Chronic kidney disease (CKD) is a major public health problem worldwide that affects more than 10% of the Spanish population. CKD is associated with high comorbidity rates, poor prognosis and major consumption of health system resources. Since the publication of the last consensus document on CKD seven years ago, little evidence has emerged and few clinical trials on new diagnostic and treatment strategies in CKD have been conducted, apart from new trials in diabetic kidney disease. Therefore, CKD international guidelines have not been recently updated. The rigidity and conservative attitude of the guidelines should not prevent the publication of updates in knowledge about certain matters that may be key in detecting CKD and managing patients with this disease. This document, also prepared by 10 scientific associations, provides an update on concepts, clarifications, diagnostic criteria, remission strategies and new treatment options.
The evidence and the main studies published on these aspects of CKD have been reviewed. This should be considered more as an information document on CKD. It includes an update on CKD detection, risk factors and screening; a definition of renal progression; an update of remission criteria with new suggestions in the older population; CKD monitoring and prevention strategies; management of associated comorbidities, particularly in diabetes mellitus; roles of the Primary Care physician in CKD management; and what not to do in Nephrology.
The aim of the document is to serve as an aid in the multidisciplinary management of the patient with CKD based on current recommendations and knowledge.
La enfermedad renal crónica (ERC) es un importante problema de salud pública a nivel mundial afectando a mas del 10% de la población española. Se asocia a elevada comorbilidad, mal pronóstico, así como a un gran consumo de recursos en el sistema sanitario. Desde la publicación del último documento de consenso sobre ERC publicado hace siete años, han sido escasas las evidencias y los ensayos clínicos que hayan mostrado nuevas estrategias en el diagnóstico y tratamiento de la ERC, con excepción de los nuevos ensayos en la enfermedad renal diabética. Esta situación ha condicionado que no se hayan actualizado las guías internacionales en este aspecto. Esta rigidez y actitud conservadora de las guías no debe impedir la publicación de actualizaciones en el conocimiento en algunos aspectos, que pueden ser clave en la detección y manejo del paciente con ERC. En este documento, elaborado en conjunto con diez sociedades científicas, se muestra una actualización sobre conceptos, aclaraciones, criterios diagnósticos, estrategias de remisión y nuevas opciones terapéuticas.
Se han revisado las evidencias y los principales estudios publicados en estos aspectos de la ERC, considerándose más bien un documento de información sobre este padecimiento. El documento incluye una actualización sobre la detección de la ERC, factores de riesgo, cribado, definición de progresión renal, actualización en los criterios de remisión con nuevas sugerencias en la población anciana, monitorización y estrategias de prevención de la ERC, manejo de comorbilidades asociadas, especialmente en diabetes mellitus, funciones del médico de Atención Primaria en el manejo de la ERC y qué no hacer en Nefrología.
El objetivo del documento es que sirva de ayuda en el manejo multidisciplinar del paciente con ERC basado en las recomendaciones y conocimientos actuales.
Chronic kidney disease (CKD) is a major public health problem worldwide, so its early detection is considered a top priority to establish strategies to prevent the progression to advanced stages of the disease and its complications.1–3
In Spain, the results of the Nutrition and Cardiovascular Risk Study (ENRICA)4 have shown a prevalence of any CKD in of its stages of 15.1% for the general population, similar to the 14.4% of the population attended in primary care in the IBERICAN study. (Identification of the Spanish population with cardiovascular and kidney risk).5 Both studies show that age and cardiovascular disease increase the prevalence of CKD. These current data differ from the 9.24% obtained in the old EPIRCE study (Epidemiology of Chronic Renal Insufficiency in Spain).6 This is partly due to substantial methodological differences between the studies, but could also indicate evolutionary changes over time. All these epidemiological data support the fact that CKD is an important health problem.
The epidemiological importance of CKD is related not only to its high prevalence but also to the significant decrease in quality of life, high morbidity and mortality, and the health and social cost that this entails. In this scenario, Primary Care (PC) is a fundamental mainstay not only in the early detection of CKD, but also in the management of progression factors and even in the management of the initial complications and, with this perception, this document is aimed at multidisciplinary collaboration for the detection and management of CKD.
Seven years have passed since the publication of the previous consensus document with the participation of ten scientific societies, and it has been a actual reference for the management of renal patients. From then until now, our KDIGO reference guide (Kidney Disease Improving Global Outcomes),7 published in 2012, has not been updated in this field. Thus, we have considered that it was appropriate to update its contents in specific areas based on the same structure of the document published in 2014. The methodology used is based on the critical review of the main published studies, clinical guidelines on CKD, the new KDIGO guides on specific topics and the few randomized clinical trials performed in CKD patients. This analysis allows to provide some recommendations or suggestions based on the best available evidence, as well as to report on new aspects that have gained importance in CKD.
Chronic kidney disease: definition and diagnosisThe international organization KDIGO defines CKD as the presence of alterations in kidney structure or function for a period longer than three months, regardless of the cause7 and with health consequences exposed by different criteria (Table 1):
- a)
The decrease in glomerular filtration rate (GFR) (< 60mL/min/1.73m 2), either measured with exogenous markers (GFRm) or estimated by equations from endogenous markers (GFR).
- b)
The presence of renal injury or damage, referred to the existence of structural or functional alterations of the kidney detected directly with a renal biopsy or indirectly by the presence of albuminuria, proteinuria, alterations in the urinary sediment, in imaging tests, hydroelectrolytic abnormalities or another type of alterations of tubular origin or history of renal transplantation.
Diagnostic criteria of chronic kidney disease (any of the following if they are present for a period > 3 months).
| GFR decrease | GFR<60mL/min /1.73m2 |
|---|---|
| Markers of kidney injury or damage | Albuminuria (UACR>30mg/g; UAE: > 30mg/24h) |
| Proteinuria (PR/CR>150mg/g; UPE > 150mg/24h) | |
| Histological changes in renal biopsy | |
| Alterations in the urinary sediment | |
| Structural alterations detected by imaging techniques | |
| Hydroelectrolytic or other disorders of tubular origin | |
| Kidney transplant history |
GFR: glomerular filtration rate ; UACR: urinary albumin to creatinine ratio in a random urine sample ; UAE: 24-h urinary albumin excretion; PR/CR: protein/creatinine ratio in a random urine sample ; UPE: 24hour urinary protein excretion.
It should be noted that a single criterion of the two above mentiones is sufficient to make the diagnose of CKD, and it should be stressed that the presence of kidney injury markers is essential to diagnose a patient as CKD if their GFR is > 60mL/min /1.73m2.8
In recent years, an important debate has arisen about whether the criterion of a decreased GFR<60mL /min/1.73m 2 should vary depending on the age of the patients, with some authors advocating that in those over 65 years of age be modified to <45mL /min/1.73m2 and if less than 40 years of age change it to <75mL /min/1.73m 2 since the range of GFR associated with increased mortality changes with the age.9,10 The use of different GFR thresholds according to age as a diagnostic criterion for CKD produces an overall decrease in the prevalence of CKD, avoids its overdiagnosis in the elderly population without other CKD criteria and low probability of CKD progression, and allows earlier detection of CKD in the younger population.
Kidney Function Assessment: Glomerular FiltrationThe GFR is the best indicator to assess renal function,7 and it corresponds to the volume of plasma from which a substance is totally eliminated by the kidney per unit of time. The GFR value varies in relation to age, sex and body mass, classically being around 125mL/min/1.73m2 in young adult individuals,7 although recent studies11 place it at around 106mL/min/1.73m2. The assessment of the GFR allows the identification and classification of CKD stages, as well as monitoring its progression. The decrease in GFR is associated with greater cardiovascular morbidity and mortality and indicates progression towards end-stage CKD.
The measurement of GFR (GFRm) requires the administration of exogenous substances (inulin, 57 Cr-EDTA, 99m Tc-DTPA, iothalamate, iohexol, etc.) and its subsequent determination in blood and/or urine. These are laborious, expensive techniques and require a methodology that is not always available in all laboratories, so their use is restricted to clinical situations that require a more precise measurement of the GFR (especially dose adjustment of drugs with high toxicity and renal elimination).
Serum creatinine concentration (endogenous marker, a product of muscle metabolism) is the test commonly used to assess renal function. However, the different causes of biological variability (age, sex, muscle mass, type of diet, etc.) that affect its serum concentration, the lack of sensitivity (to observe an increase in serum creatinine concentration above the upper reference limit the renal function must be decreased by 50% or more); furthermore the mathematical relationship between serum Cr and GFR is non-linear therefore isolated measurement of creatinine is considered to be insensitive, especially in certain population groups such as women and the elderly.
Other factors unrelated to the presence of kidney disease can modify the serum creatinine concentration and make it difficult to interpret. Thus, increases in serum creatinine concentration occur in patients treated with some drugs (eg, fibrates, rilpivirine, dolutegravir, cobicistat) that produce a moderate and generally reversible reduction in GFR or an interference with its measurement ; an increase in creatinine may occur after recent consumption of meat or fish (with a peak between 2-4hours after ingestion) or after taking creatine supplements, among others.12 In contrast, in severe liver disease (situation in which synthesis of creatinine is decreased), during pregnancy (hemodilution), or malnutrition (decreased muscle mass), there is a decrease in serum creatinine concentration.12
In recent years, the measurement of a new endogenous marker of renal function has been introduced, cystatin C, a low molecular weight protein produced by all nucleated cells and less influenced than creatinine by age, gender, muscle mass or diet, but not exempt from other sources of variability. Thus, alterations in thyroid function, administration of corticosteroids, smoking, inflammation, obesity or diabetes mellitus (DM), among others, can cause changes in the serum concentration of cystatin that are not related to alterations in renal function.12
To assess renal function, the measurement of the serum concentration of creatinine and/or cystatin C must be accompanied by an estimation equation of the GFR or estimated glomerular filtration rate (eGFR). These equations include the variables that physiologically determine its serum concentration. There have been developed multiple equations to estimate GFR. The fundamental differences between them lie in the "gold standard" used for their derivation, the characteristics of the population from which they have been obtained, the measurements included (creatinine, cystatin C or both), the use or not of methods of standardized measurement and the degree of concordance with respect to the value of the measured GFR (GFRm).
The equations developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) group have shown their superiority when applied to the adult population and are currently recommended.7,12–14 There are different equations developed by this group depending on whether it is use the measurement of the serum concentration of creatinine (CKD-EPI -creatinine), cystatin (CKD-EPI -cystatin) or both (CKD-EPI creatinine + cystatin) (Table 2).
GFR estimation equations in white adult individuals (only valid for standardized creatinine and cystatin C measurement methods).
| CKD-EPI creatinine | ||
| Women | Cr ≤ 0.7mg/dL | 144 x (Cr/0.7) – 0.329 x 0.993 age |
| Cr > 0.7mg/dL | 144 x (Cr/0.7)–1.209 x 0.993 age | |
| Men | Cr ≤ 0.9mg/dL | 141 x (Cr/0.9) – 0.411 x 0.993age |
| Cr > 0.9mg/dL | 141 x (Cr/0.9) – 1.209 x 0.993 age | |
| CKD-EPI cystatin | ||
| Women | Cys ≤ 0.8mg/L | 133 x (Cis/ 0.8) – 0.499 x 0.996 age x 0.932 _ _ _ _ |
| Cys > 0.8mg/L | 133 x (Cis / 0.8) –1.328 x 0.996 age x 0.932 | |
| Men | Cys ≤ 0.8mg/L | 133 x (Cis/0.8) – 0.499 x 0.996 age |
| Cys > 0.8mg/L | 133 x (Cis/0.8) –1.328 x 0.996 age | |
| CKD-EPI creatinine–cystatin | ||
| Women | Cr ≤ 0.7mg / dL and Cys ≤ 0.8mg / L | 130 x (Cr/0. 7) – 0.248 x (Cys / 0.8) – 0.375 x 0.995 age _ |
| Cr ≤ 0.7mg / dL and Cys > 0.8mg / L | 130 x (Cr/0 .7) – 0.248 x (Cys / 0.8) – 0.711 x 0.995 age _ | |
| Crea > 0.7mg / dL and Cys ≤ 0.8mg / L | 130 x (Cr/0. 7) – 0.601 x (Cys / 0.8) – 0.375 x 0.995 age _ | |
| Crea > 0.7mg / dL and Cys > 0.8mg / L | 130 x (Cr/0. 7) – 0.601 x (Cys / 0.8) – 0.711 x 0.995 age _ | |
| Men | Cr ≤ 0.9mg / dL and Cys ≤ 0.8mg / L | 135 x (Cr/0. 9) – 0.207 x (Cis / 0.8) – 0.375 x 0.995 age _ |
| Cr ≤ 0.9mg / dL and Cys > 0.8mg / L | 135 x (Cr/0. 9) – 0.207 x (Cis / 0.8) – 0.711 x 0.995 age _ | |
| Cr > 0.9mg / dL and Cys ≤ 0.8mg / L | 135 x (Cr/0. 9) – 0.601 x (Cis / 0.8) – 0.375 x 0.995 age _ | |
| Cr > 0.9mg / dL and Cys > 0.8mg / L | 135 x (Cr/0. 9) – 0.207 x (Cis / 0.8) – 0.711 x 0.995 age |
Cr: serum creatinine concentration (mg/dL); Cys: serum concentration of cystatin C (mg/L); age (in years).
Only the equations for white individuals are presented in the table because the inclusion of the racial factor is now a subject of new debate.
The CKD-EPI-creatinine equation is more accurate in estimating mGFR (especially between values of 60 and 90mL/min/1.73m 2) than the Modification of Diet in Renal Disease equation as well as a more capability to predict global mortality, cardiovascular mortality and the risk of presenting renal failure and it is the equation proposed as the first choice in the guidelines KDIGO on CKD.
In recent years, various equations for estimating GFR in the general population have been published, including the revised Lund-Malmö15 equation, the CAPA equation (which includes cystatin C),16 the Full Age Spectrum (FAS) equation17 or more recently the equation published by the European Kidney Function Consortium (EKFC).18 However, none of them has been globally superior (in terms of bias, precision and accuracy) to the CKD-EPI- creatinine equation.
The assessment of renal function based on serum creatinine concentration and equations that include the creatinine values is inadequate in certain clinical situations (Table 3). In these cases, the estimation of the GFR may raise greater uncertainty. The proposed strategies include: a) the determination of the serum concentration of cystatin C and use estimation equation (CKD-EPI- cystatin) if there are studies that support its use in such population group; b) the measurement of creatinine clearance, taking into account the overestimation of the GFR, especially for GFR values < 60mL/min/1.73m 2 and the problems associated with the 24-h urine collection; or c) measurement of GFR using an exogenous marker.
Limitations in the use of GFR estimation equations based on serum creatinine concentration.
| Extreme body weight: BMI<19kg/m2 or > 35kg/m2 |
| Special diets (strict vegetarians, creatinine or creatine supplements) or poor nutrition |
| Changes in muscle mass (amputations, loss of muscle mass, muscle diseases or paralysis) |
| Severe liver disease, generalized edema, or ascites |
| Pregnancy |
| Acute renal failure or acute deterioration of renal function in patients with CKD |
| Patients on dialysis |
| Dose adjustment of highly toxic drugs that are eliminated by the kidneys |
BMI: body mass index; GFR: glomerular filtration rate; CKD: chronic kidney disease.
