COVID-19 most related glomerular disease to date seems to be collapsing glomerulopathy, mostly in young Afroamerican patients with APOL1 gene risk alleles. However, in our population, predominant in elderly Caucasian patients, most biopsied pathology since the beginning of the pandemic has been IgA nephritis or Schönlein-Henoch purpura.
Since the description of the first case of this entity after SARS-CoV-2 infection by our research group, three more cases have arisen, which are described in the following article. In contrast to the rest of IgA vasculitis cases reported, our patients presented more renal function deterioration and all of them required immunosupresive therapy. Moreover, some showed incomplete recovery of renal function.
This case series strengthens the hypothesis that SARS-CoV-2 infection may be another trigger of this pathology.
La patología glomerular más relacionada con enfermedad COVID-19 hasta la fecha parece ser la glomerulopatía colapsante, principalmente en pacientes de raza afroamericana y con alelos de riesgo para el gen APOL1. No obstante, en nuestra población, conformada por pacientes añosos de raza caucásica, la patología más biopsiada desde el inicio de la pandemia ha sido la nefritis IgA o púrpura de Schönlein-Henoch.
Desde la descripción del primer caso de esta entidad tras infección por SARS-CoV-2 por nuestro grupo de investigación hemos objetivado otros tres, los cuales se describen a continuación. En contraste con el resto de los casos publicados de vasculitis IgA, nuestros pacientes presentaban mayor deterioro de función renal y todos requirieron tratamiento inmunosupresor. Además, algunos presentaron recuperación incompleta de función renal. Esta serie de casos afianza la posibilidad de que la infección por SARS-CoV-2 sea un desencadenante más de esta patología.
Since the World Health Organization (WHO) declared a pandemic due to coronavirus disease-19 (COVID-19) in March 2020, there have been described associated glomerular pathology and the most frequently associated is thecollapsing glomerulonephritis1,2 and most cases in the African-American population.
Our group has recently published the first case report on IgA vasculitis with nephritis associated with COVID-19.3 Since then, reports on other cases have been published,4–11 most in a population under 30 years of age, with only four of them presenting renal involvement.
After the index case,3 we have observed three other cases of IgA vasculitis after SARS-CoV-2 infection. Moreover, this has been the pathology most frequently biopsied in our environment since the start of the pandemic. All patients with this diagnosis had tested positive for SARS-CoV-2.
The clinical features of our patients differs widely from the rest of the published cases. They are elderly patients, with severe renal involvement and the need for immunosuppressive treatment.
Presentation of casesIndex case: Suso et al.3Case 1An 84-year-old male, a former smoker, with a history of arterial hypertension (AHT), dyslipidaemia, complete atrioventricular (AV) blockade with implanted pacemaker, tricuspid endocarditis with residual tricuspid stenosis and right heart failure with congestive liver disease, atrial flutter anticoagulated with Sintrom, mild chronic obstructive pulmonary disease (COPD), obstructive sleep apnoea syndrome (OSAS) treated with a nocturnal continuous positive airway pressure (CPAP) device, and axonal polyneuropathy. In 2019, he presented serum IgA levels of 405mg/dl (70−400mg/dl).
He was admitted to the hospital from March to June 2020 due to bilateral COVID-19 pneumonia, confirmed by nasopharyngeal polymerase chain reaction (PCR), with multiple complications during admission. He received treatment with hydroxychloroquine, lopinavir/ritonavir, azithromycin, corticosteroids, tocilizumab and therapeutic anticoagulation for right segmental pulmonary thromboembolism.
In the last 15 days of treatment, he developed acute renal failure with creatinine (Cr) of 2.39mg/dl, proteinuria 20mg/dl and microhaematuria together with purpuric skin lesions on the abdomen and limbs (previous Cr and urine sediments were normal). The immunological study (Ig, complement, antineutrophil cytoplasmic antibodies [ANCAs], antinuclear antibodies [ANAs], blood electrophoretic spectrum) showed only an increase in IgA levels of 556mg/dl (70−400mg/dl). Serological tests for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) were negative. A skin biopsy was performed, with no signs of vasculitis. The patient's renal deterioration was attributed to functional factors and he was discharged in early June 2020 with Cr 2mg/dl.
