Testosterone deficiency (hypogonadism) is common among men undergoing haemodialysis, but its clinical implications are not well characterized. Testosterone is an anabolic hormone that induces erythrocytosis and muscle synthesis. We hypothesized that testosterone deficiency would be associated with low muscle mass, physical inactivity and higher dosages of erythropoietin-stimulating agents (ESA).
MethodsSingle-center cross-sectional study of 57 male haemodialysis patients. None of the patients was undergoing testosterone replacement therapy. Total testosterone was measured in serum. Body composition (by bioelectrical impedance analysis) and physical activity (by the use of pedometers) were assessed. Patients with testosterone levels below the normal range were considered hypogonadal.
ResultsMean testosterone level was 321±146ng/dL; 20 patients (35%) were hypogonadal. Hypogonadal patients were older and had lower mean arterial blood pressure, higher interleukin-6 levels, lower lean body mass and higher fat body mass. A negative association between testosterone and normalized ESA dose was found in uni- and multivariate regression analyses. Testosterone levels directly correlated with lean body mass regardless of confounders. Hypogonadal patients had lower physical activity than their counterparts [2753±1784 vs. 4291±3225steps/day (p=0.04)]. The relationship between testosterone and physical activity was independent of age, comorbidities and inflammatory markers, but dependent on the proportion of muscle mass.
ConclusionHypogonadism is common in our male haemodialysis population and is associated with higher ESA doses, reduced muscle mass and lower physical activity. The link between low testosterone levels and physical inactivity may conceivably relate to reduced muscle mass due to inadequate muscle protein synthesis.
La deficiencia de testosterona (hipogonadismo) es frecuente en varones en hemodiálisis, pero sus consecuencias clínicas no se han caracterizado satisfactoriamente. La testosterona es una hormona anabólica que provoca eritrocitosis y síntesis muscular. Nos planteamos la hipótesis de que la deficiencia de testosterona pudiera estar asociada a una masa muscular baja, a la inactividad física y a dosis más altas de fármacos estimulantes de la eritropoyesis (FEE).
MétodosEstudio transversal de un solo centro de 57 pacientes varones en hemodiálisis. Ninguno de ellos estaba recibiendo tratamiento sustitutivo con testosterona. La cantidad total de testosterona se midió en el suero. Se evaluaron la composición corporal (mediante un análisis de impedancia bioeléctrica) y la actividad física (mediante el uso de podómetros). Los pacientes con concentraciones séricas de testosterona por debajo de los límites de normalidad se consideraron hipogonadales.
ResultadosLa concentración media de testosterona fue de 321±146ng/dl; 20 pacientes (35%) se consideraron hipogonadales. Los pacientes hipogonadales eran de edad avanzada y presentaban una presión arterial media más baja, concentraciones más altas de interleucina 6, masa corporal magra más baja y masa corporal grasa más alta. Se observó una asociación negativa entre la dosis de testosterona y de FEE normalizada en análisis de regresión univariante y multivariante. Las concentraciones de testosterona estaban directamente correlacionadas con la masa corporal magra, independientemente de los factores de confusión. Los pacientes hipogonadales presentaban una actividad física más baja que sus homólogos (2.753±1.784 frente a 4.291±3.225 pasos/día; p=0,04). La relación entre la actividad física y la testosterona fue independiente de la edad, las comorbilidades y los marcadores de inflamación, pero dependían de la proporción de masa muscular.
ConclusiónEl hipogonadismo es frecuente en la población de varones en hemodiálisis y está asociado a dosis más altas de FEE, masa muscular reducida y actividad física baja. El vínculo entre las concentraciones bajas de testosterona y la inactividad física está posiblemente relacionado con la masa muscular reducida debido a una síntesis de proteínas musculares insuficiente.
Chronic kidney disease (CKD) involves alterations in body homeostasis and metabolic disturbances (including hormone secretion disorders and altered response to hormones in target tissues), resulting in endocrine dysfunctions that may contribute to the increased mortality in CKD patients.1 Hypogonadism, hallmarked by testosterone deficiency is a common endocrine disorder among men undergoing dialysis, with a prevalence ranging from 35 to 50% in recent studies.2–4 Various studies link hypogonadism with mortality risk among hemodialysis patients,5,6 but the pathways by which this risk may be mediated are not well known.