The equation (CKD-EPI creatinine + cystatin) that includes the serum concentration of creatinine and cystatin C, shows the best diagnostic accuracy, its main indication is the confirmation of CKD in individuals with eGFR between 45-59mL/min/1.73m 2, without albuminuria or other markers of kidney injury. It has also been suggested as an alternative to mGFR in > 65 years old with a GFR<45mL/min/1.73m 2 as they often present decreased muscle mass and high consumption of medications.19
Regarding drug dose adjustment, it should be noted that the Cockcroft-Gault (C&G) equation, classically used for this purpose, has significant limitations: it overestimates mGFR (since it is based in creatinine clearance), it has not been reformulated for creatinine values obtained by standardized procedures and cannot be re-expressed for current methods of measurement. The eGFR using CKD-EPI -creatinine correlates better than the C&G with GFR values less than 60mL/min/1.73m 2, which is the GFR in most patients likely to need dose adjustment and are available in clinical laboratory reports, which is not the case for C&G.20,21 In those patients with limitations for the use of GFR estimation equations, the assessment of renal function using an exogenous marker should be considered22 and, failing that, and with the need to adjust particularly toxic drugs in patients with significant deviations of the body surface area, the eGFR should not be standardized to 1.73m 2 (eGFR x SC/1.73m 2).19,23
In any case, despite all the limitations of eGFR, it should be taken into account that a biological determination may have errors and it can be improved; however, it is widely accepted that the uniform definition of CKD with the methods available to date has assisted adequately to patients and professionals since its introduction in 2002.24
Assessment of kidney injury or damageAlbuminuria/proteinuriaThe presence of high concentrations of protein or albumin in urine constitutes, together with GFR, the basis for the diagnosis and current classification of CKD stages.7
Healthy adults the excrete less than 150mg of protein and less than 30mg of albumin in urine daily. Different studies have shown the important role of proteinuria in the pathogenesis of CKD progression, and the relationship of albuminuria with the prognosis of renal disese and with mortality in various populations, independently of GFR and other classic risk factors of cardiovascular disease. In fact, albuminuria may be an earlier marker of CKD than the actual reduction of GFR, and it is also considered to be a sign, not only of kidney injury, but also of "systemic damage" beyond the kidney (generalized endothelial dysfunction, arterial remodeling, and high cardiovascular risk).25,26 On the other hand, the decrease in proteinuria/albuminuria is clearly associated with a slower progression of CKD27 and that is why its reduction is also a therapeutic objective.
Certain situations, such as fever, stress, high protein intake, heart failure or intense physical exercise may increase proteinuria that resolves after the causative factor has disappeared. Likewise, the presence of urinary tract infections or menstruation can cause false positive results. Therefore, in these circumstances it is advisable to avoid urine collection for albuminuria/proteinuria assessment. Smoking and obesity have been associated with the presence of albuminuria and it may be also present in up to 25% of individuals older than 80 years.
The CKD is classified according to the eGFR and it is separeted in 3 categories of albuminuria (A1-A3) based on the value and its equivalents in proteinuria and even in test strips. However, the guidelines recommend stratification based on the urine albumin to creatinine ratio (UACR). Table 4 shows the values for each of the categories according to the type of sample used to assess proteinuria (24-h urine collection or urine sample) and whether it is used albumin or protein in urine.
Categories of albuminuria/proteinuria.
| A1 Normal to mild increase | A2 Moderate increase | A3 marked increase | |
|---|---|---|---|
| UACR | |||
| mg/g | < 30 | 30-300 | > 300 |
| mg/mmol | < 3 | 3-30 | > 30 |
| PR/CR | |||
| mg/g | < 150 | 150-500 | > 500 |
| mg/mmol | < 15 | 15-50 | > 50 |
| UAE (mg/24h) | < 30 | 30-300 | > 300 |
| UPE (mg/24h) | < 150 | 150-500 | > 500 |
| test strip | Negative if trace | Hints at 1 + | ≥ 1 + |
UACR: urine albumin/creatinine ratio (in a urine sample); PR/CR: protein/creatinine ratio in urine (in a urine sample); UAE: urinary albumin excretion/24h; UPE: urine protein excretion/24h.
Most guidelines recommend that in adult individuals the proteinuria should be assessed by the determination of the UACR, in a urine sample preferably the first morning urine.7,13,25 The concentration of protein or albumin in urine should always be referred to the concentration of urinary creatinine to minimize the effect of the urine concentration. This result approximates the 24-h urine determination if there is no large deviation of body surface area.1 Albuminuria is a more sensitive marker than proteinuria in the context of CKD due to DM, high blood pressure (HTN), or glomerular disease, all of which are aetiologies responsible for the majority of CKD in adults.
Significant albuminuria is considered if at least 2 out of 3 urine samples show elevated values during a period of at least three months. In CKD patients and albuminuria (UACR>300mg/g or > 30mg/mmol) monitoring could be performed by measuring urine protein/creatinine ratio (PR/CR). The use of PR/CR in urine is also recommended in patients with suspected renal interstitial pathology (hereditary kidney diseases, especially in children, Sjögren's syndrome, nephrotoxicity due to antiretrovirals -tenofovir-, etc.), since in these situations proteinuria is produced fundamentally at the expense of low molecular weight tubular proteins, different from albumin.28 The existence of a significant dissociation between the UACR and PR/CR ratio suggest the possibility of the presence of free light chains in the urine (Bence-Jones proteinuria) or immunoglobulins (as in impure nephrotic syndrome).
Alterations in the urinary sedimentDifferent elements such as cells, casts, crystals and microorganisms may be seen in the urinary sediment in a wide variety of kidney and urinary tract pathologies. Some of these elements such as dysmorphic red blood cells and/or hematic casts (proliferative glomerulonephritis, vasculitis), leukocyte casts (interstitial pyelonephritis or nephritis), lipids casts (proteinuric pathologies), renal tubular cells or granular and waxy casts are indicators of renal injury.7
The percentage of dysmorphic red blood cells to be consider as hematuria of glomerular cause is not well established, and its value as an isolated finding (without other alterations suggestive of renal pathology such as proteinuria or renal failure) is limited.
Abnormal ultrasound imagesUltrasound is an essential technique in the evaluation of patients with kidney disease, both acute and chronic, and its use is crucial to perform renal biopsy.
Renal ultrasound allows the identification of structural abnormalities that indicate the presence of kidney injury, as well as to rule out obstructive pathology of the urinary tract. The presence of simple cysts or a single calyceal stone without repercussion are not criteria of CKD (autosomal dominant polycystosis, renal dysplasia, hydronephrosis, staghorn stones, nephrocalcinosis, cortical scars, etc. are).
Histological alterationsEvidence of abnormalities in the renal parenchyma are considered criteria for CKD regardless of the GFR value or the presence of other markers of renal injury.
Biopsy indication is one of the tasks of the nephrologist and it is essential for the characterization of primary glomerular pathologies and other systemic pathologies with renal involvement, vascular, tubulointerstitial, cystic and congenital diseases.
Alterations in tubular functionAbnormal serum concentration of electrolytes and other solutes can be the result of disorders of renal tubular secretion and reabsorption. These abonormalities are not frequent but are indicative of kidney disease. We should highlight the following, renal tubular acidosis, nephrogenic diabetes insipidus, urinary losses of sodium, potassium or magnesium, Fanconi syndrome, cystinuria, etc. These are often genetic diseases, although they can also be acquired due to drugs or toxic substances.
History of Kidney transplantKidney transplant recipients are considered to have CKD, regardless of their GFR value or the presence of kidney injury markers.
Chronic kidney disease: staging and screeningAlthough the definition of CKD has remained unchanged since its initial description in 2002,29 there have been some universally accepted changes in its staging, but none since the publication of the Consensus Document in 2014.13
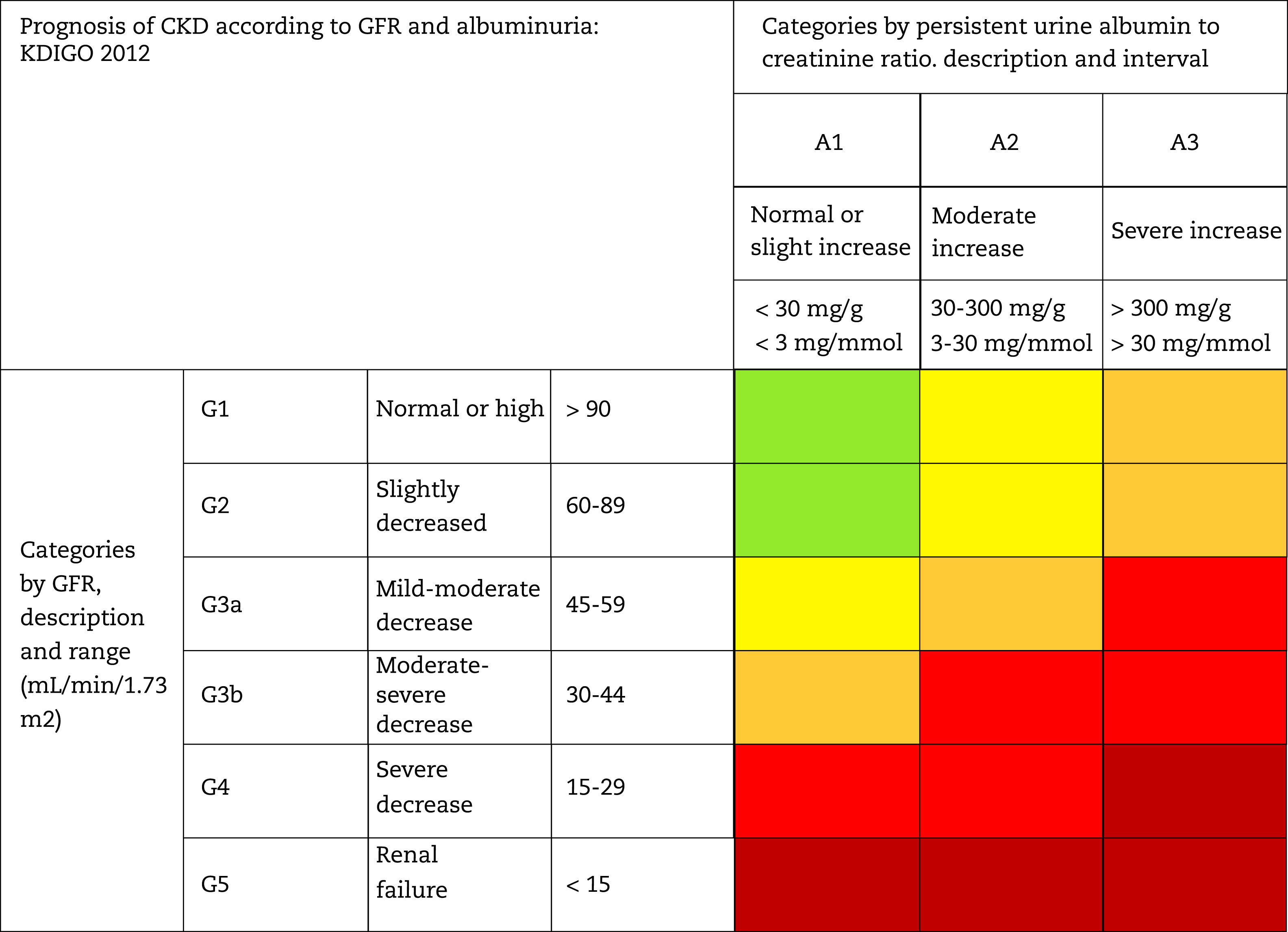
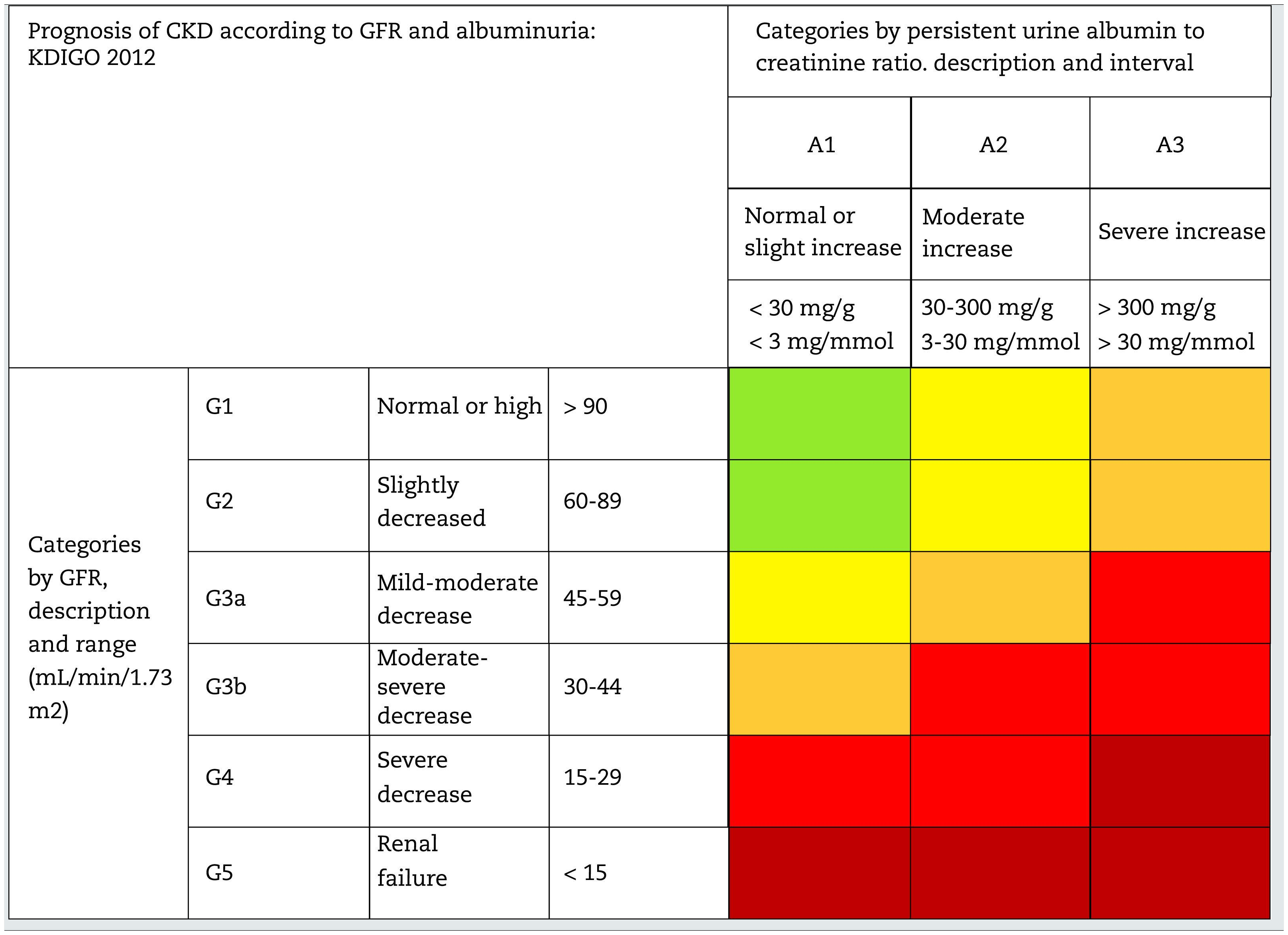
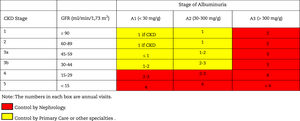
This classification considers a division of six risk categories according to the GFR (G1-G5) that are complemented by three risk categories according to the UACR (A1-A3) (Table 4). The decrease in GFR and the increase in the UACR are associated with an increase in adverse events (overall mortality, cardiovascular mortality, kidney failure requiring dialysis or transplantation, acute kidney failure and progression of kidney disease) (Table 5). The coexistence of a decreased GFR and an increased UACR multiplies the risk of adverses events. Patients that starts dialysis are included in the G5D category and, if they are transplanted they are stratified into stages G1T to G5T according to their GFR. It should be noted that in the KDIGO7 guidelines and in other studies performed in our country,4 more than 80% of the subjects with CKD (80.6%; 12.2% of the total sample) corresponded to moderate risk of cardiorenal complications, which establishes an important margin for both cardiovascular and renal prevention.