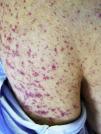
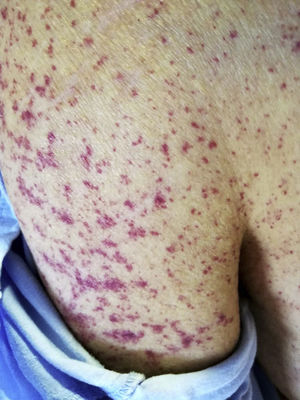
Three weeks later, he was referred to the emerging room because a Cr 5.28mg/dl with proteinuria 75mg/dl (2g/day) and macroscopic brown haematuria together with palpable purpuric lesions on the trunk and limbs, including palms and soles (Fig. 1). The patient reported several outbreaks of similar skin lesions since discharge. On examination, he was hypertensive (170/85mmHg) and presented features of volume overload in addition to the aforementioned purpuric lesions. Nasopharyngeal PCR for COVID was negative, with serology positive for immunoglobulin G (IgG) and negative for immunoglobulin M (IgM). The immunological study was very similar to the previous one. On ultrasound, the kidneys were normal in size with multiple bilateral cortical cysts.
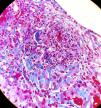
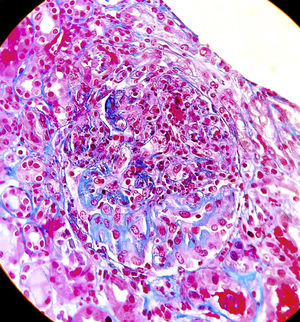
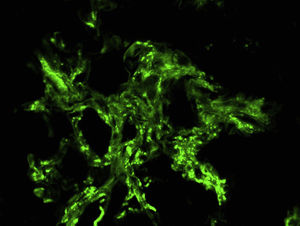
Suspecting systemic vasculitis with renal compromise, treatment was started with shock doses of 6-methylprednisolone (250mg/day, three days) followed by oral prednisone (0.8mg/kg/day) and a renal biopsy was performed. Sixteen glomeruli were identified, eight of them sclerosed. In two of them there was mesangial widening and in another two, retraction of the tuft (Fig. 2). Crescents were not observed. There were foci of interstitial fibrosis and tubular atrophy with foci of mononuclear infiltrate in the subcapsular interstitial zone showing some intraluminal red blood cells. The arteries and arterioles did not present alterations. With the immunofluorescence technique, granular mesangial deposits of IgA (Fig. 3), kappa and lambda were observed, negative for IgG, IgM, complement component 3 (C3) and complement component 1q (C1q), which were classified as vasculitis IgA (Henoch-Schönlein type) according to the International Study of Kidney Disease in Children (ISKDC) type II histological classification. The skin biopsy was repeated, which confirmed IgA deposits.
During the first month, the patient presented outbreaks of palpable purpura and reactivation of urinary sediment, coinciding with the decrease in steroids. He required new shock doses of steroids and, in order to reduce these (he had also received high doses for COVID-19), treatment was combined with mycophenolate mofetil (MMF), achieving clinical control.
At 10 months of follow-up, he has partially recovered renal function (Cr 2mg/dl), has become negative for proteinuria and maintains microhaematuria (3−5 red blood cells/field).
Case 2An 87-year-old man with a history of HTN with hypertensive cardiomyopathy, mild pulmonary hypertension, multiple admissions for community-acquired pneumonia (last one in April 2019), diverticulosis, benign prostatic hyperplasia, and moderate cognitive impairment of probable vascular aetiology exacerbated after subarachnoid haemorrhage due to head trauma in August 2019, for which he is maintained in a home care center.
He was referred to emerging room in December 2020 due to confluent purpuric lesions, predominantly in the lower limbs, distal region of the upper limbs, and buttocks. Biochemistry showed Cr of 2.29mg/dl, proteinuria of 150mg/dl on the dipstick and microhaematuria (urinary sediment and Cr during previous months were normal).
On physical examination, blood pressure was 100/70mmHg, and in addition to the purpuric lesions, the patient presented data of volume overload with hypoventilation in the right base and ipsilateral crackles. A chest X-ray showed a reticular pattern in the midfield and right base, already known, although more accentuated. The patient had a respiratory infection at the end of October 2020 that was treated with amoxicillin clavulanate, without testing for COVID-19. Upon admission, a nasopharyngeal PCR for COVID-19 was negative, while serology testing revealed positive IgG and negative IgM. The immunological study only showed an increase in IgA levels of 502mg/dl (70−400mg/dl). Syphilis, HBV, HCV and HIV serology tests were negative.