The clinical implications of hypogonadism among dialysis patients are not well characterized. Testosterone is a steroid hormone that has an important anabolic function influencing among others muscle mass, increasing both strength and size.7 In the general population, testosterone deficiency that accompanies aging has been linked to decreased physical performance and its consequent limitation of mobility.8 In non-dialysis CKD patients, endogenous testosterone is a strong determinant of both muscle mass and strength.9 It is unknown if this is still the case in individuals undergoing dialysis.
Furthermore, testosterone induces erythrocytosis10,11 and testosterone deficiency has been associated with anemia and increased resistance to erythropoietin-stimulating agents (ESA) in dialysis patients.2 However, this finding has, to date, not been confirmed. The objective of our study was to assess the prevalence of hypogonadism among men undergoing dialysis at our center. Further, we explored the clinical phenotype of hypogonadal patients. Based on the abovementioned preceding literature, we hypothesized that endogenous testosterone would associate with the percentage of muscle strength and physical activity, as well as with higher ESA requirements.
Patients and methodsPatientsThis cross-sectional study comprised clinically stable male patients with CKD attending the hemodialysis (HD) program at Severo Ochoa Hospital, Leganes, Madrid, Spain. Only patients who had been on HD for more than three months and without hospital admissions in the month preceding the investigation were invited to participate. Patients with physical limitations (amputation) and neurological impairment were not considered because of the difficulty in assessing physical activity. From 75 eligible patients, 57 patients met the inclusion criteria and all accepted to participate. None of the patients was receiving testosterone replacement therapy. All participants gave their written consent. The study was approved by the Ethics Committee of the Severo Ochoa Hospital.
Clinical data including co-morbiditiesEach patient's medical chart was reviewed to extract demographic and clinical data including comorbidities. Clinical history of cardiovascular disease (CVD) was defined as cardiac, cerebrovascular (including stroke) or peripheral vascular disease. Mean arterial pressure (MAP) was defined as [diastolic pressure+(systolic pressure−diastolic pressure)/3]. Dose of erythropoietin-stimulating agents (ESAs) for each patient was recorded as international units administered per week (U/week). Weekly doses of darbepoetin in micrograms were converted to international units of erythropoietin by multiplying with a conversion factor of 200. The median ESA equivalent dose was 6000 (0–15300) U/week, which was normalized for body weight and for hemoglobin levels and presented as U/kg/mg/mL Hb/week in the following analyses.
Laboratory analysisBlood samples were collected before midweek dialysis session. The plasma was separated within 30min, and samples were kept frozen at −70°C if not analyzed immediately. Biochemical parameters were determined by standard laboratory techniques at the Biochemical Department of the Severo Ochoa Hospital. Interleukin-6 (IL-6) concentration was assessed on plasma lithium heparin by multiplexing in a Bioplex system (X-MAP technology, BioRad, Madrid, Spain) using commercial kits (R&D Systems Europe, Ltd, UK). The concentration was calculated by interpolation from the calibration performed with the corresponding recombinant proteins provided by the kit. Total testosterone was assessed in serum with a chemiluminescence assay (Advia-Centaur, Siemens) that has sensitivity, specificity, precision and linearity according to the requirements for usage in clinical practice. The normal reference range established by our laboratory for the male population is 241–827ng/dL.
Body composition assessmentDry weight (based on clinical approach) in kilograms and height in meters of each participant was recorded. Body mass index (BMI) was calculated dividing weight in kilograms by height in square meters (kg/m2). Body composition was assessed by bioimpedance (Body Composition Monitor, Fresenius Medical Care, Bad Homburg, Germany). The measurement was performed immediately before the second dialysis session of the week. Lean body mass (LBM) and fat body mass (FBM) were expressed as percentages. Total body water (TBW), extracellular water (ECW), intracellular water (ICW) and the relation ECW/ICW were collected. Other parameters obtained were body cell mass (BCM) in kg and an estimated index of over-hydration (OH) in liters.