Staging and prognosis of CKD by glomerular filtration rate and albuminuria.
GFR: glomerular filtration rate; CKD: chronic kidney disease.
Note: Colors show the adjusted relative risk for five events (overall mortality, cardiovascular mortality, kidney failure treated with dialysis or transplantation, acute kidney injury, and kidney disease progression) from a meta-analysis of general population cohorts. The lowest risk corresponds to the color green ("low risk" category and, if there is no data on kidney damage, it cannot be classified as CKD), followed by the color yellow ("moderately increased" risk), orange ("high risk") and red (“very high risk”), which express increasing risks for the events mentioned (adapted from reference7).
Finally, it is important to take into account that the diagnosis and staging of CKD is independent of the cause of renal disease, however it is essential to bear in mind the cause of CKD in relation to the diagnosis and prognosis. For this reason, the international guidelines particularly recommend taking into account the concept of cause, grade, albuminuria (CGA).7,8 In this regard, it is emphasized that pathologies such as urinary obstruction, nephritic syndromes and/or glomerulonephritis/vasculitis, renal vascular disease, myeloma or other systemic diseases with renal involvement should have specific treatments.
In a recently published KDIGO document on the unification of nomenclature for kidney function and CKD, it is preferred to avoid the term end-stage kidney disease as it is not a patient-sensitive term and has stigmatizing connotations.24 This same document insists that albuminuria or proteinuria should not be used as equivalents of decreased kidney function, since they are not markers of kidney function but only markers of structural kidney injury.
These changes, among others, are discussed in an editorial article in the journal of the Spanish Society of Nephrology that brings together a new international initiative on the unification of nephrology nomenclature in Spanish developed by different societies.30
Risk factors and screening for CKDThe classic conceptual model of continuous CKD7,13 includes risk factors for each of the stages and are classified as factors of: susceptibility, initiating, progression and final-stage (Table 6). Obviously, some risk factors such as hypertension or DM can be at the same time susceptibility, initiators and progression factors. In addition, there have been described multiple monogenic or polygenic causes of CKD, as well as important pathophysiological associations with the development and progression of CKD thanks to the rapid growth of techniques such as Genomic Wide Association Studies (GWAS) or epigenetic studies.
CKD risk factors.
| Susceptibility factors: increase the possibility of kidney damage |
| Advanced age |
| Family history of CKD |
| Decreased kidney mass |
| Low weight at birth |
| Black race and other ethnic minorities (Afro-Caribbean and Asian) |
| Hypertension |
| Diabetes |
| Obesity |
| Low socioeconomic level |
| Initiating factors: initiate kidney damage directly |
| Acute renal failure* |
| Autoimmune diseases |
| Systemic infections (including HBV, HCV, HIV, SARS-CoV-2) |
| Urinary tract infections |
| Kidney stones |
| Lower urinary tract obstruction |
| Nephrotoxic drugs, including NSAIDs and antiretrovirals |
| Hypertension |
| Diabetes |
| Progression factors: worsen kidney damage and accelerate the decline of renal functional |
| Persistent proteinuria |
| Poorly controlled arterial hypertension |
| Poorly controlled diabetes mellitus |
| Cardiovascular disease associated with smoking |
| Obesity |
| Dyslipidemia |
| Black or Asian race |
| Chronic treatment with NSAIDs |
| Urinary tract obstruction |
| Metabolic acidosis |
| AKI and nephrotoxicity |
| Hospital admissions for heart failure |
| End-stage factors: increase morbidity and mortality in a situation of end stage renal failure |
| Low dose of dialysis (Kt/V)** |
| Temporary vascular access for dialysis |
| Anemia |
| Hypoalbuminaemia |
| Late referral to nephrology |
| Vascular calcification |
HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; CKD: chronic kidney disease; NSAIDs: nonsteroidal anti-inflammatory drugs; ARF: acute renal failure.
Presently, screening for CKD in populations at risk should be done by evaluating GFR and albuminuria at least once a year. Both diagnostic interventions have been shown to be cost-effective 1. The diagnosis should not be based on a SINGLE determination of GFR and/or albuminuria and should ALWAYS be confirmed.
We recommend CKD screening especially in patients with:
- •
HTN
- •
Type 2 DM (DM-2) or established cardiovascular disease.
- •
Older than 60 years.
- •
Obese (body mass index [BMI] > 30-35kg/m2).
- •
Type 1 DM (DM-1) with more than five years of evolution.
- •
First-degree relatives of patients with kidney disease or with hereditary kidney diseases.
- •
Obstructive urinary tract diseases or with structural alterations.
- •
Patients on prolonged treatment with nephrotoxic drugs (including calcineurin inhibitors –cyclosporine, tacrolimus–, lithium, antiretrovirals and non-steroidal anti-inflammatory drugs).
- •
Subjects with other risk factors for cardiovascular disease (smokers, dyslipidemia, metabolic syndrome).
- •
Patients with chronic infections, autoimmune diseases and neoplasms that may be associated with CKD.
- •
Patients with a medical history of acute kidney injury.
A history of acute kidney injury (Acute Kidney Injury or AKI) has acquired special relevance in recent years as a risk factor for developing subsequent CKD and/or the need for renal replacement therapy. Even the intermediate term (AKD or Acute Kidney Disease), which is not widely used, has been coined recently, referring to changes in kidney function lasting less than three months; this would define the course of kidney disease after AKI.31
Definition of CKD progressionThe average rate of annual decrease in GFR is highly variable, being higher in patients with significant proteinuria, DM or hypertension that is not optimally controlled.
Key points to consider- a)
Rate of normal renal progression: reduction of 0.7-1mL/min/1.73m 2 /year in older than 40 years.7
- b)
A patient can be considered to have renal progression if the decrease in GFR>5mL/min/1.73m 2 /year or > 10mL/min/1.73m2 in five years7,32 (functional and acute deterioration of kidney function are ruled out). There is debate about whether this rate of age-related progression is normal or pathological. In addition, the intra-individual biological variability of serum creatinine concentration (± 5%) should also be considered. Some guidelines recommend as significant progression (to consider referral to nephrology) a decrease > 5mL/min/1.73m 2 in a six month period confirmed by at least three determinations.33
- c)
Progression is defined based on the presence of any of the following points7 :
- •
Decrease in GFR:
- -
GFR decline > 5mL/min/1.73m2/year or > 10mL/min/1.73m2 in five years.
- -
Percent change as compared to baseline (> 25% deterioration in GFR), and ruling out functional factors.
- -
Accelerated progression of CKD: decrease of more than 25% of the GFR or a sustained decrease of the GFR≥15mL/min/1.73m 2 in one year, as considered by some guidelines.34
- -
- •
Increase in UACR:
- -
Increase of more than 50% in the UACR with respect to baseline.
- -
- •
Progresion to a higher category or more severe impairment in renal function or albuminuria.
- •
- d)
To assess progression of renal impairment it is recommended, estimation of baseline GFR and albuminuria (UACR) as well as to identify those factors of renal progression; this will dictate the frequency of determinations of successive analytical controls.
- e)
To analyze the rate of renal progression there are two aspects that must be considered 34 :
- •
Perform at least three determinations of GFR within a period of not less than 90 days.
- •
In the event of a new finding of GFR reduction, causes of acute deterioration of renal function must be ruled out (diarrhoea, vomiting, volume depletion due to diuretics) or initiation of treatment with drugs that affect glomerular hemodynamics (non-steroidal anti-inflammatory drugs or NSAIDs), inhibitors of the renin-angiotensin-aldosterone system inhibitors (RAASi) or sodium-glucose cotransporter 2 (SGLT2i) inhibitors.
- •
- f)
In patients with the finding of a decrease in GFR (for the first time), it is recommended repeating the measurement of estimated GFR in a period of not less than three months to rule out functional acute renal impairment. Depending on the clinical situation it may be advisable to repeat the GFR measurement in a shorter period (some guidelines recommend repeating the GFR in less than two weeks 34).
- g)
Regression:
- •
In addition to the term progression, the term regression should be considered. This aspect had already been considered in studies in patients with DM more than two decades ago.35 The definition of the term regression has been established based on recent cardiovascular safety clinical trials (CardioVascular Outcomes Trials) with SGLT2-i or GLP-1 receptor agonists (GLP1- RAs). It has been defined as a reduction in albuminuria from macro to micro or normoalbuminuria or from micro to normoalbuminuria, in at least two consecutive determinations separated by at least four weeks.36 Some treatments for DM have shown the possibility of regression of albuminuria.36,37 In individuals with baseline UACR ≥ 300mg/g, a 30% decrease in the UACR over two years confers an absolute reduction of more than 1% in the 10-year risk of requiring renal replacement therapy (RRT).38 Thus, short term changes in albuminuria (1-3 years) provide information on the long-term risk of needing RRT.
- •
Although remission has not been defined in relation to changes in GFR in recent studies, some authors have considered remission the reduction to normal values the rate of renal deterioration ≤ 1mL/min/year during the observation period, similar to the physiological rate of progression associated to aging.39 Some treatments of DM have shown the possibility of regression of albuminuria.36,37
- •
Predictors of renal progression are shown in Table 7.40–53
Predictive factors of CKD progression.34,40
| Factors |
| Proteinuria41,42 |
| Arterial hypertension43,44 |
| diabetes mellitus45 |
| Cardiovascular disease46 |
| smoking47 |
| Obesity48 |
| Black or Asian race49 |
| Chronic treatment with NSAIDs50 |
| Urinary Tract Obstruction34 |
| Metabolic acidosis51 |
| ARF and nephrotoxicity52 |
| Hospital admissions for heart failure53 |
CKD: chronic kidney disease; NSAIDs: nonsteroidal anti-inflammatory drugs; ARF: acute renal failure.
In a recent analysis of 264,296 patients with GFR<30mL/min/1.73m 2 the most relevant factors in the progression of kidney disease requiring RRT were low GFR, DM, black race, male sex, systolic BP≥140mmHg and albuminuria.54 From this analysis, a calculator based on a mathematical formulation has been built to estimate the risk of starting RRT, cardiovascular events and death: http://ckdpcrisk.org/lowgfrevents/.
FrailityFrailty is defined as syndrome that entails a decreased of reserve and reduced resistance to stressors, resulting from the accumulation of deficits in multiple physiological systems, which ends up causing vulnerability.55
The prevalence of frailty in CKD is higher than in other cardiovascular disrases; frailty increases progressively as GFR decreases, particularly from GFR<45mL/min/1.73m 2 ; it is an independent risk factor for hospitalization and mortality from any cause, especially in dialysis patients, in whom it reaches prevalences of up to 73% (preferably using Fried phenotypes) compared to 7% in stage G1-G4.56,57
There are different scales to assess frailty providing different results. One of the most used and recommended is the five-item FRAIL questionnaire (Table 8). This scale evaluates frailty according to phenotypic expression through the combination of five conditions, each one valued as one point. Patients are classified according to their health status as: frail (3-5 points), pre-frail (1-2 points), and robust (0 points).58 This quick and easy to apply questionnaire has been validated in multiple geriatric settings, although it loses discriminative capacity in populations with a high prevalence of frailty, as is the case of hemodialysis patients.
FRAIL Questionnaire.
| 1. Fatigue: Do you feel tired most of the time? (Yes, No) |
| 2. Endurance: Can you walk up one floor of stairs without pausing without assistance? (Yes, No) |
| 3. Walking: are you able to walk 100m (one block) without pausing,without assistance? (Yes, No) |
| 4. Diseases: (more than five) arthritis, diabetes, angina/heart attack, hypertension, stroke, asthma, chronic bronchitis, depression/anxiety, dementia, leg ulcers. (Yes, No) |
| 5. Weight loss: weight loss > 5% in the last 6 months? (Yes, No) |
| Evaluation of the result: 1 to 2=pre-fragile 3 or more=fragile |
In patients with CKD, the most used scale is the Fried Phenotypes55 (Table 9).
Fried Phenotypes.
| 1. Unintentional weight loss greater than 4.5kg59 or 5% of body weight during the previous year |
| 2. Low energy or exhaustion, depending on the answer to certain questions (" I feel like everything I do is an effort ", " I feel like I can't keep doing things ") at least 3-4 days a week |
| 3. Muscle weakness. Decrease in pressure muscle strength (measured with a dynamometer) < 20% adjusted according to gender and body mass index |
| 4. Reduced physical activity, as measured by calculators of weekly consumption of calorie or physical activity scales; for example, Calcumed or PASE (Abizanda P, 2013; Schuit AJ, 1997; García FJ, 2011) |
| 5. Slowness. Measured according to the walking speed test (meters/second) < 20%, adjusted according to gender and height |
| Frailty=3 or more criteria |
| Evaluation of the result: no criteria: robust ;1-2 criteria: pre-fragile; 3-5 criteria: fragile |
In summary, frailty assessment is recommended in CKD patients, as a measure of physiological reserves, to assess prognosis and help make therapeutic decisions, including replacement therapies. The optimal tool for its evaluation in patients with CKD has not been established, although the Fried phenotypes are the most used and the FRAIL questionnaire can help detect frailty in dialysis patients.
