23 levels in all experimental groups. It was found that cFGF23 increased significantly 24 h after AKI induction (Fig. 4A, p < 0.001); at 72 h post-AKI there was a in decrease in cFGF23 but it remained significantly elevated in WT mice (Fig. 4A, p < 0.05). However, in Tg-Kl mice with AKI, cFGF23 did not increase, and was significantly lower than in WT mice at 24 h post-AKI (Fig. 4A, p < 0.001). The same pattern was observed for circulating IL-6 levels in mice, with a significant increase in IL-6 in WT mice after 24 h from kidney injury (Fig. 4B, p < 0.001), decreasing after 72 h, while in Tg-Kl mice, IL-6 did not increase significantly, and its levels were significantly lower than those found in WT mice 24 h post-AKI (Fig. 4B, p < 0.001). Fig. 4C shows a comparison between IL-6 and cFGF23 levels in the two strains of mice with AKI with and without klotho overexpression, also it appears that klotho overexpression protects against the systemic increase of both molecules induced by AKI.
Relationship between IL-6 and cFGF23 levels in mice with AKI induced in WT mice and Tg-Kl mice with klotho gene overexpression. Plasma cFGF23 levels (A) and cytokine IL-6 (B) in WT mice and klotho-overexpressing mice (Tg-Kl) 24 and 72 h after AKI induction. (C) Relationship between cytokine IL-6 and cFGF23 in WT and Tg-Kl mice 24 and 72 h after induction of AKI (C). *p < 0.05, ***p < 0.001 vs WT vehicle; ###p < 0.001 vs WT group 24 h post-AKI.
Among the different pathological mechanisms associated with AKI, the strong inflammatory component triggered at the renal level stands out, leading to the release of different proinflammatory mediators into the systemic circulation. Of them, the proinflammatory cytokine IL-6 plays an important role in the onset of kidney injury. Moreover, alterations of the different components of mineral metabolism during AKI are well known, such as the release of the phosphaturic hormone FGF23, as well as the renal and systemic decrease in klotho. Our study shows that IL-6 levels are elevated in patients with AKI, especially at hospital admission, and decrease as renal function recovers. Furthermore, it is noteworthy that from the time of hospital admission the elevation of IL-6 levels are highly predictive of mortality in patients with AKI, with this prediction being total 72 h after admission. IL-6 levels are also associated with cFGF23 levels found in both AKI patients and in an experimental AKI model in mice. Furthermore, it is important to mention that mice with AKI, but with klotho overexpression, show no elevation of either IL-6 or cFGF23. As such, IL-6 is proposed as a good early biomarker for predicting AKI-associated mortality, which is, in turn, dependent on the mineral metabolism components FGF23 and klotho.
Given the high incidence of AKI among hospitalised patients, especially in critically ill patients in the ICU, who have a very high mortality rate, valid biomarkers for early detection of AKI severity and its associated mortality would be an extremely useful tool for early intervention in subjects, especially those with a high probability of their health status worsening. In light of the above, our results demonstrate that the maximum peak of systemic IL-6 concentration occurred at the time of the AKI subject’s hospital admission and, even though after 24 and 72 h the levels continued to be significantly elevated compared to the group of healthy individuals, there is a tendency for them to stabilize over time (Fig. 1A). This stabilization was associated with the recovery of renal function (Fig. 1B–D). This increase in plasma IL-6 during AKI, is due to the increase in its local synthesis in the kidneys and its subsequent release into the blood, and it could also be due to the reduction in its renal clearance, with both circumstances contributing to this increase in blood level of IL-6, especially at the time of the subject’s admission; it is therefore considered an early biomarker of inflammatory damage associated with a clear compromise of renal function.11,22,23 Our findings support previous studies in which IL-6 is also postulated as a biomarker to be taken into account after kidney injury.9,24 Analysis of IL-6 levels based on the survival of AKI patients (Fig. 2B), shows that, while IL-6 in surviving AKI patients decreased progressively over time, IL-6 values in those who died increased significantly, reaching their maximum concentration 72 h after the AKI. Furthermore, it is at 72 h when the mortality prediction of IL-6 values is 100%, with a cut-off value of 41.5 pg/ml, a threshold value that should be an alarm signal in the clinical management of AKI patients admitted to a hospital (Table 2).
