Hyperuricemia is frequently associated with gout, renal disease, arterial hypertension and high cardiovascular disease. All CKD patients with a first episode of gout should be treated with hypouricemic drugs to achieve baseline UA levels of less than 6 mg/dl (< 5 mg/dl if tophi are present). The hypouricemic drugs of choice in patients with CKD are allopurinol and febuxostat, always starting treatment with low doses that can be progressively increased according to tolerance. Asymptomatic hyperuricemia increases the risk of arterial hypertension, cardiovascular disease and renal disease, but at present published clinical trials do not support the treatment of asymptomatic hyperuricemia in patients with CKD.
La hiperuricemia se asocia frecuentemente con gota, enfermedad renal, hipertensión arterial y patología cardiovascular. Todos los pacientes con ERC que presentan un primer episodio de gota deben ser tratados con fármacos hipouricemiantes para conseguir unos niveles basales de AU menor de 6 mg/dl (< 5 mg/dl si presentan tofos). Los fármacos hipouricemiantes de elección en pacientes con ERC son el alopurinol y febuxostat, iniciando siempre el tratamiento con dosis bajas que pueden ir aumentándose progresivamente según tolerancia. La hiperuricemia asintomática aumenta el riesgo de hipertensión arterial, enfermedad cardiovascular y enfermedad renal, pero en la actualidad los ensayos clínicos publicados no avalan el tratamiento de la misma en pacientes con enfermedad renal crónica.
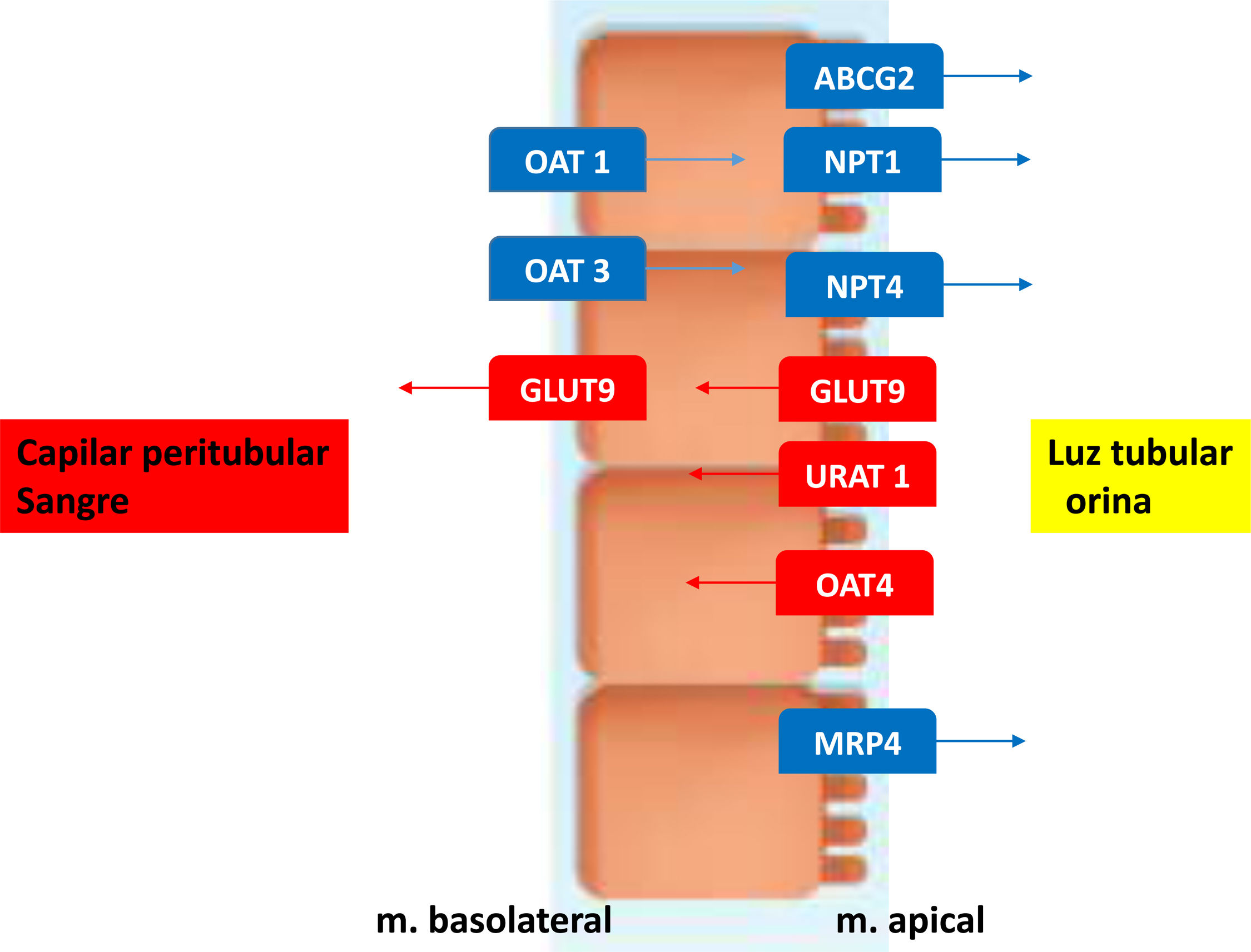
Uric acid (UA) is the end product of purine metabolism that is synthesized mainly in the liver and intestine, but also in other tissues such as muscle, endothelium and kidneys. A 2/3 of UA is eliminated by the kidney and 1/3 by the feces. Almost all UA is filtered y by the glomeruli, so the amount excreted is regulated by tubular reabsorption and secretion. In the proximal tubule the UA reabsorption is facilitated by urate anion transporter (URAT 1) and the organic anion transporter 4 (OAT4) both located on the apical membrane.
The Glucose transporter 9 (GLUT9) reabsorbs both UA and glucose by the tubular cells; it is located in both the apical tubule and the basolateral membrane and mediates the basolateral flow back into the circulation. The secretion of UA occurs in the segment S2 of the proximal tubule via transporters such as OAT1, OAT3 located in the basolateral membrane that facilitate urate entry into the renal tubules. The multidrug resistance protein(MRP4/ABCC4), the breast cancer resistance protein(BCRP/ABCG2) and the sodium-dependent phosphate transporters type 1 and type 4 (NPT1 and NPT4) are located on the apical side and contribute to the secretory transport of urate into the tubular lumen to achieve an appropriate urinary excretion of UA (see Fig. 1). Post-secretory reabsorption occurs at a more distal site of the proximal tubule. Approximately 10% of uric acid being excreted in the urine.1