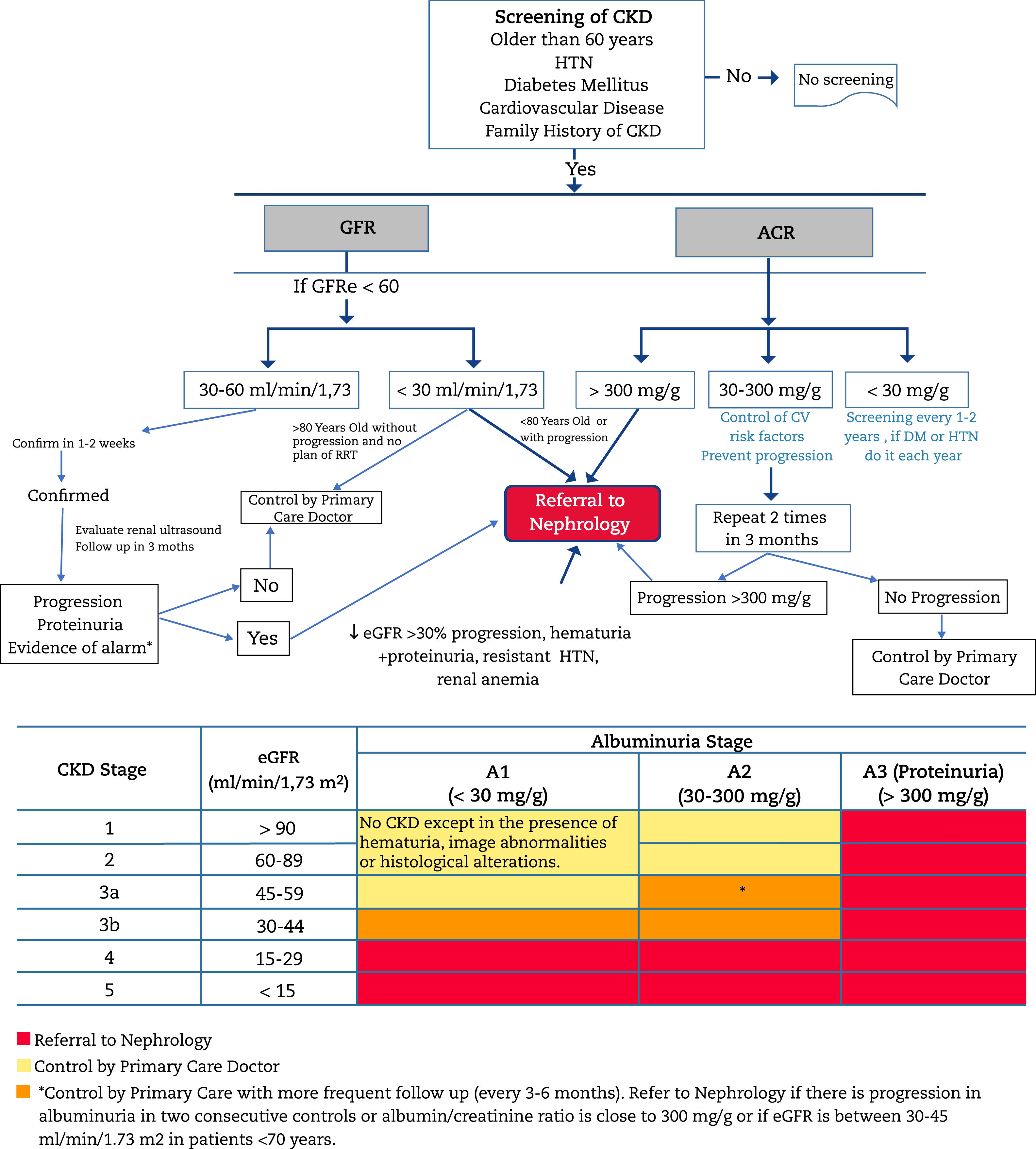
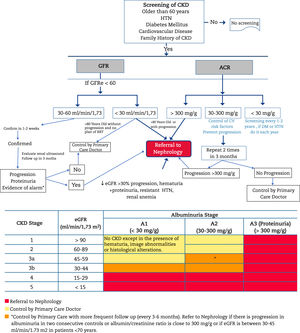
Criteria for referral to NephrologyReferral to Nephrology (Fig. 1) will be made taking into account the stage of CKD, the rate of progression of CKD, the degree of albuminuria, the presence of alarm signs, associated comorbidity and the functional status of the patient.34,60,61 In general CKD presents few sympytoms and it is often asymptomatic until very advanced stages; therefore its follow upo is highly dependent on monitoring laboratory results. The risk of cardiovascular events is higher than that of progression to dialysis.54 In many cases, renal progression is slow without requiring special measures, except for control of cardiovascular risk factors and avoidance of nephrotoxicity. For this reason, suitable circuits must be created that begin with a virtual (telematic) consultation between the doctor who is going to refer the patient and the Nephrology Department for an initial assessment, since in many cases this channel may fulfill the consultation, or clarify doubts about the follow-up of patients with advanced age, decreased GFR and little progression. In this way, the shifts of the patient and family, as well as unnecessary or repeated tests, can be avoided.
Algorithm for diagnosis and shared care between Primary Care and Nephrology.
CKD: chronic kidney disease; AHT: arterial hypertension; GFR: estimated glomerular filtration rate; UACR: urine albumin to creatinine ratio; RRT: renal replacement therapy; CVRF: cardiovascular risk factors; DM: diabetes mellitus.
In general, patients should be referred to a specialist in Nephrology if they present albuminuria > 300mg/g, albuminuria of any grade accompanied by glomerular microhematuria (hematuria of a non-urological cause) or those with CKD G4 or G5 (GFR<30mL/min/1.73m 2) (except > 80 years without renal progression, albuminuria < 300mg/g, no alarming signs and without a plan or decision to undergo RRT (see below).
According to glomerular filtration- •
All patients with GFR<30mL/min/1.73m 2, except those patients > 80 years without or very slow renal progression.
- •
In Patients > 80 years and with GFR<20mL/min/1.73m 2, the evolution of renal function and albuminuria in recent years should be previously assessed. If renal function have been stable or with a minimal progression and without renal anaemia, they can be managed jointly by telematic consultation or directly by the family doctor with occasional telematic consultation to Nephrology. If necessary, a single evaluation will be carried out with physical presence and subsequently the successive visits will be agreed with the family doctor.
- •
Patients < 70 years with GFR between 30-45mL/min/1.73m 2 should be monitored more frequently (every 3-6 months), and be referred to Nephrology in case of progression of renal function and/or albuminuria in two consecutive controls, especially with UACR>300mg/g once they have received RAAS blockade as well as optimal blood pressure (BP) and glycaemia control in the case of patients with DM, and always ruling out functional renal impairment (depletion, diuretic, hypotension, NSAID, etc.).
- •
It is recommended that the candidate patient to receive RRT be referred to Nephrology at least one year before the start the treatment. Candidate patients for RRT are those who meet any of the following characteristics :
- o
Older than 80 years with a GFR<20mL/min/1.73m 2.
- o
Between 70-80 years with a GFR<30mL/min / 1.73m2
- o
In < 70 years with a GFR<45mL/min/1.73m 2, especially if accompanied by albuminuria.
- o
These criteria may vary if there is renal progression or accelerated renal deterioration (GFR progression of > 5mL/min/year for two consecutive years).
- o
The objective is to prevent unscheduled dialysis (which would require implantation of a central catheter) in a patient that is candidate of RRT. In the case of scheduled dialysis, the patient can choose the dialysis technique that best suits ther needs (peritoneal dialysis, in-center hemodialysis, or home hemodialysis) and it can even consider early kidney transplantation.
- o
- -
ACR UACR>300mg/g which is approximately equivalent to albuminuria > 300mg/24hours,patients should be referred to Nephrology especially if there is no no apparent cause of albuminuria (DM, hypertension…) and with optimal BP control (including RAAS blockade). In patiets of advanced age >80 years, and especially >90 years, the progression of albuminuria should be assessed and patients should be referred only if the albuminuria > 300mg/g is accompanied by a decrease in GFR. If renal function is preserved, action will be taken on renal progression factors (BP, HbA1c, obesity) and it will only be referred in the event of renal progression.
- -
ACR 30-300mg/g: Patients with urine UACR between 30-300mg/g and CKD G3b (GFR: 30-45mL/min/1.73m 2) have a higher probability of progression.62 In these patients, more frequent monitoring will be implemented by their family doctor. These patients will be referred to Nephrology in case of progression of albuminuria during its evolution (> 300mg/g). It is important to take into account the variability of albuminuria and the factors that can influence it, as mentioned before. For this reason, with values albuminuria with remission criteria, the measurements should be repeated to confirm and rule out causes that may influence the temporal occurrence of albuminuria.
- •
Acute deterioration in kidney function or acute kidney injury (AKI):
- o
Increase in serum creatinine concentration >30% or decrease in GFR>30% in less than one month.
- o
AKI: increase in serum creatinine concentration ≥ 50% in 7 days or an increase in creatinine ≥ 0.3mg/dL (≥ 26.5μmol/L) in 48hours or the presence of oliguria.
- o
- •
In both cases, exogenous factors (excessive control of BP, diarrhoea, vomiting, volume depletion due to the use of diuretics, NSAIDs, initiation of treatment with iRAAs or SGLT2-i) must have been ruled out.
- •
Patients with renal progression (> 5mL/min/year) especially if it is accelerated (decrease in GFR>30% or > 15mL/min/year) or if it is required diagnostic clarification (DM of short duration, unexplained non-urological hematuria, autoimmune diseases).
- •
CKD and HTN (> 130/80mmHg) refractory to treatment with three drugs at full doses, one of them being a diuretic.
- •
Suspected renal artery stenosis.
- •
Alterations in serum potassium concentration (> 5.5 mEq/L or < 3.5 mEq/L without receiving diuretics).
- •
Anemia: Hb < 10.0g/dL with CKD despite correcting iron deficiency (transferrin saturation index [ISAT]) > 20% and ferritin > 100ng/mL).63
- •
Presence of signs of alert :
- o
Presence of non-urologic hematuria, especially if associated with albuminuria. In the event that the patient presents glomerular hematuria (not of urological cause – rule out kidney stones, urinary tract infection, urinary tract neoplasia or other urinary tract injuries). The relative importance of albuminuria will be greater the greater the associated deterioration in renal function and, especially, if it is accompanied by glomerular hematuria. If microhematuria is detected in the absence of albuminuria, neoplasia of the urinary tract should be ruled out in populations with risks by performing urinary cytology and renal and urinary tract ultrasound.34
- o
Referral to Nephrology will be made taking into account the above criteria and comments, and any patient with increased albuminuria despite following an adequate treatment. In all the above cases, the subsequent follow-up will be agreed between the family doctor and the nephrologist. The drop in GFR after starting an SGLT2-i (as with RAAs-i) should not be a reason for referral to Nephrology or to withdraw treatment if the drop in GFR is ≤30%. To start with, functional causes of GFR reduction should be ruled out (volume depletion, excess dose of diuretics, or low BP before initiation of tratment). If after ruling out these causes or adjusting the dose of diuretics, the drop in GFR is maintained > 30% of basal values it will be sent to Nephrology.64–66
Octogenarian or nonagenarian patients- •
Will perform Telematic consultation for joint management. In this consultation, frailty will be assessed and, if the patient is not a frail elderly person, it will be considered whether the patient needs to be present in the visit.
- •
In frail elderly people or those with little life expectancy (<1year) with CKD G4-G5 and who are not candidates for RRT, both diagnostic and therapeutic actions will be agreed upon. If necessary, a joint renal palliative care will be established.67
- •
Family physicians will be informed about the possibility of exacerbation episodes, most cases will be of prerenal cause (excessive diuretics, excessive BP control, associated acute comorbidities) that may justify renal deterioration that can be solve without Nephrology assistance.
There is a score to calculate the risk of requiring RRT at five years in patients > 65 years and GFR<60mL/min/1.73m 2 (Kidney Failure risk equation or “KFRE”: http://ckdpcrisk.org/kidneyfailurerisk/). The result of the equation when applying these variables indicates that the patient should be referred to Nephrology with an index between 3-5%. This equation is recommended by the European Working Group on good renal clinical practice in patients with GFR<45mL/min / 1.73m2.19 As compared with the SEN criteria, this equation overestimates the percentage of patients who should be referred to Nephrology, especially in patients over 80 years of age. There are also other options, such as the Nefroconsultor application for mobile devices, developed in our country, which in this case is more consistent with the SEN criteria and also provides recommendations according to the stage of CKD. Access to the application: https://www.senefro.org/modules.php?name=apps&op=detail&id=6.
Indications for requesting renal ultrasound in Primary CareWhether the purpose is the follow-up by the family doctor or for referral to Nephrology, a request to perform an ultrasound is considered pertinent in the study of CKD. Its indications are34 :
- -
Rapid progression of CKD.
- -
Macroscopic hematuria (or microscopic if it is persistent).
- -
Symptoms of urinary tract obstruction.
- -
CKD with proteinuria.
- -
Age >20 years and family history of polycystic kidneys disease.
- -
CKD stage G4 or G5.
- -
Recurrent urinary tract infections involving the kidney.
If Nephrology consider to perform a kidney biopsy, this department will request an ultrasound.