Physical activity measurementPhysical activity (PA) was measured by a Geonaute-onstep-400® pedometer. Each patient was requested to wear the device for six consecutive days (two HD days, two HD-free midweek days, and two HD-free weekend days). Information on PA was obtained from the device memory. The data were recorded as number of steps per day. We used the pedometer-determined PA classification for healthy adults12 as a benchmark of PA degree. This classification considered that more than 10000 steps per day is the goal for an active life13 and participants with PA recorded below 5000 steps/day are considered sedentary.14
Statistical analysisAll statistical analyses were performed using SPSS version 12 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±SD or median (range of 10th to 90th percentile) or percentage, as appropriate. Statistical significance was set at the level of p<0.05.
Comparisons between two groups were assessed with the Kruskal–Wallis test for continuous variables and a χ2 test for nominal variables. Univariate analysis was performed using Spearman correlation. Different multivariate linear regression analyses were performed to test the independence of the association between testosterone and the three main dependent variables: ESA dose, percentage of lean body mass and physical activity (number of steps/day). All models were adjusted for a priori decided biological confounders. Because bioimpedance measurement was performed before the dialysis session, LBM% may have been overestimated due to overhydration. As a sensitivity analysis we tested further including OH in the studied multivariate regression models. Since the variable ESA dose had a non-normal distribution, the Loge of the variable was calculated and used in the multivariate analysis. Data are presented as standardized coefficient (β) and standard error (SE).
ResultsBaseline characteristicsAmong the 57 patients included, the median age was 65 years (49–80) with a median dialysis vintage time of 30 (7–183) months. Twenty-two (39%) patients were diabetics and 36 (66%) patients had a history of cardiovascular disease (CVD). Mean arterial pressure (MAP) at inclusion was 91 (76–109) mmHg. The median body mass index (BMI) was 25 (20–29)kg/m2.
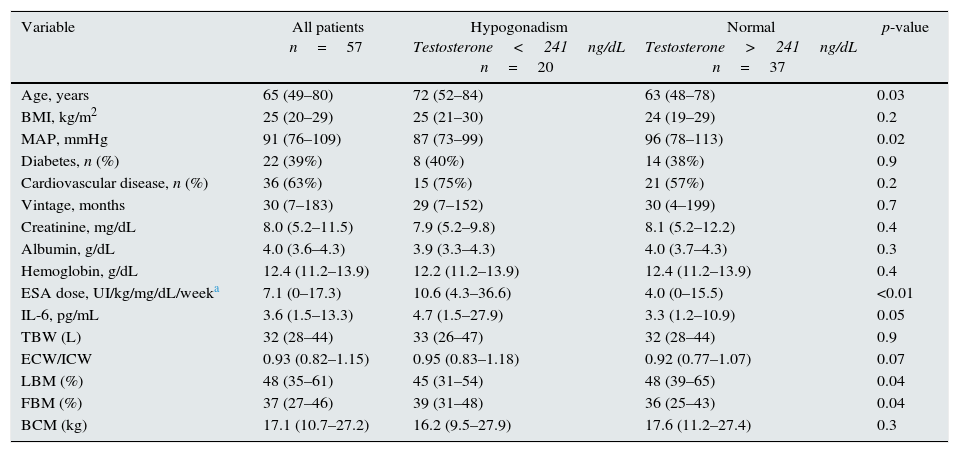
Testosterone levels and clinical and laboratory dataThe mean testosterone level was 321±146ng/dL. Twenty patients (35%) had a testosterone concentration below the normal range established in our laboratory and were classified as having hypogonadism. The general characteristics of all patients, and when placed in two groups according to testosterone levels, are summarized in Table 1. Patients in the hypogonadism group were older and had lower MAP, higher IL-6, lower LBM and higher FBM. Other variables did not differ.