Hyperuricemia is defined as an increase in UA concentration above its level of solubility in water (6.8 mg/dl) and it may occur as a result of overproduction, decreased excretion, or both processes.
Hyperuricemia may result in a variable clinical spectrum: acute gouty arthritis due to precipitation of monosodium urate crystals at the joints; tophaceous gout due to precipitation of crystals in skin and subcutaneous tissue; urate nephrolithiasis; acute UA nephropathy due to precipitation of intratubular crystals (tumor lysis syndrome) and chronic UA nephropathy due to deposition of urate crystals in the medullar interstitium producing inflammation and interstitial fibrosis. In addition, there are congenital alterations that affect the uromodulin gene leading to familial juvenile hyperuricemic nephropathy.2
Treatment of symptomatic hyperuricemiaTreatment of gout is based on four basic principles regardless of whether the patient has CKD:
(a) reduction of the concentration of UA, (b) administration of prophylaxis when UA is acutely reduced, (c) treatment of the acute “attack” of gout, and (d) non-pharmacological hygienic and dietary measures. The goal of the treatment is to maintain UA levels below 6 mg/dL (or 5 mg/dL in case of tophaceous gout); the aim is to decrease the frequency and duration of gout attacks. Ninety percent of the causes of hyperuricemia are due to a reduced excretion of UA, and only 10% are caused by increased UA production. In the latest published guidelines, it is recommended to initiate treatment with hypouricemic drugs in patients presenting ≥ 2 gout outbreaks per year, or the presence of tophi and/or nephrolithiasis. However, in patients with CKD stage 2 or greater the treatment should be initiated after the first of gout attack.3
Regarding hygienic-dietary measures, it is recommended to all patients with gout a diet low in foods containing high purine, weight loss, avoid alcohol (especially beer), and the prohibition of drinks with high fructose content (soft drinks). The complete prohibition of foods with high purine content is not recommended, since its impact only entails the reduction of 1 mg/dl of uric acid and therefore, these measures can never replace the pharmacologic treatment of gout.4
In CKD patients with gout attack acute treatment with non-steroidal anti-inflammatory drugs are contraindicated and colchicine should be used with caution since it is eliminated by the kidney and its half-life may increase two to three times as compared to normal renal function patients. In addition colchicine is metabolized by cytochrome P453A therefore caution should be exercised when using other drugs such as macrolides (clarithromycin), cyclosporine or statins. The dose of colchicine in patients with CKD stage 1 or 2 initially should be 1 mg, followed by 0.5 mg one hour later and 0.5 mg/12 h during a 3−5 day period. If the eGFR is less than 60 ml/min, the recommended dose is less than 0.5 mg per day. In patients with advanced CKD the most effective treatment is a short course of corticosteroids, prednisone 20−30 mg for 3−5 days.