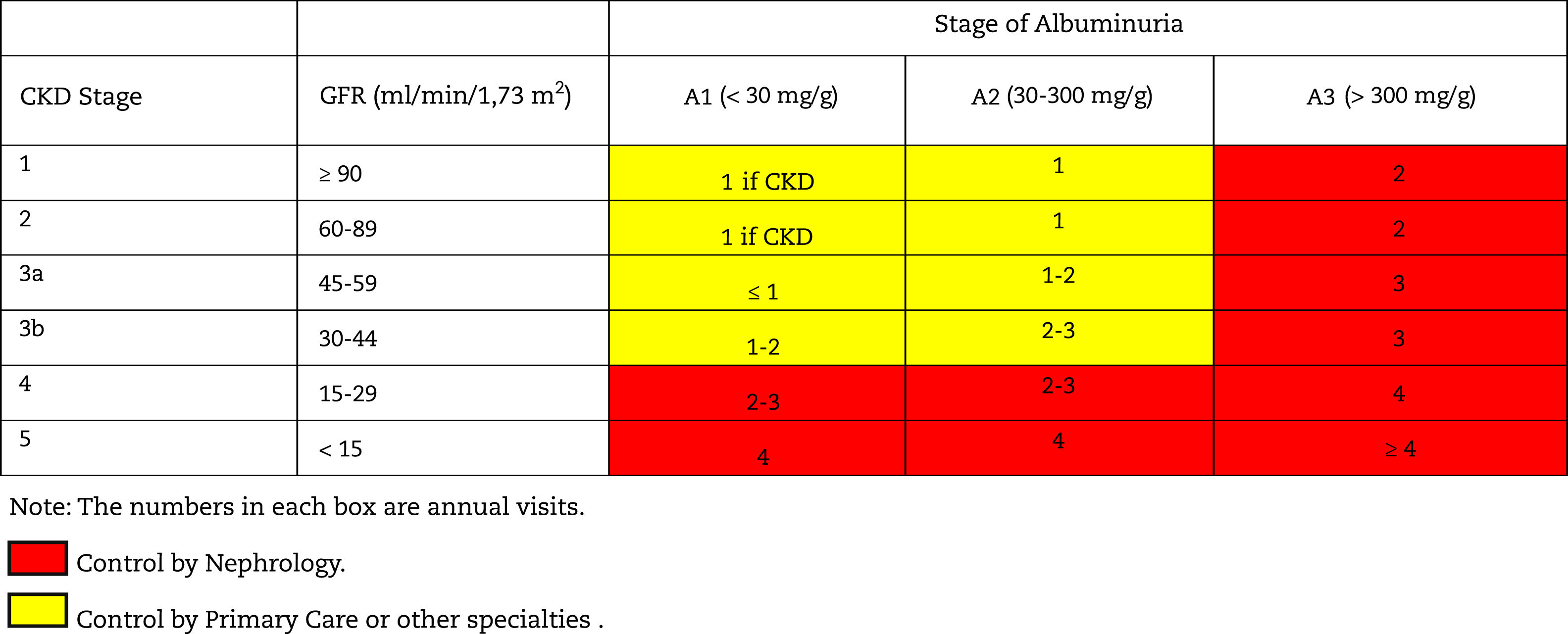
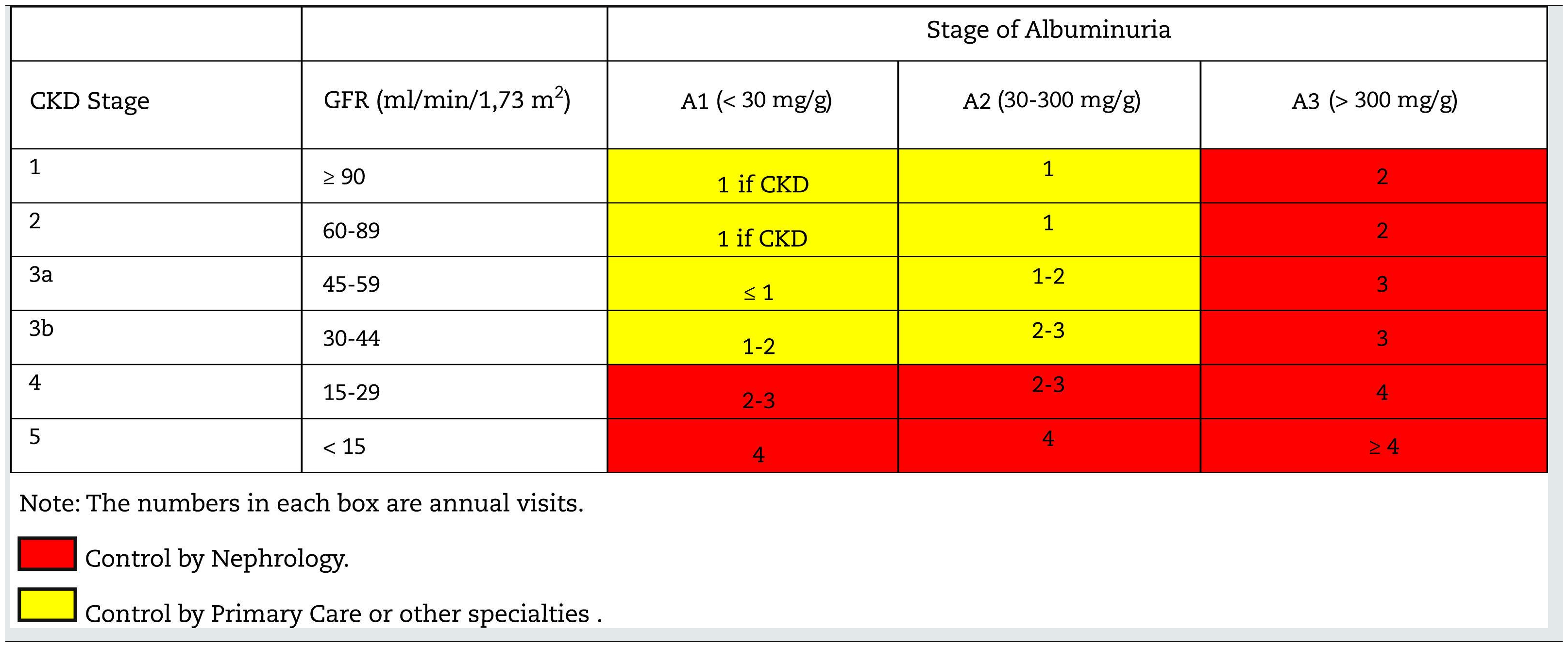
Monitoring and follow-up of patients with CKDThe frequency of monitoring and visits for patients with CKD is shown in Table 10; it will depend on the cause of the CKD, the the rate of change of GFR/creatinine and albuminuria (easily visible in computer applications of health systems), the presence of comorbidities, acute intercurrent illnesses or hospitalizations, especially heart failure, and changes made in treatment. In this regard, it should be remembered that in some situations monitoring should be performed after the introduction of some drugs such as RAAS inhibitors, SGLT2 inhibitors, increased doses of diuretics, suspicion of renal impairment due to NSAIDs, adjustment of direct-acting oral anticoagulants (DOACs) or after occurrence of unexpected hypoglycemia in diabetics.
In patients who have presented acute renal failure with hospitalization, monitoring of renal function will be required for at least two years, even if renal function has returned to baseline.34
In any case, it is necessary to individualize these general criteria.
In each clinical visist to the family doctor it is recommended:
- -
Control BP and adjust the treatment. BP target < 140/90mmHg (using self-measurement of BP at home). Try to reach BP values of 130/80mmHg, if it is tolerated, in patients with UACR>30mg/g and especially in those with values > 300mg/g. Avoid a reduction of systolic BP below 120mmHg, especially in elderly patients, if orthostatic hypotension or autonomic neuropathy are present.68 Treatment should be individualized according to tolerability and the impact on renal function and electrolytes.69
- -
Monitor the presence of renal anemia: If CKD 3-5 and Hb < 10.0g/dL (after ruling out iron deficiency: IST>20 % and ferritin > 100ng/mL), consider referral or and earlier follow-up visit in Nephrology to evaluate treatment with erythropoiesis-stimulating factors according to the established protocols.63
- -
Review the medication, adjusting the dose according to the GFR (oral antidiabetics of renal elimination, direct-acting oral anticoagulants). In CKD 3-5, avoid the use of NSAIDs and iodinated contrast agents.
- -
Review dietary habits, recommending appropriate physical exercise for each age and clinical situation, achieving the ideal weight, as well as quitting smoking and guiding the patient on the type of diet to follow according to the GFR:
- o
CKD 1-3: Moderate sodium restriction is only recommended in the case of hypertension or volume overload.7
- o
CKD 4-5: Dietary recommendations for moderate sodium, potassium, phosphorus and protein restriction (0.8g/kg/day).
- o
- -
Laboratory tests in each follow-up visit for patients with CKD G3 or more advanced CKD* (in italics the minimum recommended):
- o
Complete blood count
- o
Serum concentration of glucose, creatinine, urea, sodium, potassium, calcium, phosphate, albumin, cholesterol, triglycerides, and urate. eGFR by CKD-EPI-creatinine.
- o
UACR in the first morning urine sample.
- o
Urine sediment.
- o
- -
Review criteria for referral to nephrology in teach of he follow-up visits.
* Efforts will be made to combine the request for laboratory tests with other specialties or with Primary Care so as not to repeat them.
Table 11 shows the objectives in the monitoring and follow-up of patients with CKD according to stages.
Follow-up of patients with CKD. Objectives by specialty.
| CKD stage | Primary Care | Nephrology |
|---|---|---|
| 1-2-3a (GFR>45mL/min/1.73m 2) | Identify and treat CKD risk factors | Assess renal diseases subsidiary to specific treatment: |
| Rule out functional causes of renal impairment (excessive control of blood pressure, NSAIDs, volume depletion) | Primary or secondary glomerulonephritis | |
| Detect CKD progression | Ischemic kidney disease | |
| Deterioration of GFR | Detect and treat CKD progression | |
| Increase in proteinuria | ||
| Control of factors associated to progression of renal disease | ||
| 3b GFR: 30-45ml/min/1.73m2) | To Detect progression of CKD | Assess kidneys diseases that are subsidiary of specific treatment. |
| Rule out functional causes of renal deterioration (excessive control of blood pressure, NSAIDs, volume depletion) | Monitor and treat factors associated with renal progression | |
| Treat and maintained controlled factors associated renal progression. | Evaluate and treat complications of CKD: | |
| Adjustment of Drug dose according to GFR. Search for of nephrotoxic drugs (eg NSAIDs) | Alterations of bone-mineral metabolism | |
| Advice on Hygienic and dietary habits. | Anemia | |
| Vaccinate against pneumococcus, influenza and HBV | Electrolyte disturbances | |
| Detect complications of CKD: | ||
| -Anemia | ||
| -Electrolyte disorders | ||
| 4 (GFR<30mL/min/1.73m 2) | Drug dose adjustment according to GFR. Search for of nephrotoxic drugs (eg NSAIDs) and rule out functional causes of renal impairment (excessive control of blood pressure, NSAIDs, volume depletion) | Prepare, if appropriate, for renal replacement therapy. |
| Advice on Hygienic and dietary habits. | Organize treatment palliative if replacement therapy is not appropriate. | |
| Detect complications of CKD: | Evaluate and treat complications of CKD: | |
| Anemia | Alterations of bone-mineral metabolism | |
| Electrolyte disturbances | Anemia | |
| Electrolyte disturbances | ||
| Metabolic acidosis |
CKD: chronic kidney disease; NSAIDs: nonsteroidal anti-inflammatory drugs; GFR: glomerular filtration rate; tto.: treatment.
Once the patient is diagnosed with CKD, the priority is to prevent progression of of CKD by acting on the renal progression factors and be aware that there are drugs used in daily practice that are nephrotoxic and may cause progression of CKD (Table 7).
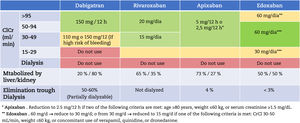
Avoid nephrotoxic drugsAvoid the unnecessary use of NSAIDs, due to the risk of renal function deterioration, as well as other potentially nephrotoxic drugs (aminoglycosides, some antivirals –acyclovir, cidofovir). The list of drugs that need dose adjustment may be consulted at the following link: https://www.nefrologiaaldia.org/es-articulo-adjustment-farmacos-enfermedad-renal-cronica-325
Minimize the use of intravenous contrast agents and be aware of the risk factors that favor the renal toxicity- •
Contrast-induced nephropathy (loss of renal function >30% or absolute increase in creatinine of 0.5mg/dL as compared to baseline that occurs during the first three days after contrast administration and is not due to any other cause). It is more frequently in patients with advanced age, heart failure, DM, CKD, especially if GFR<30mL/min/1.73m 2, previous acute renal failure, dehydration, acute myocardial infarction, shock, high volume of contrast, anemia, hypotension, use of nephrotoxic drugs and high doses of diuretics. Detection of these risk factors is key to prevention of contrst induced nephropathy. The need for contrast administration should be confirmed and, given an unfavourable clinical situation, consider the whether alternative diagnostic imaging without the use of intravenous contrast is sufficient for diagnostic purpose. If the administration of contrast is essential, the minimum necessary dose will be administered, avoiding, if possible, repeated administrations.
- •
The best treatment is prevention, avoiding risk situations. The suspension of diuretics is recommended at least 4-6 days before contrast administration, as well as intravenous fluid therapy and oral hydration.
- •
Some drugs, such as metformin,can be potentially toxic after the administration of contrast. Metformin should not be administered in patients with a GFR<30mL/min/1.73m 2 in accordance with the recommendations of the data sheet. Although there has been controversy, according to the consensus of the American Society of Nephrology and the National Kidney Foundation, on whether metformine should be maintained at the time of a contrast study in patients with eGFR between 30-59mL/min/1, 73m 2 the decision should be individualized according to clinical situations, assessing the risk factors for presenting lactic acidosis and the mentioned eGFR level.70 Likewise, there are doubts about the possible adjuvant effect on contrast nephrotoxicity in patients receiving SGLT2-i.71,72 Although there are no established recommendations, it is advisable to wear in mind this possibility, especially in patients with multiple risk factors of nephrotoxicity, especially the patient has received NSAIDs.
Some drugs can alter glomerular hemodynamics, favoring renal hypoperfusion and enhancing the possible nephrotoxicity of other drugs. The vast majority of these drugs are cardioprotective and nephroprotective, so they should not be discontinued (SGLT2 inhibitors, RAAAs, including mineralocorticoid receptor antagonists [MRAs] such as spironolactone), but special care should be taken to prevent the use of high doses or a potentiation of their effects due to various circumstances, generally of a functional nature (sudden volume depletion, excess dose of diuretics, excessive BP control or administration of NSAIDs), since they can favor the appearance of hypotension, acute kidney injury or hyperkalaemia, as well as possible nephrotoxicity of other drugs.
Hygienic-dietary measures. attitudes and lifestyleThe initiation, evolution and prognosis of CKD is influenced by various risk factors that are frequently present in the general population.73 The recommendations in this regard are as follows:
- •
Physical exercise: 30-60minutes of moderate exercise is recommended, 4 to 7 days per week (minimum of 150min/week), with moderate-intensity aerobic and/or strength exercises to avoid a sedentary lifestyle and with individualized programs based on the patient's characteristics and a gradual introduction in time and intensity.74
- •
Diet: Mediterranean-type, with foods rich in fiber, avoid saturated and trans fats and individualize according to existing risk factors, low sodium diet 6g of salt (equivalent to 2.4g sodium) in case of hypertension and/or heart failure.75 There is controversy regarding potassium restriction in CKD, especially in patients who do not have hyperkalaemia, since some studies have shown that diets with moderate or high potassium content can reduce kidney damage,76 but the important point is to establish strategies to avoid severe hyperkalemia. In advanced phases of CKD (G4-G5) will be recommended dietary content of sodium, phosphorus, potassium and proteins. In patients with CKD G4-G5 not on dialysis it is recommended protein restriction of 0.8 is g/kg/day (at least half must be animal proteins of high biological value).77 In patients with CKD on hemodialysis, protein intake may be increased to 1.2g/kg of weight to favor an adequate protein balance and avoid caloric-energy wasting and maintain an adequate nutrition.78,79
- •
It is recommended to avoid alcohol intake above 12-14grams/day (approximately 300 cc of beer or 150 cc of wine), and to avoid smoking due to its deleterious cardiovascular and renal effects.80–83 The issue of staying away from tobacco consumption should be discussed in all the clinic visits. Empathetic, firm and motivated advice will be given to smokers to quit smoking using Systematized Minimum Intervention, cognitive-behavioral techniques or pharmacological treatment (bupropion at lower doses than usual –150mg/24h in advanced stages of CKD, or varenicline at usual doses or reduced to half (1mg/24h) in patients with decreased renal function (CKD G4 and G5).
