The recent publication on the review of the KDIGO guidelines1 in their recommendations for the management of chronic kidney disease (CKD) in patients with diabetes mellitus (DM) has been as always welcome and very clarifying on the peculiarities in the management and treatment of these patients. Its key recommendations include the use of 3 pharmacological groups and the determination to contraindicate the use of tobacco. The last few years in the management of DM have been key for the increase in the number of effective and safe therapeutic groups with a clear cardioprotective and nephroprotective profile beyond their efficacy as antihypertensives, antihyperglycemic agents or diuretics.
These new drugs whose randomized clinical trials (RCT) never cease to surprise us because they are generalizing the inclusion of more and more patients similar to those in real life (RL), participants who on many occasions are at very serious moments in the evolution of their disease (CKD + DM). Using outcome criteria that include total, renal and overall cardiovascular mortality. In that aspect we are seeing that there are patients included with advanced CKD, a high range of proteinuria and heart failure in NYHA grades IV. Patients who complete the studies in a sufficiently representative number to make the appropriate clinical considerations.
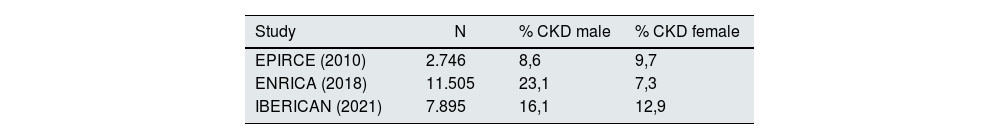
In our Primary Care setting, according to the results of the most extensive epidemiological studies published in the last 10 years and included and consensus document for the detection and management of CKD published in this journal,2 the prevalence of CKD is around 15% on average, increasing with age as supported in all the studies them, and has an undefined behavior with respect to sex. The studies EPIRCE, ENRICA and the more recent the IBERICAN study, all representative of the Spanish population, do not reach the same results (Table 1).3–5
Nor do we have a clear idea of the prevalence of diabetic nephropathy, the most prevalent cause of CKD, the percentage of which is unknown at the present time, and above all, its possible differentiation by age or sex.
That is why we wanted to show what is the true value of gender as an independent risk marker in the RCT that substantiate the most important conclusions of the KDIGO 2022 guidelines. It is clear that if the studies do not show proven evidence on differences in prevalence, predisposing factors, initiating factors, factors causing progression or evolution to replacement therapy, gender should be considered an independent factor to be controlled and treated or managed separately. We should at least consider that those RCTs with drugs that substantiate our daily practice with CKD patients with DM should be a reflection of our real population. Or close enough to avoid making spurious decisions.
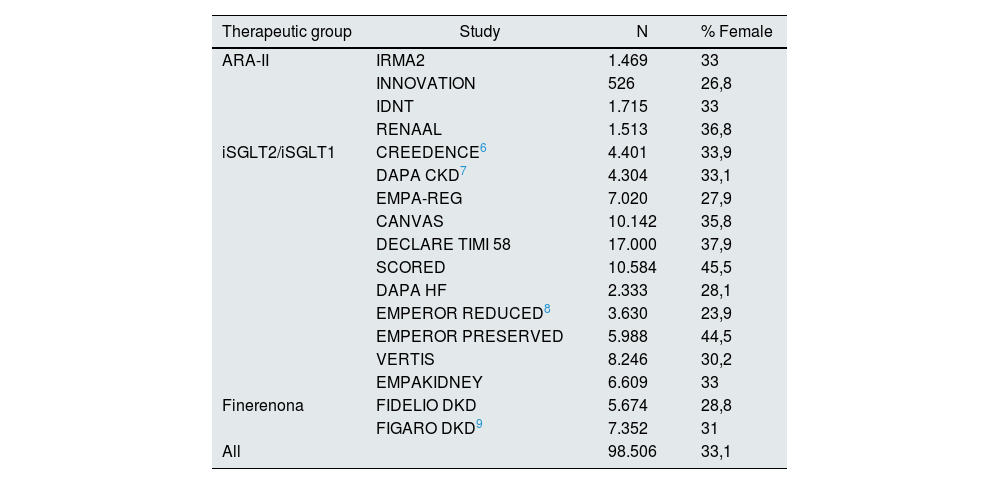
The KDIGO guidelines in the March 2022 update for DM and CKD make a series of very powerful recommendations on the use of 3 types of drugs in patients with CKD and DM to avoid greater renal damage, cardio- and renoprotection, and possible regression of filtration loss or proteinuria. These studies are shown in Table 2 (17 in total): 4 on ARA-II, 11 on iSGLT2/iSGLT1, 2 on finerenone.6,7–9
RCTs analyzed in the KDIGO guidelines. Number of participants. Percentage of female participants.
| Therapeutic group | Study | N | % Female |
|---|---|---|---|
| ARA-II | IRMA2 | 1.469 | 33 |
| INNOVATION | 526 | 26,8 | |
| IDNT | 1.715 | 33 | |
| RENAAL | 1.513 | 36,8 | |
| iSGLT2/iSGLT1 | CREEDENCE6 | 4.401 | 33,9 |
| DAPA CKD7 | 4.304 | 33,1 | |
| EMPA-REG | 7.020 | 27,9 | |
| CANVAS | 10.142 | 35,8 | |
| DECLARE TIMI 58 | 17.000 | 37,9 | |
| SCORED | 10.584 | 45,5 | |
| DAPA HF | 2.333 | 28,1 | |
| EMPEROR REDUCED8 | 3.630 | 23,9 | |
| EMPEROR PRESERVED | 5.988 | 44,5 | |
| VERTIS | 8.246 | 30,2 | |
| EMPAKIDNEY | 6.609 | 33 | |
| Finerenona | FIDELIO DKD | 5.674 | 28,8 |
| FIGARO DKD9 | 7.352 | 31 | |
| All | 98.506 | 33,1 |
A total of 98,506 patients were included of which only 33.1% were women ranging from 45.5% (SCORED) to 26% (INNOVATION). The mean for ARA-II was 32%, 34% for iSGLT2/iSGLT1 and 29% for finerenone.
It is therefore worth asking the recruiters, protocol developers, evaluators from various agencies and ethics committees, regulators from the Food and Drug Administration and the European Medicines Agency, guideline developers (KDIGO), why this overrepresentation of men? Is it an assumed bias? Why do most of the studies reach 33%? Are women more difficult to recruit, are they less adherent to protocols?
These are questions that are not easily answered and that I suggest to future researchers. Perhaps the correction will come when we see these drugs in RV. Perhaps the actual prevalence by recruitment site should be a criterion of good clinical practice in drug research.