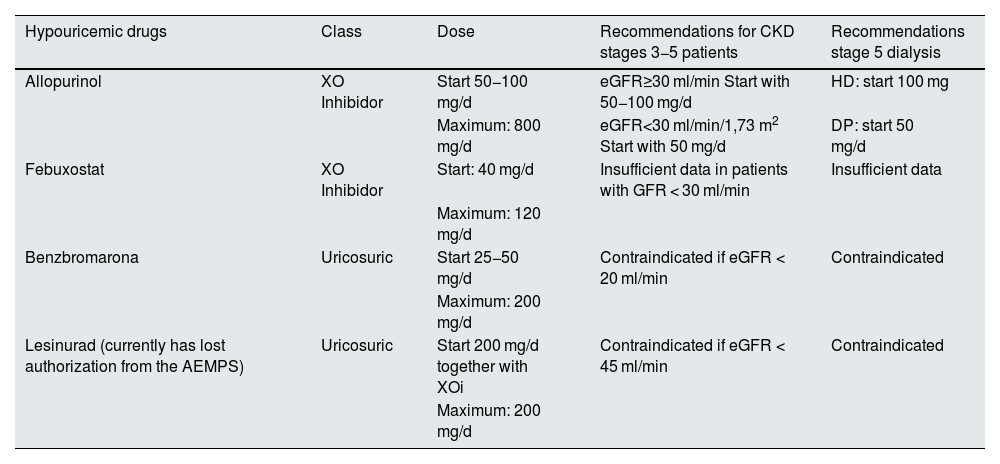
The hypouricemic drugs currently available include: (a) inhibitors of xanthine oxidase (XO) that block purine metabolism; (b) uricosurics that act on the main cause of hyperuricemia, which is a reduced renal excretion of UA, and 3) uricase, which oxidizes urate through an enzymatic reaction to allantoin. Table 1 shows a list of the different hypouricemic drugs marketed in Spain, the mechanism of action and their doses in the different stages of CKD. Although dialysis in principle is removes UA, and reduces the number of gout attacks, sometimes it is necessary to add hypouricemic drugs in patients with tophaceous gouty arthropathy.
Hypouricemic drugs and recommended doses in renal patients.
| Hypouricemic drugs | Class | Dose | Recommendations for CKD stages 3−5 patients | Recommendations stage 5 dialysis |
|---|---|---|---|---|
| Allopurinol | XO Inhibidor | Start 50−100 mg/d | eGFR≥30 ml/min Start with 50−100 mg/d | HD: start 100 mg |
| Maximum: 800 mg/d | eGFR<30 ml/min/1,73 m2 Start with 50 mg/d | DP: start 50 mg/d | ||
| Febuxostat | XO Inhibidor | Start: 40 mg/d | Insufficient data in patients with GFR < 30 ml/min | Insufficient data |
| Maximum: 120 mg/d | ||||
| Benzbromarona | Uricosuric | Start 25−50 mg/d | Contraindicated if eGFR < 20 ml/min | Contraindicated |
| Maximum: 200 mg/d | ||||
| Lesinurad (currently has lost authorization from the AEMPS) | Uricosuric | Start 200 mg/d together with XOi | Contraindicated if eGFR < 45 ml/min | Contraindicated |
| Maximum: 200 mg/d |
XO: xantino-oxidasa, XOi: XO inhibitor. HD: Hemodialysis. PD: Peritoneal Dialysis.