Confirm the diagnosis of HTN and detect whether it meets the criteria for hard-to-control or resistant hypertension84 using BP measurenet in the clinic, self-measurement of BP at home (AMPA) or ambulatory blood pressure monitoring (ABPM). The latter prevents overtreatment and helps to optimize the control of BP, an important factor that influences the progression of renal and cardiovascular disease.85,86
Target BP values are different according to guidelines,87 with heterogeneity of recommendations in CKD:
- •
ACC (American College of Cardiology 2017): < 130/80mmHg, regardless of the degree of proteinuria.88
- •
NICE (National Institute for Health and Care Excellence) guidelines: < 140/90mmHg, in the presence of proteinuria < 1g/day. If proteinuria > 1g/day: 130/80mmHg.34
- •
KDIGO 2021: systolic BP<120mmHg, using iRAAS if GFR<60mL/min/1.73m 2 or UACR>30mg/g.89
- •
ESC/ESH 2018 (European Society of Cardiology/European Society of Hypertension): Systolic BP<140mmHg regardless of the level of proteinuria.69
The available evidence does not show a clear consensus on the most appropriate BP target in CKD patients; therefore the guidelines recommendations are not uniform either, and the BP control strategy in patients with CKD will have to be established individually, considering the global cardiovascular risk, the rate of decrease in GFR, and the presence of other comorbidities. In addition, the targets and objective may change as the patient ages, becomes frail or develops more severe CKD.87
There is much more consensus on the use of antihypertensives, and those that block the actions of the RAAS are recommended as drugs of first choice, either angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists. Likewise, the use of combinations of antihypertensive drugs is recommended to achieve the objectives. This combination should include a diuretic, thiazide or loop, depending on the severity of the CKD.69,88
Detection and management of hyperglycemia in CKDManagement goals- •
How to assess metabolic control?
Use of glycosylated hemoglobin A1c (HbA1c) to assess metabolic control in patients with CKD with GFR down to 30mL/min/1.73m2.90–95 Below these value GFR the HbA1c is less reliable and this must be taken into account.96 In these situations, continuous glucose monitoring (CGM) may be useful in those patients in whom hemoglobin A1c is not consistent with the routine values of blood glucose or with clinical symptoms.97
- •
Glycemic control targets
The appropriate individualized targets for HbA1c may range from as low as < 6.5% to as high as < 8%, depending on the characteristics of the patient (severity of CKD, macrovascular complications, comorbidities, life expectancy, perception of hypoglycemia, treatments with risk of hypoglycemia).96 There is no evidence indicating what is the optimal HbA1c level for dialysis patients. HbA1c levels >6.5% have been associated with an increase in microvascular complications.98 This is a strict objective to consider if there is no risk of hypoglycemia, especially in young patients and DM-2 with long life expectancy.
Antidiabetic drugs99The recent KDIGO 2020guideline96 recommends that glycemic management for patients with DM-2 and CKD should include lifestyle therapy, first-line treatment with metformin and an SGLT-2 inhibitor, and additional pharmacological therapy, as necessary to achieve glycemic control. In general, glucagon-like peptide receptor type 1 agonists (GLP1 RAs) are the preferred additional drugs because of their demonstrated benefit in reducing cardiovascular events, particularly among people with prevalent atherosclerotic CVD, and also because of their potential to prevent macroalbuminuria and reduce the rate of the progression of CKD, in addition to being able to contribute to weight reduction.
- •
Metformin
In patients with DM-2, CKD and GFR≥30mL/min/1.73m 2, it is recommended to use metformin as first-line treatment for hyperglycemia (along with SGLT2-i), with adjustment according to renal function and use the same regimen in kidney transplant patients:
- o
Do not adjust with GFR>45mL/min/1.73m 2.
- o
Reduce to half if GFR is between 30 and 45mL/min/1.73m 2.
- o
Discontinue metformin if GFR<30mL/min/1.73m 2.
It is recommended to watch for the occurrence of vitamin B12 deficiency if patients have been treated with metformin for more than four years. It is also important to advise patients with eGFR between 30-40mL/min/1.73m 2 to contact the family doctor if acute complications are observed (diarrhoea, hypotension, vomiting, etc.) that may cause functional deterioration of renal function with complications derived from the accumulation of metformin resulting from kidney failure.
- •
SGLT2 inhibitors
Treatment with SGLT2i is accompanied by important cardiovascular and renal benefits in DM-2 patients with different ranges of renal function deterioration and albuminuria. These has been demonstrated in the following clinical studies: EMPA-REG OUTCOME, CANVAS, DECLARE and CREDENCE.36,37,100,101 The benefits were disproportionate to the reduction in HbA1c and did not appear to be dependent on glucose reduction.
Based on this information, the KDIGO Work Group96 considered that for most patients with DM-2, CKD and GFR≥30mL/min/1.73m2 would use SGLT2-i, regardless of the stage of CKD or the level of glycemic control. The choice of an SGLT2-i should prioritize drugs with documented renal or cardiovascular benefits, taking into account the GFR, since the degree of renal function influences the antihyperglycemic efficacy, but not the cardiovascular and renal benefits.
If the patient is being treated with insulin or sulfonylureas and currently maintain glycemic targets, the addition of SGLT2-i may increase the risk of hypoglycemia; therefore it may be necessary to discontinue or reduce the dose of an antihyperglycemic agent other than metformin to facilitate the addition of an SGLT2-i.
The possibility of side effects should be kept in mind and the concomitant treatments need to be adapted (history of recurrent genital candidiasis, episodes of volume depletion or excess of diuretics or especially in patients with risk factors for development of diabetic ketoacidosis).102
As mentioned, the reversible drop in GFR with the initiation of SGLT2i is not per se an indication to discontinue treatment, it this is associated with the hemodynamic effect of reducing intraglomerular hypertension. Once an SGLT2-i is started, it is reasonable to maintain it even if the FG falls below 30mL/min/1.73m2, unless the reduction in GFR is precipitating uremic symptoms or other complications of CKD.
The technical data sheets of the SGLT2i are being updated to include evidences from studies showing benefit until the start of dialysis in patients with diabetic kidney disease. For this reason, it is necessary to revise the the data sheets shortly, relative to the initiation and maintenance of SGLT2-i in patients with low eGFR, and their use in non-diabetic CKD or in heart failure with low eGFR.
The SGLT2i have not been sufficiently studied in kidney transplant recipients, who may benefit from SGLT2i but who are immunosuppressed and potentially at increased risk of infections, therefore presently the recommendation to use SGLT2-i is not extended to kidney transplant recipients.
Glucagon-like peptide type 1 receptor agonistsThe use GLP-1 RAs have shown cardiovascular benefit and reduction in proteinuria. It is recommended in patients with DM-2 and CKD who have not achieved individualized glycemic goals despite the use of metformin and SGLT2-i, or in those in whom these drugs cannot be used. It is recommended in those that have achieved cardiovascular and renal benefit. Lliraglutide, Semaglutide and Dulaglutide are available in Spain, Albiglutide is not. These three drugs can be used in patients with GFR of 15mL/min/1.73m 2, although experience in patients with CKD G4 (GFR between 15 and 30mL/min/1.73m 2) is limited.
In addition to the cardiovascular and renal benefits, there are significant reductions in HbA1c with a low risk of hypoglycemia and with additional benefits such as weight and BP reduction.103 The risk of hypoglycemia with GLP-1 RAs is generally low when it is used alone, but the risk increases if used concomitantly with other antidiabetics, therefore, the administration of a GLP-1 RA, should be accompanied by reduction in the dose of sulfonylureas or insulin as the risk of hypoglycaemia is increased. To minimize the gastrointestinal side effects of GLP-1 RAs, it should be started with a low dose and titrated slowly, probably more slowly than indicated in the data sheet.
The GLP-1 RAs should not be used in combination with inhibitors of dipeptidyl peptidase 4 (DPP-4-i). In our country, GLP-1 RAs are financed exclusively for the treatment of DM-2 patients with with a body mass index greater than 30kg/m2.104
- •
Dipeptidyl peptidase 4 inhibitors
These drugs are safe in terms of risks of hypoglycemia and very well tolerated. However, unlike the GLP-1 RAs, the studies performed have not shown to offer cardiovascular or renal protection. They can be used as monotherapy and in association with any other drug, except GLP-1 RAs; iDPP4 will have to be suspended if a GLP-1 RA is administered.
The dose adjustments according to renal function of the different iDPP4 are shown in Table 12.
- •
Secretagogues
Dose of DPP4-i according to degree of renal function. Daily Doses are Shown
| DPP4-i dose according to the range of estimated glomerular filtration rate (mL /min/1.73m2) | |||||
|---|---|---|---|---|---|
| i-DPP4 (daily dose) | > 60 | 45-59 | 30-44 | 15-29 | < 15 |
| Sitagliptin (adjusted dose) | 100mg/d | 50mg/d | 50mg/d | 25mg/d | 25mg/d |
| Alogliptin (adjusted dose) | 25mg/day | 12.5mg/day | 12.5mg/day | 6.25mg/d | 6.25mg/d |
| Vildagligtin (adjusted dose) | 100mg/d | 50mg/d | 50mg/d | 50mg/d | 50mg/day |
| Saxagliptin (adjusted dose) | 5mg/d | 5mg/d | 2.5mg/d | 2.5mg/d | No |
| Linagliptin | 5mg/d | 5mg/d | 5mg/d | 5mg/d | 5mg/d |
Sulfonylureas (SU) are not the drug of first choice in renal failure. Glibenclamide and glimepiride are metabolized in the liver to less active metabolites, but they are excreted in the urine, so their use, even at low doses, is no longer advisable in patients with CKD. Glipizide is metabolized to inactive metabolites, therefore, it would be the only SU that can be administered in CKD, but its use is not allowed with lower GFR (<30mL/min/1.73m 2). Repaglinide is metabolized in the liver with less than 10% renal elimination, yet it should be started with a low dose, 0.5mg.
- •
Glucosidase inhibitorsα
Both acarbose and miglitol, as well as their metabolites, are not excreted in patients with renal failure therefore there is body accumulation of these compounds. Furthermore there are limited studies in patients with CKD. They have low hypoglycemic potency and significant gastrointestinal side effects, so their use is not recommended.105
- •
Glitazones
Glitazones are metabolized by the liver, and less than 2% is excreted in the urine. Consequently, there is no accumulation of active metabolites in renal failure. However, its use is limited because increases the risk of edema, heart failure, and osteoporosis. It is contraindicated in patients on dialysis.
- •
Insulin
Insulin requirements are highly variable, so it is essentialthe individualization of treatment. As initial guidelines, that must be adapted to each patient through glucose monitoring, we can point out:
- o
GFR>50mL/min/1.73m 2 : no dose adjustment is required.
- o
GFR 50-10mL/min/1.73m 2 : will require a 25% reduction in the previous insulin dose.
- o
GFR<10mL/min/1.73m 2 : will require a 50% reduction in the previous dose of insulin.
The insulin regimen will be adapted to the objective of glucose control and can be conventional therapy or intensive treatment, although it should be remembered that the basal-bolus regimen is the one with the lowest rate of hypoglycemia.
An interesting phenomenon is the so-called " diabetes burn-out ", well described among some patients (approximately 15-30%) with advanced CKD (GFR<20mL/min/1.73m 2) and DM-2. These patients that had been previously treated with insulin or other anti-hyperglycemic agents, as CKD progresses to end-stage CKD they need less or no medication for glycemic control.106 Several factors have been proposed to explain this changes: a prolonged half-life of endogenous and exogenous insulin, decreased insulin resistance resulting from the removal of uremic toxins by dialysis, decreased gluconeogenesis, and poor nutritional status.107
Detection and management of dyslipidemia in CKDDyslipidemia increases cardiovascular risk therefore control of lipids is an objective in patients with CKD. Dyslipidemia is considered to be of high (GFR 30-59mL/min/1.73m 2) or very high cardiovascular risk (GFR<30mL/min/ 1.73m2 ). The suggested LDL-c targets are a ≥ 50% reduction as compared to baseline and, values of 70mg/dL in CKD G3 and 55mg/dL in CKD G4.108
There is evidence of the benefit of treating dyslipidemia in CKD in stages prior to dialysis (G5D),109 but the benefits in dialysis patients are not so clear.
Treatment will be based on dietary measures and the administration of statins alone or associated with ezetimibe.110 Ezetimibe does not require dose adjustment for renal insufficiency. The statins of choice would be those with hepatic removal (fluvastatin, atorvastatin and pitavastatin). In the case of kidney transplant patients, certain interactions must be taken into account,111 particularly between cyclosporine and atorvastatin, lovastatin, and simvastatin, since it can increase their levels and therefore the risk of myopathy. Fluvastatin, pravastatin, pitavastatin, and rosuvastatin are less likely to present interactions. Although tacrolimus is also metabolized by CYP3A4, it seems to have less risk of interacting with statins. In these patients, statins should be started at low doses, titrated cautiously, and monitorinteractions.112
Being the control of cholesterol the primary objective, the KDIGO113 guidelines recommend the use of fibrates with triglyceride concentrations > 1,000mg/dL to avoid pancreatitis. Changes in lifestyle are recommended if values > 500mg/dL. In the case of association with statins, fenofibrate is preferred to gemfibrozil due to the lower risk of myopathy and rhabdomyolysis and the dose should be adjusted to the renal function (67-100mg/24h if the GFR is between 30-60mL/min/1.73m 2). However, it is expected to observe a decrease in GFR of still uncertain significance, probably related to the inhibition of tubular secretion of creatinine, increased production of creatinine by fibrates or an increase in vasodilator prostaglandins, resulting in a false decrease in GFR.114
There is no evidence in the general population on the use of Omega 3 fatty acids for the treatment of dyslipidemia115 and no studies in CKD that support their use.
The inhibitors of Proprotein convertase subtilisin/kexin type 9 (PCSK9) may be an alternative in patients with CKD who have not responded to other treatments and have had previous cardiovascular events. Evolocumab and alirocumab have shown their ability to reduce the levels of LDL cholesterol with a reduction of major cardiovascular events in secondary prevention in patients with high or very high cardiovascular risk in the FOURIER and ODYSSEY-OUTCOMES studies.116,117 It has been published one study on efficacy and safety in patients with CKD included in the FOURIER study. The results of such study show that these patients present a reduction in major cardiovascular events similar to those with normal renal function, and do not present a higher rate of adverse events or changes in renal function, except for patients who develop rhabdomyolysis.118 None of these studies has been specifically designed for patients with CKD. In addition, patients with GFR below 20mL/min/1.73m 2 and transplant patients were excluded. However, the Spanish Society of Atherosclerosis recommends treatment with PCSK9i in primary prevention in patients with CKD grade 3b or higher if they do not achieve LDL cholesterol <130mg/dL with statins.119 The indication of these drugs in our country is subject to the therapeutic positioning report.120,121
Other strategies in nephroprotectionUntil now, RAAS blockade had been the only evidence in the treatment and prevention of CKD, both in diabetics and in non-diabetic patients.7,68,89,122,123 In DM-2, treatment with SGLT2-i has, to date, the greatest evidence of nephroprotection36,37,100,101,124 and there is also evidence of a reduction in albuminuia with GLP-1 RAs.125–127
Recently finerenone, a more specific and selective mineral corticosteroid receptor antagonist without hormonal effects and with less tendency to hyperkalaemia than spironolactone and eplerenone, has shown nephroprotective benefit when compared with placebo in DM-2 patients treated with iRAAs. Finerenone is not marketed at the moment.128
The DAPA-CKD study has shown the benefit of an SGLT2-i (dapagliflozin) not only in diabetics but also in non-diabetic patients, including IgA nephropathy.124,129
Based on this study, dapagliflozin has recently been indicated not only for diabetic kidney disease but also for non-diabetic kidney disease patients, being able to be maintained until eGFR of 25mL/min/1.73m2. Likewise, the data sheet for empagliflozin has recently been modified, and it can be started with eGFR>45mL/min/1.73m 2, and it can be used in diabetics and non-diabetics with heart failure and with eGFR > 20mL/min/1, 73m2. Therefore the SGLT2-i can be used even in reduced GFR, each one with its specific characteristics indicated in their technical data sheets. The development of new studies will probably lead to a broadening of the spectrum of indications for these drugs and even in greater ranges of eGFR.