Given the progression of renal function deterioration (Cr 3.6mg/dl) on admission and the suspicion of systemic vasculitis, treatment was started with shock doses of 6-methylprednisolone (250mg/day, three days), followed by oral prednisone (0.8mg/kg/day). Due to the patient's age and comorbidity, renal biopsy was not considered. Skin biopsy revealed leukocytoclastic vasculitis with perivascular granular deposits of IgA in direct immunofluorescence, confirming the diagnosis of IgA vasculitis.
At five months of follow-up, renal function recovery is complete with Cr of 1.07mg/dl. Urine is not available due to incontinence.
Case 3A 64-year-old woman with a history of HTN, hypothyroidism on replacement therapy, and stage 3B A1-A2 chronic kidney disease with mild intermittent microhaematuria and slightly elevated IgA (486mg/dl), probably related to chronic glomerulonephritis (not biopsied), in follow-up by nephrology department since 2013. The patient have remained stable with Cr 1.37mg/dl at the last visit (October 2020), without proteinuria or microhaematuria.
In March 2020, she presented unilateral COVID-19 pneumonia that was treated with hydroxychloroquine and azithromycin on an outpatient basis.
From mid-December 2020, she had reported high blood pressure values of 200/100mmHg, requiring antihypertensive treatment with enalapril and manidipine. Analytical tests revealed impaired renal function with Cr 2.48mg/dl, proteinuria 100mg/dl with a protein/creatinine index of 3mg/mg and 20–40 red blood cells/field in the sediment. Ten days later, her condition had deteriorated, with Cr 3.41mg/dl, proteinuria 4.3g/day (without nephrotic syndrome) and microhaematuria. The patient denied having previous skin lesions, arthritis/arthralgia and gastrointestinal symptoms. With the suspicion of rapidly progressive glomerulonephritis, she was admitted for renal biopsy.
Upon admission, a COVID-19 PCR was negative with positive SARS-CoV-2 serology. The immunological study only showed increased IgA levels (434mg/dl [70−400mg/dl]). Syphilis, HBV, HCV and HIV serology tests were negative. The biopsy identified seven glomeruli, one of them sclerosed. Diffuse mesangial thickening in the mesangial matrix and cellularity, forming synechiae. Two glomeruli with epithelial crescents and one with fibrous crescent. Foci of interstitial fibrosis and tubular atrophy with interstitial mononuclear inflammatory infiltrates were observed. The arteries showed slight intimal fibrous thickening. IF revealed granular mesangial and glomerular deposits of IgA (+++), C3 (+), kappa (+) and lambda (+). The histopathological diagnosis was IgA nephropathy, Oxford classification M1 E1 S0 T0.
Given the clinical behaviour of a rapidly progressive glomerulonephritis that presented almost 50% crescents in the renal biopsy, immunosuppressive treatment was started with methylprednisolone shock doses (250mg/day for four days), followed by oral prednisone combined with cyclophosphamide (500mg fortnightly).
At four months of follow-up, in the active treatment phase, improvement in renal function has been observed, up to Cr 2.4mg/dl, proteinuria 1.33g/day and 10−20 red blood cells/field in the sediment.
DiscussionMultiple pathologies associated with COVID-19 disease have been described, including kidney disease. We currently know that kidney failure and sediment abnormalities lead to higher in-hospital mortality.12 Although the most frequent finding in renal biopsies performed has been acute tubular necrosis,13 glomerular pathology has been also described, mainly podocytopathies, in a middle-aged African-American population, with mild respiratory symptoms and APOL1 gene risk alleles.14 Another common cause of kidney involvement is thrombotic microangiopathy (TMA).15 Cases of systemic vasculitis have also been described, such as Kawasaki disease16 and p-ANCA vasculitis,17 some of which have renal involvement.
In October 2020, our group published the first case report on IgA vasculitis with nephritis after SARS-CoV-2 infection.3 In addition, in the first year of COVID, we identified the three cases now described. In fact, between March 2020 and March 2021, IgA vasculitis with nephritis (Henoch-Schönlein purpura) was the most biopsied pathology in our centre, representing 33% of the biopsies performed. In all these IgA nephropathies, both the clinical picture and the alterations in the biopsy motivated the start of immunosuppressive treatment.