Regardless of the hypouricemic drug to be used, there are common rules about all of them: a) always start treatment associated with prophylaxis, b) start with the lowest dose and monitor UA levels until the target is reached, c) the hypouricemic drug should not be withdrawn or change its dose during a gout attack and 4) in the case of additional new treatments, the drug should be introduced after resolution of the acute gout attack.
Xanthine oxidase inhibitors (XOI)Xanthine oxidase (XO) is an enzyme that catabolizes the transformation of hypoxanthine into xanthine and xanthine into UA. XOIs are considered the treatment of choice in patients with gout.
AllopurinolAllopurinol is a structural analog of hypoxanthine, which causes a competitive inhibition of XO. In addition, allopurinol is a substrate of XO that catabolizes its transformation to oxypurinol. For years, it has been established that the dose of allopurinol should be corrected for the reduction of renal function to achieve the same level of oxypurinol as would be achieved by a dose of 300 mg, in a patient with normal renal function.5 This dose adjustment was developed to reduce the occurrence of allopurinol hypersensitivity syndrome (AHS), which is characterized by rash, eosinophilia, leukocytosis, fever, hepatitis, acute renal failure and high mortality. However, with this strategy, less than 50% of subjects with CKD, achieve the target of UA. Recent studies6 have shown that the maximum peak dose of allopurinol is not associated with increased AHS. However AHS is associated with the initial dose of allopurinol and also with genetic variants (HLA B5801); furthermore, the risk is higher during the first 6 month of treatment.7,8 Thus, the latest recommendations indicate that the dose of allopurinol can be increased in patients with CKD. It should be started with a low dose of 50 mg/day in CKD patients stage 4 and 5 (GFR < 30 ml/min) and with a dose of 100 mg/day in the remaining CKD stages. Allopurinol may be used in patients on hemodialysis and peritoneal dialysis. Starting with the lowest dose, the numbers of gout attacks are reduced with less incidence of AHS and other allergic reactions. Subsequently the dose should be titrated progressively being increased by 50−100 mg/day every 2–5 weeks until the desired level of UA is achieved.
FebuxostatFebuxostat is a potent non-purinic XO inhibitor that inhibits both, the oxidized and reduced forms of the enzyme. Clinical studies have demonstrated a greater efficacy in the reduction of UA with the use of febuxostat than with 200−300 mg allopurinol.9 Febuxostat can be administered to CKD patients with GFR less than 30 ml/min/1.73 m2. However, data on its efficacy in these cases are scarce.10 With regard to adverse effects, severe skin reactions and even Stevens-Johnson syndrome have been reported in patients who have had in the past a reaction to allopurinol. Therefore the European and Canadian Medicines Agency have issued a warning about these adverse effects and it should not be used in patients who have had previous serious reactions.11,12 Unlike allopurinol, no association has been found with HLAB5801.13
The CARES study (Cardiovascular Safety of Febuxostat and Allopurinol in patients with gout and cardiovascular comorbidities)14 included 6,190 patients with gout who were randomized to receive allopurinol or febuxostat. Patients who received febuxostat did not have more cardiovascular events such as acute myocardial infarction, arrhythmias, unstable angina or hospitalization due to heart failure. However, cardiovascular mortality was significantly higher in the febuxostat-treated group as compared with allopurinol-treated group HR 1.34 (1.03–1.73), similar findings were obtained regarding all-cause mortality (HR 1.22 (1.01−1.47). This clinical trial included patients with mild CKD (stage 1–3) and one of its main limitations was the high dropout rate due to different reasons. These results led to the generation of a warning from the FDA (https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat) and from the AEMPS (https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2019/NI_MUH_FV-10-2019-febuxostat.htm) warning that febuxostat increases overall and cardiovascular mortality, so it should not be