General characteristics of 57 prevalent male HD patients and stratified according to the presence of hypogonadism (median and range of 10th to 90th percentile).
| Variable | All patients n=57 | Hypogonadism Testosterone<241ng/dL n=20 | Normal Testosterone>241ng/dL n=37 | p-value |
|---|---|---|---|---|
| Age, years | 65 (49–80) | 72 (52–84) | 63 (48–78) | 0.03 |
| BMI, kg/m2 | 25 (20–29) | 25 (21–30) | 24 (19–29) | 0.2 |
| MAP, mmHg | 91 (76–109) | 87 (73–99) | 96 (78–113) | 0.02 |
| Diabetes, n (%) | 22 (39%) | 8 (40%) | 14 (38%) | 0.9 |
| Cardiovascular disease, n (%) | 36 (63%) | 15 (75%) | 21 (57%) | 0.2 |
| Vintage, months | 30 (7–183) | 29 (7–152) | 30 (4–199) | 0.7 |
| Creatinine, mg/dL | 8.0 (5.2–11.5) | 7.9 (5.2–9.8) | 8.1 (5.2–12.2) | 0.4 |
| Albumin, g/dL | 4.0 (3.6–4.3) | 3.9 (3.3–4.3) | 4.0 (3.7–4.3) | 0.3 |
| Hemoglobin, g/dL | 12.4 (11.2–13.9) | 12.2 (11.2–13.9) | 12.4 (11.2–13.9) | 0.4 |
| ESA dose, UI/kg/mg/dL/weeka | 7.1 (0–17.3) | 10.6 (4.3–36.6) | 4.0 (0–15.5) | <0.01 |
| IL-6, pg/mL | 3.6 (1.5–13.3) | 4.7 (1.5–27.9) | 3.3 (1.2–10.9) | 0.05 |
| TBW (L) | 32 (28–44) | 33 (26–47) | 32 (28–44) | 0.9 |
| ECW/ICW | 0.93 (0.82–1.15) | 0.95 (0.83–1.18) | 0.92 (0.77–1.07) | 0.07 |
| LBM (%) | 48 (35–61) | 45 (31–54) | 48 (39–65) | 0.04 |
| FBM (%) | 37 (27–46) | 39 (31–48) | 36 (25–43) | 0.04 |
| BCM (kg) | 17.1 (10.7–27.2) | 16.2 (9.5–27.9) | 17.6 (11.2–27.4) | 0.3 |
BMI: body mass index, MAP: mean arterial pressure, TBW: total body water, ECW: extracellular water, ICW: intracellular water, LBM lean body mass, FBM: fat body mass, BCM: body cell mass.
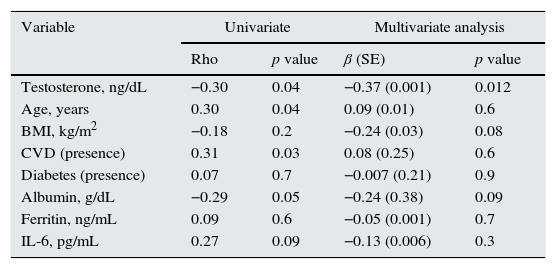
The required weekly dose of EPO (normalized by weight and by hemoglobin levels) was significantly higher in the group with lower testosterone levels (Table 1). This negative association was also observed after adjustment for potential confounders in a multivariable regression analysis (Table 2). Additional adjustment for OH or dialysis vintage did not alter the results (sensitivity analysis not shown).
Univariate y linear regression multivariate models for weekly ESA dose normalized by weight and by hemoglobin level (loge, UI/kg/week) (R2 0.27).
| Variable | Univariate | Multivariate analysis | ||
|---|---|---|---|---|
| Rho | p value | β (SE) | p value | |
| Testosterone, ng/dL | −0.30 | 0.04 | −0.37 (0.001) | 0.012 |
| Age, years | 0.30 | 0.04 | 0.09 (0.01) | 0.6 |
| BMI, kg/m2 | −0.18 | 0.2 | −0.24 (0.03) | 0.08 |
| CVD (presence) | 0.31 | 0.03 | 0.08 (0.25) | 0.6 |
| Diabetes (presence) | 0.07 | 0.7 | −0.007 (0.21) | 0.9 |
| Albumin, g/dL | −0.29 | 0.05 | −0.24 (0.38) | 0.09 |
| Ferritin, ng/mL | 0.09 | 0.6 | −0.05 (0.001) | 0.7 |
| IL-6, pg/mL | 0.27 | 0.09 | −0.13 (0.006) | 0.3 |
BMI: body mass index, CVD: cardiovascular disease.