Management of obesity in CKDObesity is a factor of renal progression and should be considered as a target to be treated to reduce cardiovascular risk and CKD progression.130 It is a paradox to observe an inverse relationship between obesity and mortality in dialysis patients and it seems that a high BMI protects in the short term, but this benefit is not maintained over time.131
There is no solid evidence on treatments for obesity in CKD. Liraglutide, a GLP1 RA, is currently available and, according to the data sheet, is indicated for the treatment of obesity in combination with a low-calorie diet and increased physical activity. Its mechanism of action includes delayed gastric emptying and a central action decreasing appetite, so it increases the feeling of satiety. The GLP-1 RAs marketed in our country that have shown renal benefits (liraglutide, semaglutide, and dulaglutide) can be administered with GFR of 15mL/min/1.73m2.132 Pharmacokinetics studies indicate that in CKD G5 it does not increase the area under the curve, not even in dialysis patients. But there are no studies to support its use in CKD G5.
Detection and management of hyperuricemia in CKDHyperuricemia may produce a variable clinical spectrum: acute gouty arthritis due to the precipitation of monosodium urate crystals in the joints, tophaceous gout due to the precipitation of crystals in the skin and subcutaneous cellular tissue, uric nephrolithiasis, Acute uric acid nephropathy and chronic uric acid nephropathy.
Currently there is not sufficient evidence for the treatment of asymptomatic hyperuricemia. Decisions to initiate treatment will be based on each patient's risk of developing crystal deposits, gout, or uric acid nephropathy, as well as treatment-related side effects. The decision regarding the establishment of lifestyle-based or pharmacological therapies for asymptomatic hyperuricemia should be individualized based on estimates of the risk of hyperuricemia-related clinical events and the potential benefits and risks of the intervention.
Uric acid-lowering drugs include: a) xanthine oxidase (XO) inhibitors that block purine metabolism, b) uricosurics that act on the main cause of hyperuricemia, which is low renal excretion, and 3) treatment with uricase which oxidizes urate, through an enzymatic reaction, to allantoin. It is recommended to start treatment with urate-lowering drugs after the first attack of gout in patients with CKD≥G2. The goal is to keep uric acid levels below 6mg/dL (5mg/dL in tophaceous gout).
Table 13 includes a list of the different urate-lowering drugs marketed in Spain, their mechanism of action and dose for the different CKD stages.
Hypouricemic drugs marketed in Spain. Recommended doses in patients with CKD.
| Drugs (hypouricemic) | Class | Dose | Recommendations in patients with CKD (G3-5) | Recommendations G5-Dialysis |
|---|---|---|---|---|
| Allopurinol | XO inhibitor | Start 50-100mg/d | GFR≥30mL/min start 50-100mg/d | HD: start 100mgpost-dialysis |
| Maximum: 800mg/d | GFR<30mL/min/1.73m2 | PD: start 50mg/d | ||
| Start 50mg/d a | ||||
| Febuxostat b | XO inhibitor | Start: 40mg/d | Insufficient data in patients with GFR<30mL/min c | insufficient data |
| Maximum: 120mg/d | ||||
| Benzbromarone | Uricosuric | Start 25-50mg/d | Contraindicated if GFR<20mL/min | contraindicated |
| Maximum: 200mg/d | ||||
| Lesinurad | Uricosuric | Start 200mg/d together with iXO | Contraindicated if GFR<45mL/min | contraindicated |
| Maximum:200mg/d |
HD: hemodialysis, PD: peritoneal dialysis.
Regardless of each of the uric acid-lowering drug, there are common rules in the use of all of them: a) always start treatment with prophylaxis, b) start with the lowest dose and monitor the levels until the target is reached, c) the drug should not be withdrawn or its dose modified during a gout attack, and 4) in the case of a new treatments, the drug should be introduced after the resolution of the acute gout attack. The guidelines recommend prophylactic treatment for three months or six months in cases of tophaceous gout when hypouricemic agents are started. Colchicine is contraindicated in patients with a GFR less than 30mL/min/1.73m 2, and in patients with CKD stage 3 the patients should receive a 0.5mg tablet daily.
Table 14 shows the drugs that can be used during an acute attack of gout and their dose adjustment in patients with CKD.133
Drugs indicated for the treatment of acute gout attack and dose adjustment in CKD.
| Normal kidney function | CKD (G3-G5) | Dialysis | |
|---|---|---|---|
| Colchicine | 1-2mg/d. Maximum dose of 2mg/d. Do not exceed 6mg in 4 days. Washout period of three days, before repeating treatment | Stage G3 reduce dose by half and increase intervals | Contraindicated |
| GFR<30 contraindicated | |||
| NSAIDs | Any type at its usual dose | In stage G3 use with caution and decreasing the dose | Any type at its usual dose |
| In Stage G4 it is contraindicated | |||
| Corticosteroids (use in patients with contraindication to colchicine and/or NSAIDs) | 0.5mg/kg/d, tapering 5mg every 2 days | Same as in normal kidney function | Same as in normal kidney function |
| ACTH analog (tetracosactide depot) | 25-40mg (im or sc) in patients who do not respond to other treatments. | Does not require dose adjustment | Does not require dose adjustment |
| IL-1 inhibitors (refractory gout attack) | Anakinra dose 100mg sc/d * | Stage G4: Anakinra 100mg/48 h | Anakinra 100mg/48 h |
| Canakinumab 150mg sc, one single dose | Canakinumab: no adjustment needed | Canakinumab: no need for dose adjustment |
NSAIDs: nonsteroidal anti-inflammatory drugs; ACTH: corticotropin; IL-1: interleukin 1; im: intramuscular; sc: subcutaneous.
Anemia associated with CKD is generally normocytic and normochromic and is not usually associated with iron deficiency (ferritin > 100ng/mL and IST>20%). Otherwise, it is advisable to make a differential diagnosis with other causes of anemia. Nevertheless, anemia of renal cause is a diagnosis of exclusion.
In the presence of anemia in a patient with CKD, other causes of anemia should to rule out, a study should be performed including: complete blood count, percentage and count of reticulocytes, ISAT, ferritin, B12 and folic acid.63
The indications for the referral of a patient with CKD and anemia will be63 :
- o
Indication of intravenous iron therapy (failure/intolerance of oral iron therapy).
- o
Indication for treatment with ESA-EPO.
- o
In the patient treated with ESA-EPO and confirmed Hb ≥ 13g/dL or Hb ≤ 9g/dL (for dose adjustment).
These aspects are specified in more detail in the consensus document for the detection and management of renal anemia by the SEN and the three societies family physicians.63
Detection and management of bone and mineral metabolism disorders in CKDAlterations in calcium and phosphorus metabolism in CKD are associated with various complications that go beyond simple bone involvement (previously called renal osteodystrophy) involving other systems, especially the cardiovascular system (for example, vascular and valvular calcifications, arterial stiffness, left ventricular hypertrophy, etc.), clearly associated with an increase in cardiovascular and global morbidity and mortality.
The best known clinical manifestation is the increase in parathyroid hormone (PTH) [(secondary hyperparathyroidism (SHPT)] produced, among other factors, by a multifactorial tendency to hypocalcaemia, a deficiency of active vitamin D (calcitriol) or the retention of phosphate (with or without hyperphosphatemia). In fact, it is common to find normal plasma levels of calcium and phosphorus at the expense of a significant elevation of PTH, so they should always be assessed as a whole.134,135 The appearance of hypercalcemia and / or hypophosphatemia with elevated PTH, in these cases primary hyperparathyroidism (parathyroid hyperplasia or adenoma) should be considered, even in patients with CKD The management of hyperparathyroidism secondary to CKD is carried out by Nephrology.
The goals of screening and treatment are:
- o
Avoid hyperphosphatemia
- o
Avoid vitamin D deficiency (calcidiol or 25-OH-vitamin D)
- o
Avoid persistent and progressive increase in SHPT
Hyperphosphatemia should be initially treated with an adequate and balanced diet (with special care to reduce the intake of inorganic phosphorus contained, for example, in the additives of prepared foods) and/or with the use of phosphate binders.134,135 Serum vitamin D concentration should be corrected to at least 20-30ng/mL (50-75nmol/L) by supplementation that will contribute to the pleiotropic hormonal actions of vitamin D and control mild cases of HPS.
Especially in stages G4 and G5, it is even recommended to maintain PTH at values slightly above normal, so that intact PTH levels classically considered adequate in a patient with CKD G5 are between 150-300 pg/ml (two to five times the upper limit of normal for the assay used). A small degree of stable SHPT is not worrisome and represents an adaptive phenomenon (ie PTH is a phosphaturic hormone), but progressive SHPT, with PTH values two or three times higher than the reference value despite adequate levels of calcidiol, they could require at least an initial consultation with a Nephrology specialist and/or the initiation of active forms or analogs of vitamin D (calcitriol, paricalcitol, alfacalcidol). Persistently high phosphate levels would also be worthy of dietary advice and/or nephrology consultation.
Treatment is based on:
- o
Dietary advice to avoid hyperphosphatemia.
- o
Phosphate binders or binders: calcium carbonate, calcium acetate or its association with magnesium, sevelamer (hydrochloride or carbonate), lanthanum carbonate or sucroferric oxyhydroxide.
- o
Native” vitamin D (cholecalciferol or calcifediol).
- o
In case of persistence and especially progression of HPS (trends are assessed), calcitriol or vitamin D analogues.136 Calcimimetics can be used in dialysis patients.
Osteoporosis has recently gained relevance as an added problem in patients with CKD. It can be senile, postmenopausal or associated with steroid treatment, among other causes. In addition, it is now known that the frequency of fractures and their morbidity and mortality are higher in patients with CKD137 and that densitometry (DEXA) has a predictive value for fractures in these patients as well. Densitometry would be indicated if the results impact therapeutic decision-making. There is no clear evidence on the treatment of osteoporosis in CKD and there are only retrospective experiences that these drugs are effective in patients with CKD.138,139 However, current guidelines advise adopting an active attitude in this regard.134,140
Detection and management of hyperkalaemia in CKDHyperkalemia is defined as a serum potassium concentration above the upper limit of normal (5 mEq/L). It is considered mild hyperkalaemia if potassium concentrations are between 5 and 5.5 mEq/L, moderate between 5.5 and 6 mEq/L, and severe if it is greater than 6 mEq/L. Hyperkalaemia is generally associated with a decrease in GFR (reduced ability to excrete potassium), the use of iRAAs and/or MRA, or abnormal redistribution of potassium (intra-extracellular). An important exception is hyporeninemic hypoaldosteronism (type IV renal tubular acidosis) in whom hyperkalaemia may occur with moderate reduction in GFR. These are characterized by hypoaldosteronism and hyperkalemia and it may be present in various types of kidney diseases such as diabetic nephropathy, interstitial nephropathy and obstructive uropathy, as well as with the use of NSAIDs, among other causes.
Cation exchange resins remove potassium by an exchange for another cation in the GI tract. Its main drawback is its limited effectiveness and the delay in the onset of its action that could be of several hours, in addition it produces GI intolerance. Recently, two new potassium chelating agents have been developed (patiromer and sodium zirconium cyclosilicate), which offer better tolerance and efficacy. The chronic use of these new drugs may allow the maintenance of the cardio-renal benefit of iRAAS with much better tolerance than the classic calcium polystyrene sulfonate.
The measures for the treatment of asymptomatic moderate-mild hyperkalemia are shown in Table 15. More details on the treatment of chronic hyperkalemia can be found in the following publication.141
Treatment of mild-moderate chronic hyperkalaemia.
| Treatment of mild-moderate chronic hyperkalaemia | |
|---|---|
| Dietary interventions to avoid exogenous increase in potassium levels | Avoid foods high in potassium (> 250mg per 100g) |
| Low potassium diet (≤ 3g/d) | |
| Avoid potassium salts | |
| Avoid potassium supplements | |
| Prevent cellular release of potassium | Correct acidosis |
| Control blood glucose | |
| Adjust beta-blockers | |
| Correct digoxin levels | |
| Rule out urological pathology | Urinary tract obstruction |
| Urinary infection | |
| Assess and adjust the dose of drugs that can cause hyperkalaemia | Mineralocorticoid receptor antagonists |
| Amiloride/triamterene | |
| Nonsteroidal anti-inflammatory drugs | |
| Calcineurin inhibitors: tacrolimus, cyclosporine | |
| RAAS inhibitors | |
| Co-trimoxazole/pentamidine | |
| Heparin | |
| Use drugs that increase renal excretion of potassium | Loop diuretics: furosemide, torasemide |
| Use drugs that decrease intestinal absorption of potassium | Ion exchange resins |
| Patiromer | |
| Sodium Zirconium Cyclosilicate | |
RAAS: renin-angiotensin-aldosterone system.
Metabolic acidosis in CKD is usually a chronic normochloremic acidosis or with an increased anion gap that frequently appears if the GFR<20mL/min/1.73m 2 due to an increased acid load due to the renal inability to eliminate them. It is important to treat it to avoid long-term bone demineralization, as well as the progression of CKD itself, among other deleterious effects.142
Oral sodium bicarbonate can be used if blood bicarbonate is less than 22mmol/L.7 There are also other measures such as diets rich in plant-based proteins that produce bases such as fruits and vegetables143 and other recently marketed drugs such as veverimer, a non-absorbable polymer that binds selectively to hydrochloric acid, eliminating it from the intestinal lumen through through feces.144 Obviously, sodium overload induced by classic bicarbonate should be avoided, as well as overcorrection and metabolic alkalosis.
Management of HCV hepatitis in CKDThe HCV infection is associated with a 23% increased risk of CKD development and progression, the hidden mechanisms of this association are currently under investigation.145
The different guidelines and scientific societies, including the Strategic Plan for the Management of Hepatitis C 2015 recommend antiviral therapy for all patients with chronic hepatitis due to HCV with CKD with/without RRT, including candidates to kidney transplant. The only exception is patients with specific contraindications or with limited life expectancy due to other intercurrent diseases. There are no recommendations for the management of acute hepatitis C.146,147
Treatments based on interferon and ribavirin have been poorly tolerated as they are eliminated by the kidney and have significant side effects, with a low rate of sustained viral response.