recommended in patients with cardiovascular risk. However, the CARES study, published in the NEJM, has some limitations. First, as previously mentioned, there was a dropout rate of almost 50% in the treated group; secondly, there were very different findings in relation to fatal and non-fatal cardiovascular events, with no significant differences in non-fatal cardiovascular events in the febuxostat-treated group. And finally, without a placebo group, these results do not answer the question of whether treatment with febuxostat increases the risk of fatal cardiovascular events, or whether treatment with allopurinol decreases them. Furthermore, subsequent studies did not support these results. An observational study from the MEDICARE registry15 in 24,936 patients older than 65 years with gout treated with febuxostat vs allopurinol found no increased cardiovascular risk or mortality in patients treated with febuxostat as compared to those treated with allopurinol. In June/2019 it was published the clinical trial FREED (Febuxostat for Cerebral and Cadiorenovascular events Prevention Study) in which 1,070 elderly patients with hypertension and hyperuricemia were randomized to treatment with febuxostat or standard treatment. Treatment with febuxostat decreased the primary composite endpoint (HR 0.750 (0.592−0.950), p = 0.017), and did not increase cardiovascular mortality.16 In 2020, the results of the FAST study (Febuxostat vs Allopurinol Streamlined Trial) were published; the study compared the effect of febuxostat vs allopurinol in the prevention of cardiovascular events, showing no superiority of one drug over the other, and once again the study confirmed the long-term cardiovascular safety of febuxostat vs allopurinol.17 Therefore, there is insufficient data to support the contraindication of this drug, although it is recommended to be used with caution in patients with history of ischemic heart disease and heart failure.18 In Spain, both XO inhibitors are in the first line of treatment in patients with gout.
UricosuricsUricosurics decrease the concentration of UA by increasing the renal excretion of UA. Uricosurics act through transporter proteins that are involved in the reabsorption and/or secretion of UA. Among these transporters are URAT1, GLUT9, and transporters of organic anions: OAT 1, OAT 3, OAT 4 and OAT10. Currently only benzbromarone has AEMPS approval. Probenecid, sulfinpyrazone and lesinurad are not approved in Spain.
Benzbromarone is a potent uricosuric that can be administered in all patients with different degrees of renal disease. This drug is not approved by the FDA and by some European countries because of its hepatotoxicity.19 Lesinurad is a URAT1 and OAT4 inhibitor that must be administered together with an XOI. The use of lesinurad has been associated with acute renal failure and it is contraindicated in subjects with GFR less than 30 and is not recommended if the GFR less than 45 ml/min/1.73 m2.20 It was marketed and approved by the AEMPS and subsequently withdrawn from the market due to high risk of acute renal failure. Uricosurics should be avoided in patients with nephrolithiasis and uricosuria greater than 800 mg/24 h.
UricaseHominids do not express the gene encoding uricase, an enzyme that degrades UA to allantoin, a molecule with greater solubility than urate and it is eliminated more easily. Pegloticase is a pegylated porcine uricase which is indicated in patients with gout refractory to XOI. It is administered intravenously every two weeks for a six-month period. It is not approved by the EMA which withdrew its authorization in 2016, however it is approved by the FDA. As it is a porcine molecule it has a high risk of immunogenicity and produces allergic reactions during the infusion, and also there are cases described of heart failure. Recently, a study has been published using methotrexate associated with pegloticase to decrease immunogenicity and favor its efficacy.21 It can be administered in patients with any degree of chronic renal disease and also on dialysis.22
Rasburicase is approved in Spain for the prevention of tumor lysis syndrome associated with chemotherapy treatment21. It has a very short half-life (about 20 h), in contrast to pegloticase with half-life of about 7 days.