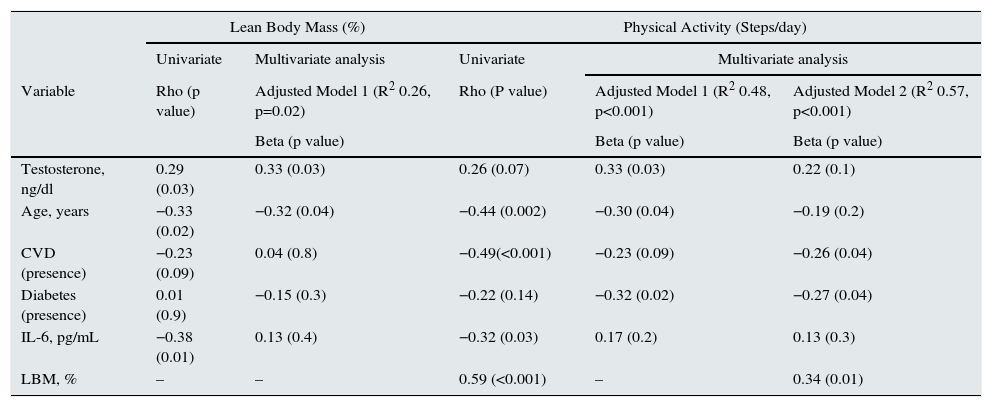
In uni- and multivariate analysis, testosterone positively correlated with the percentage of LBM (Table 3). Additional adjustment for dialysis vintage and OH did not alter the results (data are not shown). The average PA registered in the group with normal testosterone levels was 4291±3225steps/day compared to an average of 2753±1784 (p=0.04) in the group of patients with hypogonadism. In multivariate regression models considering confounders, testosterone levels remained positively associated to physical activity (Table 3, physical activity adjusted model 1). However, when the percentage of lean body mass was further included in the model (presumed within the causal pathway), the association between testosterone and physical activity ceased to be statistically significant, reducing the regression coefficient of the association (β) by approximately 30% (from 0.33 to 0.22) (Table 3, Physical activity adjusted model 2). No changes in the model were found when we additionally adjusted for dialysis vintage or OH (data not shown).
Linear regression model for lean body mass (percentage) and physical activity (number of steps/day).
| Lean Body Mass (%) | Physical Activity (Steps/day) | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate analysis | Univariate | Multivariate analysis | ||
| Variable | Rho (p value) | Adjusted Model 1 (R2 0.26, p=0.02) | Rho (P value) | Adjusted Model 1 (R2 0.48, p<0.001) | Adjusted Model 2 (R2 0.57, p<0.001) |
| Beta (p value) | Beta (p value) | Beta (p value) | |||
| Testosterone, ng/dl | 0.29 (0.03) | 0.33 (0.03) | 0.26 (0.07) | 0.33 (0.03) | 0.22 (0.1) |
| Age, years | −0.33 (0.02) | −0.32 (0.04) | −0.44 (0.002) | −0.30 (0.04) | −0.19 (0.2) |
| CVD (presence) | −0.23 (0.09) | 0.04 (0.8) | −0.49(<0.001) | −0.23 (0.09) | −0.26 (0.04) |
| Diabetes (presence) | 0.01 (0.9) | −0.15 (0.3) | −0.22 (0.14) | −0.32 (0.02) | −0.27 (0.04) |
| IL-6, pg/mL | −0.38 (0.01) | 0.13 (0.4) | −0.32 (0.03) | 0.17 (0.2) | 0.13 (0.3) |
| LBM, % | – | – | 0.59 (<0.001) | – | 0.34 (0.01) |
Dashes indicate not included. CVD: cardiovascular disease, LBM: lean body mass.
This study shows that hypogonadism is rather common among men undergoing HD and that, independently of age and other potential confounders, lower levels of endogenous testosterone associate with higher doses of ESA, lower lean body mass and poorer physical activity.
The observed prevalence of hypogonadism among male dialysis patients in our study (35% prevalence) is in line with recent reports,15,16 although larger dialysis cohorts document up to 70% of their population to present with testosterone deficient levels.4,17 Inconsistencies in this information may be affected by different inclusion criteria, age-ranges, different cut-off points for hypogonadism and overall, differences in clinical practice between countries.