Abnormalities in mineral metabolism, such as hypocalcemia, hyperphosphataemia, increased FGF23 and decreased renal and systemic expression of klotho are characteristic of CKD, and many of these abnormalities are also common in AKI.25 Furthermore, other studies have shown that in CKD, elevated levels of FGF23 correlate with the inflammatory status of these subjects, where IL-6, C-reactive protein and FGF23 are also independent risk factors in terms of mortality.26 In this study, we analysed whether there was a relationship between plasma IL-6 levels and the components of mineral metabolism cFGF23 and klotho. In our cohort, the maximum peak concentration of cFGF23 coincided with the maximum peak of IL-6, which was at the time of the AKI subject’s admission to hospital (Fig. 3B), finding a significant positive significant correlation between both (Fig. 3D). The maximum drop in plasma klotho levels also occurred at the time of admission, coinciding with the highest concentration of IL-6 (Fig. 3A), and although renal function recovered and IL-6 levels decreased over time, klotho continued to decrease at 72 h post-admission. These results point to the existence of a close relationship between the worsening of renal function, the inflammatory phenomenon and the regulation of mineral metabolism after AKI. To verify this relationship, we then studied IL-6 in an experimental nephrotoxic mouse model of AKI, which is well known to be associated with a strong reduction in klotho expression and increased levels of FGF23.27 Our results demonstrate that, in WT mice, the plasma increase of both cFGF23 and IL-6 was reproduced 24 and 72 h after the induction of AKI, as occurred in our patients, recoding the peak concentration of both molecules after 24 h. In contrast, in Tg-Kl mice with genetic overexpression of klotho, neither IL-6 nor cFGF23 increased significantly (Fig. 4A–C), demonstrating the protective role of klotho at both the inflammatory and mineral metabolism levels. Previous studies have also shown that inflammation is capable of activating the synthesis of FGF23 and, specifically, cytokine IL-6 is one of the inflammatory mediators that contributes to its increase by activating the transcription of FGF23.28,29 Furthermore, FGF23 has also been described to promote inflammation by creating a positive feedback loop, aggravating kidney injury.28 Therefore, IL-6 could be one of the possible contributors to the elevation of systemic FGF23 levels beyond hyperphosphataemia in renal disease, and particularly in the context of AKI, where the acute inflammatory component is very important. Klotho is a transmembrane protein: in addition to acting as a co-receptor of the FGF23 receptor (FGFR1) facilitating the phosphaturic function of FGF23, the cleavage of its extracellular domain gives rise to the soluble form of klotho (sKl). sKl is released into the systemic circulation as an endocrine factor, and can exert its antiapoptotic, antifibrotic and anti-inflammatory functions on different organs of the body.25,30 In relation to its actions on inflammation, klotho is capable of decreasing the expression of certain proinflammatory cytokines and increasing the secretion of anti-inflammatory factors.31–33 Since we have found that, in mice with Klotho overexpression, IL-6 does not increase in to the same extent as in WT mice, it can be postulated that, by inhibiting the synthesis of IL-6 and, therefore, attenuating the renal injury induced by folic acid in conditions of klotho overexpression, the renal function of these mice is protected. One of the main limitations of this study is the inability to conclude whether the decrease in circulating IL-6 that we see in Tg-Kl mice is due to the action of the transmembrane form of klotho, or whether it is a systemic effect on the inflammatory status thanks to its soluble form. Previous studies have shown that klotho-overexpressing mice maintain renal klotho levels after AKI induction.27 Furthermore, other studies have shown that these transgenic mice have elevated systemic klotho levels.34 These results suggest that maintaining klotho levels, whether renal, systemic or both, under AKI circumstances, could be responsible forthe inhibition of IL-6 synthesis, as we observed in our klotho-overexpressing mice after AKI induction. Similarly, with the results obtained in subjects, we cannot conclude whether it is the increase in IL-6 or the decrease in klotho, or even both, that determines the severity of AKI. It could be speculated that, in conditions where klotho is decreased, this factor predisposes to a greater inflammatory state and that this determines greater renal severity and a worse prognosis. Future studies will need to resolve these questions. In the same line of the results presented it has been shown in clinical studies that higher levels of klotho correlate with lower levels of IL-6 in subjects on peritoneal dialysis,35 as well as that klotho deficiency seems to contribute to increased inflammation in patients with end-stage CKD, especially in those on haemodialysis.36
In conclusion, the present study demonstrates that there is a significant association between acute kidney dysfunction and the increase of the proinflammatory cytokine IL-6 and FGF23, in a context of AKI, particularly at hospital admission. Measuring IL-6 upon arrival of an AKI patient to hospital is of particular relevance, since its predictive capacity for mortality is very high, both at the time of admission and in the first few days thereafter. Furthermore, this study also shows how klotho levels may determine renal protection against the triggered inflammatory processes that can lead to increased IL-6 synthesis.
FundingThis study was primarily funded by one of the research grants from the Sociedad Española de Nefrología [Spanish Society of Nephrology]/Fundación SENEFRO 2021 [SENEFRO 2021 Foundation], and partially by the Instituto de Salud Carlos III [Carlos III Health Institute] (ISCIII) (PI20/00763, CPII20/00022, FI21/00212, PI23/00182) and co-financed by the European Union, Ministry of Universities (FPU20/03005), Department of Education of the Community of Madrid (PEJ-2021-AI/SAL-21426), Biomedical Network of the Community of Madrid (P2022/BMD-7223 CIFRA_COR-CM), National Research Networks (RED2022-134511-T) and the Sociedad Española de Cardiología [Spanish Society of Cardiology] (SEC)/Fundación Española del Corazón [Spanish Heart Foundation] (SEC/FEC-INV-BAS 23).
The following is Supplementary data to this article:
Valoración de los parámetros de la función renal en los pacientes que sobrevivieron al FRA frente a los que no. Valores de la creatinina sérica (A), urea (B) y TFGe (C) en pacientes con FRA que sobrevivieron (no exitus) frente a aquellos que fallecieron (exitus) en el momento de la admisión hospitalaria, tras 24 y 72 horas desde el diagnóstico del FRA. *p < 0.05, **p < 0.01, ***p < 0.001.