IL1β antagonistsMonosodium urate crystals activate inflammasome 3 releasing interleukin 1β. Anakinra is a short-acting IL-1 receptor antagonist that improved acute gout flares in 94% of 551 patients, of whom 53% had CKD and 3% were on dialysis. Most of these patients had been resistant to treatment with corticosteroids and colchicine. Anakinra is not approved by FDA and EMA for the treatment of acute gout flares, but can be used on a compassionate basis in patients resistant to classical treatments. Its usual dosage is 100 mg daily for 3–5 days until flare reduction, without having to modify the dose in patients with CKD. Its most frequent side effect is neutropenia.23
Canakinumab is a long-acting monoclonal anti-IL-1β, which reduces pain in acute gout attack and reduces flares. It is approved by the FDA and EMA for the treatment of patients with gout and frequent flares refractory to other treatment. The dose is 150 mg s.c., and is not adjusted in patients with CKD. Its high price makes its use very limited.24
Drugs that modify uric acid levelsThe use of diuretics is the most frequent cause of secondary hyperuricemia. Loop diuretics and thiazides inhibit the organic anion transporters (OAT1 and OAT3) that are involved in the active uptake of UA in the renal proximal tubules. Hydrochlorothiazide also significantly increases UA uptake through the organic anion transporter OAT4. In addition, diuretics induce salt and water loss, which causes volume contraction, stimulating UA reabsorption. In contrast, many drugs currently used to treat various cardiovascular diseases have a positive secondary effect on UA metabolism and levels. Among them it should be highlighted that fenofibrate25 and atorvastatin increase the fractional excretion of UA.26