Since the first case report, others have been published in the literature in the last year (Table 1).4–11
IgA vasculitis in the literature.
| Author | Patient | Comorbidities | COVID clinic | Latency | Vasculitis clinic | Kidney involvement | Kidney/skin biopsy | Treatment | Clinical course |
|---|---|---|---|---|---|---|---|---|---|
| Suso et al.3 Oct 2020 | Male, 78 years old | Moderate alcohol consumption, AHT, DL, aortic stenosis | Mild bilateral pneumonia | 3 weeks | Kidney involvement | ARF (Cr 1.96mg/dl) | 7 glomeruli: 1 sclerotic, 4 mesangial expansion, 1 synechiae. | MP 250mg x3 | 9 months: Cr 1.13mg/dl |
| Caucasian | Cutaneous purpura | Nephrotic proteinuria (10g/day) | 2 epithelial crescents. | Rituximab 750 mg | Proteinuria 0.62g/day | ||||
| Arthritis | Haematuria (moderate with dysmorphia) | Granular IgA deposits in the mesangium. | Oral CG | 5−10 red blood cells/field | |||||
| ME: pedicel fusion and mesangial electron-dense deposits. | |||||||||
| Huang et al.4 Nov 2020 | Female, 65 years old | AHT | Flu-like symptoms | Concomitant | Kidney involvement (probable reactivation of previous glomerular pathology) | ARF (eGFR 53ml/min) | 16 glomeruli: 5 sclerosed, 1 segmental sclerosis, 1 fibrocellular crescent | Valsartan | 3 months: eGFR 74.7ml/min |
| Asian | Proteinuria and haematuria at 14 months | Bilateral pneumonia | Proteinuria (1.07g/day) Haematuria (worsening, 2815/mcl) | Mesangial hypercellularity | MP 40mg x3 | 28 red blood cells/mcl | |||
| Mild | Interstitial fibrosis with mononuclear infiltrate | ||||||||
| Diarrhoea | NTA data | ||||||||
| IF: granular deposit Iga, C3, kappa, lambda | |||||||||
| ME: mesangial electron-dense deposits. | |||||||||
| Allez et al., 5 Nov 2020 | Male, 24 years old | Crohn's under treatment with adalimumab | No respiratory symptoms | Concomitant | Cutaneous purpura | No | Cutaneous: leukocytoclastic vasculitis with perivascular IgA deposits | MP 0.8mg/kg | |
| Previous ileocecal resection | No fever | Arthralgia | Oral CG | ||||||
| Digestive involvement (ileitis) | |||||||||
| Sandhu et al.,6 Nov 2020 | Male, 22 years old | Fever | 2 days | Kidney involvement | Proteinuria (2g/day) | Mesangial and focal endocapillary proliferative nephropathy with mesangial IgA deposits | MP 1mg/kg x10 days | 2 weeks: Normalisation of parameters and clinical resolution | |
| No respiratory symptoms | Palpable purpura | Haematuria not described. | Cellular crescents | MFM 3 months | |||||
| Arthralgia, arthritis | IF: granular mesangial IgA deposits | Oral CG | |||||||
| Abdominal pain, vomiting | |||||||||
| AlGhoozi et al.7 Dec 2020 | Male, 4 years old | Fever | 37 days | Palpable purpura | No | No | Paracetamol | ||
| Flu-like symptoms | Arthralgia | ||||||||
| Li et al., 8 Jan 2021 | Male, 30 years old | Former smoker | Fever | Concomitant | Kidney involvement | Proteinuria (I pro/Cu 0.57) | Renal: 22gl., 1 cellular crescent with segmental necrosis, 1 gl global sclerosis (5%), 1 gl segmental sclerosis (5%) | Prednisone 40mg/day | 6 weeks: RF preserved |
| Caucasian | Flu-like symptoms | Painful palpable purpura | Haematuria (11−20 red blood cells/field with dysmorphia) | Focal tubular atrophy and interstitial fibrosis. | Losartan | I alb/Cr 128.6mg/mmol | |||
| Diarrhoea | Arthralgia | IF: mesangial deposit of IgA, IgM; IgG, C3 | Persistent haematuria 30/field | ||||||
| Abdominal pain | ME: mesangial and subendothelial deposits | ||||||||
| Cutaneous: leukocytoclastic vasculitis with negative IF. | |||||||||
| Hoskins et al.9 Jan 2021 | Male, 2 years old | No fever | Concomitant | Cutaneous purpura | No | Cutaneous: IgA+perivascular neutrophilic infiltrate. | IV CG | Clinical resolution | |
| No respiratory symptoms | Abdominal pain, hematemesis, haematochezia | LMWH | |||||||
| Jacobi et al.10 Feb 2021 | Male, 3 years old | Hirschsprung's disease with surgical correction in treatment with metronidazole and enemas | No fever | Concomitant | Purpura | No | No | NSAIDs | |