Recently, low testosterone levels have been associated with lower levels of hemoglobin in ESA-naïve CKD patients and with higher ESA dosages in hemodialysis patients.18 These data are now corroborated by the findings of our study, where the prescribed dose for ESA was negatively associated with serum testosterone levels. Testosterone is known to stimulate erythropoiesis through the production of hematopoietic growth factors19 and through the improvement of iron bioavailability via the hepcidin pathway.11 Considering that male hypogonadism may represent a contributing factor to renal anemia, restoration of this deficiency could potentially be of value in the treatment of anemia.20–22
One of the most important actions of testosterone regards its effect on body composition and metabolism. In our study, we observed an independent direct association between testosterone levels and muscle mass, which agrees with community studies23,24 and expands to hemodialysis our previous observation in non-dialyzed men with CKD stages 3–5.9 Testosterone increases muscle mass by multiple mechanisms; including, differentiation of pluripotent stem cells toward the myogenic lineage, as well as, stimulation of muscle protein synthesis and inhibition of muscle protein degradation.7 A novel finding in our report is the, perhaps expected, positive and independent association between endogenous testosterone levels and physical activity as determined by number of steps per day. Because the relationship between physical activity and testosterone levels ceased to be significant when muscle mass was included in the model (adjustment in the causal pathway), it would seem congruent to speculate that this association appears to be explained by the association between testosterone and muscle mass status. It is not well known whether testosterone supplementation affects muscle function over and above its effects on muscle mass. Some interventions have tested the effects of supraphysiological dosages of testosterone supplementation in HD patients (both men and women) with normal or unknown testosterone levels, and described muscle growth effects.25,26 The study of Johanssen et al., however, did not see any measurable improvement in physical performance coupled to this anabolism. The intervention period, however (3 months) may have been too short to discern this. Nevertheless, no study has yet addressed the impact that restoration of hypogonadism could have in improving or maintaining muscle mass in this patient population subjected to a strong catabolic environment.27
Our study has some limitations that should be pointed out, starting by a reduced sample size and a cross-sectional design, which does not allow establishing causal relationships. The diagnosis of hypogonadism was performed based on a single testosterone determination, and signs and symptoms of this condition were not considered. However, because most hypogonadal symptoms coincide with those of uremia, reliance on testosterone levels in the setting of chronic diseases such as CKD is indicated by some guidelines.28 The use of total rather than free testosterone concentrations might result in under-diagnosis because the concentration of sex-hormone binding globulin increases with age. We collected samples before the dialysis session, with some patients attending the afternoon rounds. Because testosterone has a certain circadian variation, this may have influenced our findings, however, toward the null. On the other hand, performing the bioimpedance measurement before the dialysis session could introduce a bias on the value of LBM due to overhydration, in order to reduce this possible bias the OH value was included in the different models without finding any modification on the results.
To conclude, we show that hypogonadism is common among our population of men undergoing hemodialysis and it is related to higher doses of ESA, reduced muscle mass and poorer physical activity. Conceivably, these associations reflect inadequate erythropoiesis and low muscle protein synthesis due to low levels of circulating testosterone, while the observed link between low testosterone levels and physical inactivity is most likely explained by reduced muscle mass due to hypogonadism. The results of the current study can be of value to raise the awareness of male hypogonadism in CKD and increase interest on the potential of treating this endocrine deficiency. Although pharmacokinetics of testosterone administration seem unaffected in dialysis patients29 and we are proposing restoration of clinical deficiencies rather than supraphysiological administration, safety of this therapy should not be taken for granted and needs to be confirmed by randomized controlled trials.
Support and financial disclosuresGabriela Cobo is a beneficiary of a PhD scholarship from the Ecuadorian Government. This work also received a publication aid granted by the Spanish Nephrology Society. Bengt Lindholm is employed by Baxter Healthcare. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. Juan Jesus Carrero is co-investigator of an ongoing investigator-driven randomized trial on testosterone restoration in dialysis patients that is partially funded by Bayer, the manufacturer of Nebido©.
Conflict of interestNone of the other authors declare any conflict of interest.
We thank the patients for their willingness to participate in this study and the staff of the hemodialysis unit for their collaboration. Also we want to express our gratitude to Dr. Fernando García Lopez, member of Carlos III Public Health Institute of Madrid, for his invaluable help with this manuscript.