Furthermore, inhibitors of the sodium-glucose cotransporter type 2 (iSGLT2) in the post hoc analysis of clinical assays of the DAPA-HF and EMPEROR-reduce it was observed that are associated with a decrease in UA levels.27,28
Asymptomatic hyperuricemiaExperimental work, has shown that prolonged hyperuricemia produces hemodynamic and histological changes in the kidneys can lead to the development of de novo chronic kidney disease unrelated to the deposition of urate crystals in the medullary interstitium or accelerate the progression of an existing nephropathy. The main lesions produced by chronic hyperuricemia at the renal level are glomerulosclerosis, arteriolopathy and interstitial fibrosis.29 The mechanism of this injury is the development of a glomerular arteriolopathy that impairs the renal autoregulatory response and causes glomerular hypertension.30
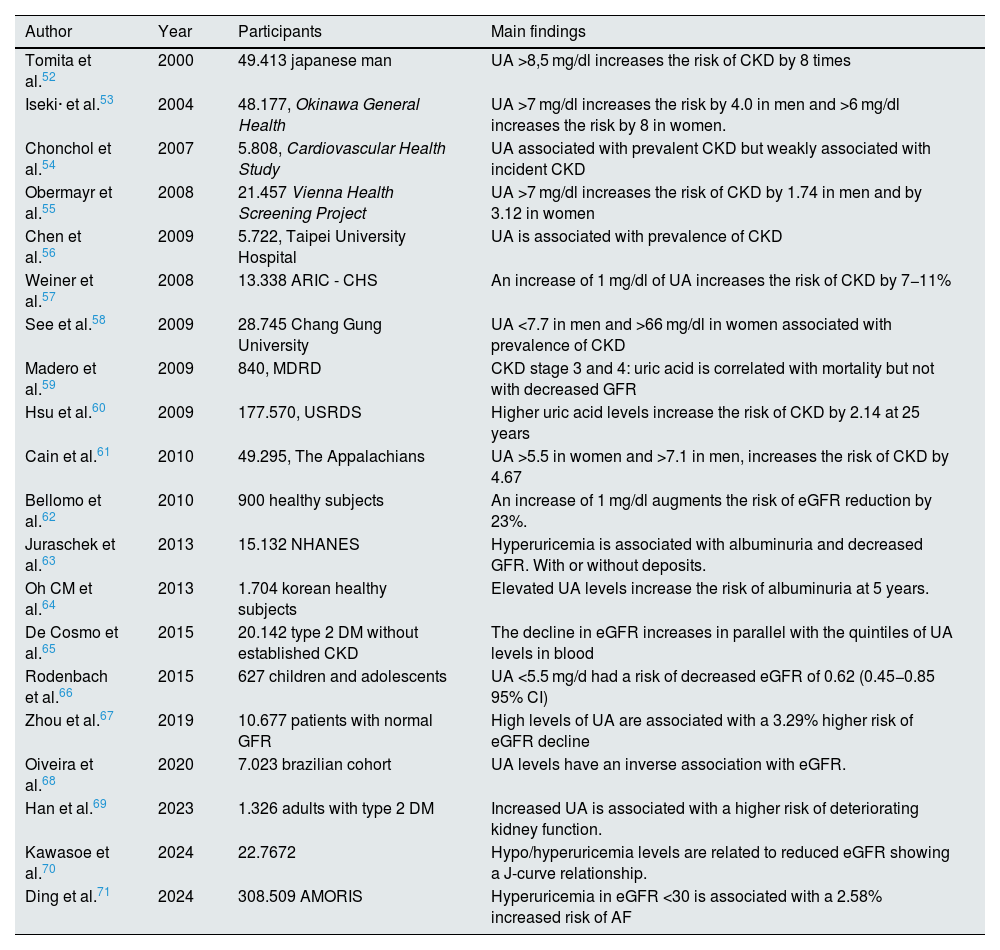
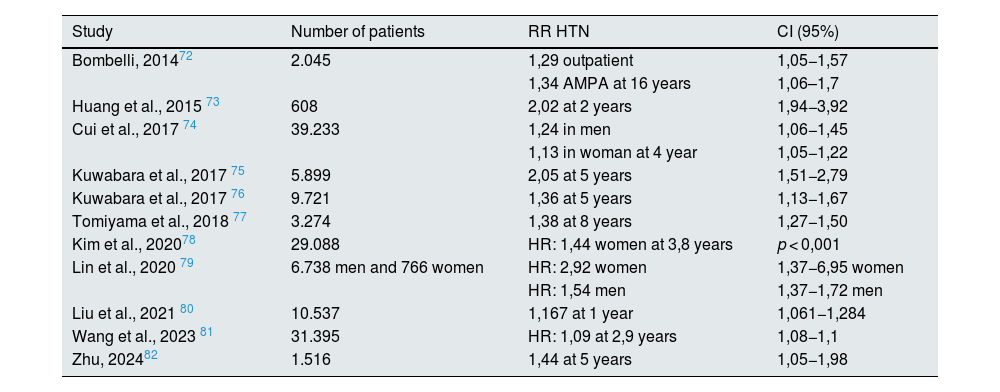
Many observational studies have shown an association between hyperuricemia and increased incidence of chronic kidney disease in the general population (Table 2). These results have been confirmed in the diabetic population31–34 and in renal transplant recipients.35 In recent years, sufficient evidence has been published to establish an association of asymptomatic hyperuricemia with arterial hypertension and cardiovascular risk (Tables 3 and 4). However, interventional studies showing a benefit of treatment of asymptomatic hyperuricemia on renal and cardiovascular risk are scarce and controversial, and the question remains as to whether hyperuricemia plays a causal role in the progression of CKD or it is simply an indirect marker of renal disease. The following sections will summarize the published scientific evidence regarding treatment with allopurinol and febuxostat in patients with asymptomatic hyperuricemia and its effect on renal and cardiovascular risk.
Hyperuricemia and risk of kidney disease.