| No respiratory symptoms | Abdominal pain and vomiting | ||||||||
| Barbetta et al.11 Mar 2021 | Male, 62 years old | Unknown | Fever | 10 days | Kidney involvement | Proteinuria | Cutaneous: perivascular and interstitial lymphocytic infiltrates IF IgA+ | MP 1mg/kg/day | Clinical remission and normalisation of renal alterations |
| Bilateral pneumonia | Cutaneous purpura | Haematuria | |||||||
| Abdominal pain, vomiting, haematochezia |
IgA vasculitis is an immune-mediated small-vessel vasculitis that manifests as non-thrombocytopenic purpura, arthritis or arthralgia, abdominal pain and/or kidney involvement. Despite being much more frequent in childhood, 10% of the affected population are adults. These develop more severe forms in which kidney involvement is more frequent, leading to a worse prognosis.18 The annual incidence in adults ranges between 0.8 and 1.8 per 100,000 inhabitants, with a predominance in those over 65 years of age, men and diabetics.19,20 In addition, it is more common in Caucasians and Asians than in African-Americans.21
Although its pathophysiology is not completely known, the influence of genetic, epigenetic and environmental factors is stipulated. The “four-hit” model is currently accepted, according to which a dysregulation of the immune system would lead to formation of abnormally glycosylated IgA1, which would be recognised by IgG/IgA autoantibodies forming immunocomplexes deposited together with complement factors at the mesangial level.18 Pathologies that predispose to the development of this condition have been described, such as liver disease (hepatic clearance deficiency and generation of elevated baseline levels of IgA), inflammatory bowel disease (overproduction of IgA in the intestinal tract)18 or underlying glomerular pathology (IgA nephropathy). Among the triggers of IgA vasculitis, infections stand out, with respiratory and gastrointestinal infections being the most frequently associated.22
Respiratory infection by SARS-CoV-2, like other infections, generates antigens that are recognised by the immune system and in the presence of predisposing factors, enhance the already known cytokine storm22 and lead to immune dysregulation that triggers the aforementioned vasculitis. Direct renal damage is unlikely, as evidence of particles at the renal level has not been demonstrated.23 Two cases of IgA nephropathy have recently been described that, after COVID-19 vaccination, have presented an outbreak of macroscopic haematuria.24 All this supports the idea that COVID-19 infection may be a trigger for IgA vasculitis.
Taking into account the first published case,3 75% of our patients with this diagnosis were male, elderly (>64 years) and Caucasian. They all started with renal function deterioration and one of them also with a clinical and biochemical nephrotic syndrome,3 and in all of them the clinical course and the biopsy findings supported the need for immunosuppressive treatment. Three of them presented extrarenal involvement, mainly cutaneous. The latency time between infection and the onset of vasculitis ranged from three weeks to nine months, in all cases, with serological evidence of past infection. This data supports the theory of immune dysregulation as the main aetiopathogenic factor. All had presented pulmonary involvement secondary to SARS-CoV-2 infection. One patient had chronic congestive liver disease (case 1) and another (case 3) had chronic glomerular disease, not biopsied, with a high clinical suspicion of IgA mesangial nephropathy as predisposing factors. All except the index case showed elevated IgA levels at onset. During follow-up (four to 10 months), recovery of renal function was incomplete in 50% of the cases. The data of our patients are summarised in Table 2.
IgA vasculitis in our setting.