Direct-acting antivirals achieve a sustained viral response greater than 90-95%, presenting an excellent safety and tolerance profile.148–150 Adverse effects are infrequent, but and interactions with commonly used drugs are very common, to be taken into account especially in renal transplant recipients.151
The current regimens with greater efficacy and safety for patients with advanced CKD or on dialysis, free of interferon, include: elbasvir/grazoprevir (genotypes 1 and 4), ombitsavir/dasabuvir/paritaprevir/ritonavir (genotype 1), ledipasvir/sofosbuvir (genotypes 1, 4, 5, and 6), glecaprevir/pibrentasvir, and sofosbuvir-velpatasvir (all genotypes).152–154 In some cases it is necessary the administration of ribavirin.
Achieving sustained viral response or undetectable viral RNA level after 12-week treatment is considered cure in 99% of patients with long-term follow-up. However, a second viral RNA determination is required during the first year after completion of therapy.146
Management of direct-acting anticoagulants (DOACs) in CKDThe prevalence of atrial fibrillation (AF) is significantly increased in subjects with CKD and HD, and cardioembolic strokes is one of the most frequent complications.155 Patients with AF and CKD are at increased risk of thrombotic complications due to the frequent coexistence of other factors such as advanced age, DM, vascular comorbidities, or RRT itself. However, hemorrhagic events are also very frequent, as a consequence of the alteration in the generation, activation and function of platelets in kidney patients.156
There are different DOACs approved for the prevention of stroke in patients with AF. They act through the inhibition of factor Xa (rivaroxaban, apixaban, edoxaban), or thrombin (dabigatran).
An important aspect to consider is that the recommendations of the pivotal studies with DOACs have been made based on the use of the C&G formula and not CKD-EPI. Although scientific societies recommend the latter equation, for the time being the prescription should be based on C&G, since this is what the dosage was based on in the pivotal studies. The scientific societies must pronounce themselves in this regard.
In pivotal clinical trials, DOACs achieved non-inferiority criteria as compared to warfarin in patients with creatinine clearance (CrCl) (calculated using the C&G equation) of 30-50mL/min (20-50mL/min in the case of apixaban). However, there is not enough evidence to recommend one DOAC over the others, since no trials have been carried out to compare some drugs with others.
Although the efficacy in the prevention of stroke and systemic embolism may be non-inferior to warfarin, the overall safety profile of DOACs is superior to vitamin K antagonists (VKAs). In all pivotal trials, DOACs have been associated with significant reductions (about 50%) in the risk of intracranial hemorrhage compared with warfarin.
Anticoagulant treatment in patients with CKD according to CrCl:
- o
CrCl >50mL/min: The decision to anticoagulate these patients with non-valvular AF should be based on clinical trials, and in these cases DOACs are superior to VKAs that in Spain are dicoumarins.
- o
ClCr between 15-30mL/min: In these patients, anticoagulation will be assessed considering the risk/benefit of the treatment.
- o
CrCl<fifteen mL/min: There is insufficient evidence from clinical trials to support anticoagulation in these patients. Some trials have shown benefit from the use of DOACs in these patients. The use of anticoagulant treatment and the type of pharmacological treatment or closure of the left atrial appendage by endovascular procedure, should be decided on an individual basis. In this group of patients and in those on dialysis, the use of DOACs versus VKAs should be considered when there is calciphylaxis, risk of relevant vascular calcification, or a history of warfarin-induced nephropathy, and always making an appropriate dose adjustment.156,157 There are everal clinical trials underway testing DOACs in this population that will provide us with answers about the treatment needs in this very high-risk population.
Table 16 shows the current recommendations for the administration of DOACs in CKD.158
Characteristics of the different direct acting oral anticoagulants (DOACs) (dosage in CKD, metabolism and elimination by dialysis). Modified from references.156–158
- o
Control of cardiovascular risk factors and monitoring variables that favor CKD progression, especially BP, body weight and blood glucose.
- o
Monitoring nephrotoxicity to avoid iatrogenesis in any process, paying special attention to:
- -
Avoid the use of NSAIDs whenever possible.
- -
Avoid hyperkalaemia associated with the use of drugs.
- -
Avoid/adapt the use of oral antidiabetics according to GFR.
- -
Avoid as much as possible the use of iodinated contrast agents, adjusting any drug to the patient's GFR.
- -
In the event of a deterioration in renal function, always rule out functional causes (excessive control of blood pressure, NSAIDs, volume depletion) before referring to Nephrology.
- -
- o
Participation in therapeutic compliance and referral to Nephrology in case of unexplained acute worsening of renal function or the presence of complications.
- o
Follow-up of elderly patients, with stable GFR, who, for reasons of age, quality of life or others, are not eligible for RRT and do not receive erythropoiesis-stimulating agents (ESAs) and/or complex medication for SHPT of CKD.
- o
In the case of dialysis patients, learn about the different options of dialysis technique and their implementation (place to perform the dialysis, periodicity, structural needs and possible complications), especially the techniques that the patient performs at home. The family doctor plays especial role in the management of the dialysis patient who, for reasons of clinical deterioration and after a consensus decision, it is decided to withdraw dialysis treatment and provide his care al home.
- o
In the case of kidney transplant patients, in addition to knowing the types and their peculiarities, immunosuppressive treatment is of special interest due to the complications, side effects and interactions that may occur.
- o
When a patient opts for conservative management of their CKD, we will continue to actively treat the complications that may occur throughout its evolution (anemia, hyperkalaemia, metabolic acidosis) until reaching the terminal phase of uremia, working in a coordinated manner: Nephrologist, Family doctors, Home Care and palliative care if required.
- o
Ensure and participate actively in the vaccination of patients with CKD159 :
- -
General recommendations: tetanus (complete five doses, dose received, dose that counts), triple viral (two-dose regimen, if not previously vaccinated) and varicella-zoster virus if susceptible (two intramuscular doses separated by two to six months).
- -
Specific recommendation: Annual flu. Pneumococcus (VNP23 sequential pattern+PCV13) if the patient has not been vaccinated 1 °PCV13 and at 12 months VNP23 (accelerated regimen only wait two months), if vaccinated with PCV23 wait 12 months to administer PCV13. Hepatitis B (vaccine of 40μg HBsAg, regimen: 0, 1 and 6 months). Perform serological control at four to eight weeks to verify seroconversion. Booster dose if in annual follow-up anti-HBs are <10 mIU/mL and also vaccination against COVID-19.
- -
- o
Maintain communication between the family doctor and Nephrology using all means that technology offers us, all in a virtual manner:
- -
Via telephone: with pre-established schedules and direct pone calls.
- -
Via the Internet: specific consultations such as "telenephro", intranephro, etc. The family doctor would refer doubts, queries about specific patients and the nephrologist would respond in less than 24-48hours about the strategy to follow.
- -
Videoconferences, about specific consultations or online training.
- -
- o
Maintain continuing medical education in collaboration with Nephrology on the following issues:
- -
Control of cardiovascular risk factors.
- -
Follow-up and monitoring of patients with CKD. Preferably assess the GFR and evaluate the presence of albuminuria in urine, to classify the patient's risk.
- -
Recognize the different stages of kidney function loss and understand the criteria for referral to Nephrology.
- -
Prevention of nephrotoxicity and avoid iatrogenesis, especially in polymedicated elderly patients.
- -
To avoid delays in referrals to appropriate specialists, certain pathologies that are listed that should not be referred to Nephrology.
- •
Urological pathology:
- o
Renal colic/renal lithiasis.
- o
Simple renal cysts (do not refer), or complex cysts (Bosniak IIF, III and IV: refer to Urology).
- o
Urinary tract infections with normal renal function.
- o
Renal tumors/masses.
- o
- •
Non-urological pathology:
- o
Non-renal anemia.
- o
Primary hyperparathyroidism with normal renal function or secondary to vitamin D deficiency.
- o
There are factors that accelerate renal deterioration regardless of the specific rate of progression of CKD. Since many of these factors are reversible, it is essential to recognize and correct them.
General measures:
- 1)
Hydration: abundant fluid intake should not be recommended in all patients with CKD. It is important to limit salt intake. Adequate fluid intake must be ensured, especially in elderly patients and in the summer time, but this recommendation must be applied with caution and should be individualized. It is not applicable to patients with cardiorenal syndrome, at risk of hydrosaline retention and congestive heart failure.
- 2)
A low-potassium and protein-restricted diet should not be generalized to all patients with CKD. It should be individualized according to laboratory results, aetiology, symptoms and individual characteristics of each patient.
- 3)
Referral to Nephrology: a CKD patient should not be diagnosed with a single measurement of creatinine, GFR and/or albuminuria.
- 4)
The presence of kidney injury markers is essential to classify a CKD patient if their GFR is > 60mL/min/1.73m 2.
Pharmacological interventions:
Drugs should not be used without adjusting the dose to the GFR. In general, the most important recommendations are the following:
- 1)
NSAIDs: should be avoided at any stage of kidney disease, although occasional use may be necessary in certain situations.
- 2)
RAAS:
- •
Double RAAS blockade with ACE inhibitors associated with ARBs or direct renin inhibitors is not recommended in diabetic and/or CKD patients.
- •
RAAS should not be discontinued if there is a slight increase in creatinine with the corresponding decrease in GFR. After introducing the drug, the serum creatinine should be monitored 7 to 10 days thereafter; elevations of up to 20-30% are tolerable, given its antiproteinuric/cardio and nephroprotective benefits.
- •
Do not suspend these drugs if there is a slight-moderate increase in serum potassium. Hyperkalaemia should be treated with dietary and pharmacological measures, avoiding the suspension of the drug that offers cardio and nephroprotective benefits.
- •
Only suspend treatment with these drugs in patients with infectious or acute gastrointestinal processes that involve dehydration or hypotension, trying to reintroduce them slowly after recovery.
- •
Do not administer RAASi to patients with bilateral renal artery stenosis (which is rare), or with severe diffuse distal renal vascular lesions, as they may reduce GFR markedely (>30%).
- •
- 3)
Diuretics:
- •
The use of thiazide diuretics is not recommended in patients with moderate-advanced CKD. In these situations, loop diuretics (furosemide, torasemide) should be used preferably. Thiazide diuretics are generally ineffective with GFR<30mL/min/1.73m 2. They could be associated with loop diuretics, in patients with cardiorenal syndrome and episodes of heart failure, with hydrosaline retention, in whom a potentiation of the diuretic effect is observed.160
- •
The uncontrolled use of AMR (spironolactone, eplerenone) is not recommended in patients with moderate-advanced CKD due to the risk of hyperkalaemia. If indicated to treat heart failure with reduced ejection fraction or in patients with resistant hypertension, with normal values of potassium, it should be started with low doses and with frequent monitoring of serum potassium concentration. Special attention should be paid in periods of exacerbation.
- •
- 4)
Digoxin should not be administered without dose adjustment according to the GFR especially in patients with GFR less than 30mL/min/1.73m 2.
- 5)
Fibrates associated with statins should not be administered in patients with CKD stage G3b -G5 (GFR<45mL/min/1.73m 2).
- 6)
No antibiotic should be given without adjusting the dose for the stage of CKD.
- 7)
Do not administer antidiabetic drugs without taking into account the stage of CKD and modify te dose if when there are changes in FGFR.
- 8)
Radiological contrast agents: As far as possible, the administration of iodinated contrast agents should be avoided in patients with CKD G3b -G5 (GFR<Four. Five mL/min/1.73m 2). Risk-benefit should always be weighed. If the administration of contrast agents is essential, we should recommend adequate volume replacemet of the patient, suspension of metformin in diabetics, avoid diuretics whenever possible, correcting metabolic acidosis by administering sodium bicarbonate and also give of N-acetylcysteine (600mg the day before and the day of the radiological test). Avoid magnetic resonance imaging with gadolinium in patients with CKD with GFR<30mL/min/1.73m 2 because of the risk of developing nephrogenic systemic fibrosis.
- 9)
Do not use intestinal preparations rich in phosphorus to perform colonoscopy.
Dr. Rafael García Maset declares that he has received fees for lectures from Amgen, Boehringer-Ingelheim, Esteve and Fresenius Medical Care, not related with present work.
Dr. Jordi Bover Sanjuán declares that he has received honoraria for lectures and consultancies from Abbvie, Amgen, Rubió, Sanofi and Vifor Pharma, not related with present work.
Dr. Julián Segura de la Morena declares that he has received fees for conferences from AstraZeneca, Esteve and Medtronic, not related with present work.
Dr. Marian Goicoechea Diezhandino declares that she has received conference and consultancy fees from Astellas, AstraZeneca, Boehringer-Ingelheim, Novonordisk, Sanofi and Vifor Pharma, not related with present work.
Dr. Javier Escalada San Martin declares that he has received conference fees from AstraZeneca, Boehringer-Ingelheim, Lilly, Mundipharma and Novonordisk, not related with present work.
Dr. Lorenzo Fácila Rubio declares that he has received fees for lectures from AstraZeneca, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo and Pfizer, not related with present work.
Dr. Lisardo García-Matarín declares that he has received fees for conferences and consultancies from AstraZeneca, Bial, Boehringer-Ingelheim, GlaxoSmithKline, Menarini, Novartis, Novonordisk, Pfizer, Sanofi, Teva and Vifor Pharma, not related with present work.
Dr. María Isabel Gutiérrez Pérez declares that she has received conference fees from GlaxoSmithKline, not related with present work.
Dr. Pilar Mazón Ramos declares that she has received fees for conferences and consultancies from AstraZeneca, Boehringer-Ingelheim, MSD and Novonordisk, not related with present work.
Dr. Manuel Muñoz Torres declares that he has received conference fees from Amgen, Lilly and Novonordisk, not related with present work.
Dr. Manuel Pérez-Maraver declares that he has received fees for conferences and consultancies from Abbot, AstraZeneca, Lilly, Novonordisk and Sanofi, not related with present work.
Dr. José Luis Górriz Teruel declares that he has received fees for conferences and consultancies from AstraZeneca, Boehringer-Ingelheim, Lilly, Mundipharma, Novartis, Novonordisk and Vifor Pharma, not related with present work.
Conflict ofinterestsDrs. Jesús Cebollada del Hoyo, Javier Gamarra Ortiz, Jose A. García-Donaire, Sílvia Gràcia Garcia, Julio Hernández Moreno, Rosario Montañés Bermudez, Pedro de Pablos-Velasco, Carmen Suárez Fernández and Salvador Tranche Iparraguirre declare that they have no conflict of interests.
Please cite this article as: García-Maset R, Bover J, Segura de la Morena J, Goicoechea Diezhandino M, Cebollada del Hoyo J, Escalada San Martin J, et al. Documento de información y consenso para la detección y manejo de la enfermedad renal crónica. Nefrologia. 2022;42:233–264.
The complete affiliations of the authors are detailed at the end of the article.