| Case | Background | COVID clinic | COVID treatment | Vasculitis latency | Vasculitis clinic | Kidney involvement | Kidney/skin biopsy | Treatment | RF progression | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Male, 78 years old | Moderate alcohol consumption, AHT, DL, aortic stenosis, possible past HBV infection | Mild bilateral pneumonia | Dolquine | 3 weeks | Kidney involvement | ARF (Cr 1.96mg/dl) | 7 glomeruli: 1 sclerotic, 4 mesangial expansion, 1 synechiae. | MP 250mg x3 | 9 months: Cr 1.13mg/dl |
| Caucasian | Lopinavir/ritonavir | Cutaneous purpura | Nephrotic proteinuria (10g/day) | 2 epithelial crescents. | Rituximab 750 mg | Proteinuria 0.62g/day | ||||
| Dexamethasone | Arthritis | Haematuria (moderate, with dysmorphia) | Granular IgA deposits in the mesangium. | Oral CG | Microscopic haematuria (5−10 red blood cells/field) | |||||
| Ceftriaxone | IgA at diagnosis 367mg/dl | ME: pedicel fusion and mesangial electron-dense deposits. | ||||||||
| Azithromycin | ||||||||||
| Tocilizumab x1 | ||||||||||
| 1 | Male, 84 years old | Right CHF with congestive liver disease | Bilateral pneumonia | Dolquine | 8 weeks | Kidney involvement | ARF (Cr 5.51mg/dl) | Kidney: 16 gl. 8 sclerosed, 2 mesangial widening, 2 plume retraction. Not crescent. Fibrosis foci and tubular atrophy; intraluminal tubular red blood cells. Granular mesangial IgA deposits in IF. | MP 250mg x3 | 10 months: Cr 2mg/dl |
| Segmental PTE | Kaletra | Recurrent palpable purpura | Subnephrotic proteinuria (2g/day) | Cutaneous: perivascular PMN infiltrate, DIF+IgA. | Oral CG | Negative proteinuria | ||||
| Steroids | IgA at diagnosis 556mg/dl (baseline 405) | Macroscopic haematuria (25−30 red blood cells/field) | MP 125mg x3 | Microscopic haematuria (3−5 red blood cells/field) | ||||||
| Tocilizumabx1 | MFM | |||||||||
| Ceftriaxone | ||||||||||
| Azithromycin | ||||||||||
| O2 w/reservoir | ||||||||||
| 2 | Male, 87 years old | Past HBV infection? | Doubtful interstitial disease | Amoxicillin? | Not confirmed, 2 months | Kidney involvement | ARF (Cr 3.6mg/dl) | Cutaneous: leukocytoclastic vasculitis with granular perivascular IgA deposits | MP 250mg x3 | 5 months: Cr 1.07mg/dl |
| Cutaneous purpura | Proteinuria (I Pro/Cr 0.74mg/mg) | Oral CG | ||||||||
| IgA at diagnosis 502mg/dl | Haematuria (25−30 red blood cells/field) | |||||||||
| 3 | Female, 64 years old | Previous glomerular pathology not biopsied (suspected IgA) | Unilateral pneumonia | Dolquine | 9 months | Kidney involvement | ARF (Cr 3.41mg/dl) | 7 gl.: 1 sclerosed, 2 epithelial crescents, 1 fibrous crescent. Mesangial thickening and synechiae with Bowman's capsule | MP 250mg x5 | 4 months: Cr 2.4mg/dl |
| Azithromycin | IgA at diagnosis 434mg/dl | Nephrotic proteinuria (4.3g/day) | IF: mesangial granular deposits IgA, C3, kappa and lambda. | Oral CG | Proteinuria 1.33g/day | |||||
| Haematuria (10−20 red blood cells/field, with dysmorphia) | CF 500mg fortnightly | 10−20 red blood cells/field | ||||||||
Compared to the majority of published cases, our patients showed an older age at diagnosis. They all had severe kidney damage, requiring prolonged high-dose immunosuppressive treatment. Of the eight reported cases, excluding our index case, 75% were children and young people, and only 50% had kidney involvement, which was milder, with less need for immunosuppressive treatment and in shorter regimens. Only the case reported by Huang et al.4 is superimposable to ours: advanced age, greater renal involvement and presence of previous glomerular pathology with greater chronicity in the biopsy. Extrarenal involvement was frequent, both at the cutaneous, digestive and joint levels. The latency period was much shorter. In fact, many of the patients in the literature showed symptoms concomitant with the infection. In three patients there were predisposing factors: Allez et al.5 and Jacobi et al.10 describe a history of intestinal disease, and Huang et al.4 of previous glomerulopathy. In the five patients for which the information was available, renal progression was favourable.
In conclusion, like other infections, COVID-19 seems to be a trigger of glomerular pathology. In our setting, with a predominantly elderly Caucasian population, it has become the glomerular pathology most frequently associated with this infection. All patients required immunosuppressive treatment, given the clinical presentation and biopsy findings. In half of the cases, recovery of renal function was incomplete.
Conflicts of interestThe authors declare that they have no conflicts of interest.