| Author | Year | Participants | Main findings |
|---|---|---|---|
| Tomita et al.52 | 2000 | 49.413 japanese man | UA >8,5 mg/dl increases the risk of CKD by 8 times |
| Iseki· et al.53 | 2004 | 48.177, Okinawa General Health | UA >7 mg/dl increases the risk by 4.0 in men and >6 mg/dl increases the risk by 8 in women. |
| Chonchol et al.54 | 2007 | 5.808, Cardiovascular Health Study | UA associated with prevalent CKD but weakly associated with incident CKD |
| Obermayr et al.55 | 2008 | 21.457 Vienna Health Screening Project | UA >7 mg/dl increases the risk of CKD by 1.74 in men and by 3.12 in women |
| Chen et al.56 | 2009 | 5.722, Taipei University Hospital | UA is associated with prevalence of CKD |
| Weiner et al.57 | 2008 | 13.338 ARIC - CHS | An increase of 1 mg/dl of UA increases the risk of CKD by 7−11% |
| See et al.58 | 2009 | 28.745 Chang Gung University | UA <7.7 in men and >66 mg/dl in women associated with prevalence of CKD |
| Madero et al.59 | 2009 | 840, MDRD | CKD stage 3 and 4: uric acid is correlated with mortality but not with decreased GFR |
| Hsu et al.60 | 2009 | 177.570, USRDS | Higher uric acid levels increase the risk of CKD by 2.14 at 25 years |
| Cain et al.61 | 2010 | 49.295, The Appalachians | UA >5.5 in women and >7.1 in men, increases the risk of CKD by 4.67 |
| Bellomo et al.62 | 2010 | 900 healthy subjects | An increase of 1 mg/dl augments the risk of eGFR reduction by 23%. |
| Juraschek et al.63 | 2013 | 15.132 NHANES | Hyperuricemia is associated with albuminuria and decreased GFR. With or without deposits. |
| Oh CM et al.64 | 2013 | 1.704 korean healthy subjects | Elevated UA levels increase the risk of albuminuria at 5 years. |
| De Cosmo et al.65 | 2015 | 20.142 type 2 DM without established CKD | The decline in eGFR increases in parallel with the quintiles of UA levels in blood |
| Rodenbach et al.66 | 2015 | 627 children and adolescents | UA <5.5 mg/d had a risk of decreased eGFR of 0.62 (0.45−0.85 95% CI) |
| Zhou et al.67 | 2019 | 10.677 patients with normal GFR | High levels of UA are associated with a 3.29% higher risk of eGFR decline |
| Oiveira et al.68 | 2020 | 7.023 brazilian cohort | UA levels have an inverse association with eGFR. |
| Han et al.69 | 2023 | 1.326 adults with type 2 DM | Increased UA is associated with a higher risk of deteriorating kidney function. |
| Kawasoe et al.70 | 2024 | 22.7672 | Hypo/hyperuricemia levels are related to reduced eGFR showing a J-curve relationship. |
| Ding et al.71 | 2024 | 308.509 AMORIS | Hyperuricemia in eGFR <30 is associated with a 2.58% increased risk of AF |
AMORIS: Swedish Apolipoprotein-Related Mortality Risk; ARIC: Atherosclerosis Risk Communities, CHS: Cardiovascular Health Study, GFR: Glomerular Filtration Rate, CKD: Chronic Kidney Disease.
Association between hyperuricemia and risk of arterial hypertension (last 10 years).
| Study | Number of patients | RR HTN | CI (95%) |
|---|---|---|---|
| Bombelli, 201472 | 2.045 | 1,29 outpatient | 1,05−1,57 |
| 1,34 AMPA at 16 years | 1,06–1,7 | ||
| Huang et al., 2015 73 | 608 | 2,02 at 2 years | 1,94−3,92 |
| Cui et al., 2017 74 | 39.233 | 1,24 in men | 1,06−1,45 |
| 1,13 in woman at 4 year | 1,05−1,22 | ||
| Kuwabara et al., 2017 75 | 5.899 | 2,05 at 5 years | 1,51−2,79 |
| Kuwabara et al., 2017 76 | 9.721 | 1,36 at 5 years | 1,13−1,67 |
| Tomiyama et al., 2018 77 | 3.274 | 1,38 at 8 years | 1,27−1,50 |
| Kim et al., 202078 | 29.088 | HR: 1,44 women at 3,8 years | p < 0,001 |
| Lin et al., 2020 79 | 6.738 men and 766 women | HR: 2,92 women | 1,37−6,95 women |
| HR: 1,54 men | 1,37−1,72 men | ||
| Liu et al., 2021 80 | 10.537 | 1,167 at 1 year | 1,061−1,284 |
| Wang et al., 2023 81 | 31.395 | HR: 1,09 at 2,9 years | 1,08−1,1 |
| Zhu, 202482 | 1.516 | 1,44 at 5 years | 1,05−1,98 |
AMPA: self-measurement of blood pressure; HR: hazard ratio; HTN: arterial hypertension; IC: confidence interval; RR: relative risk.
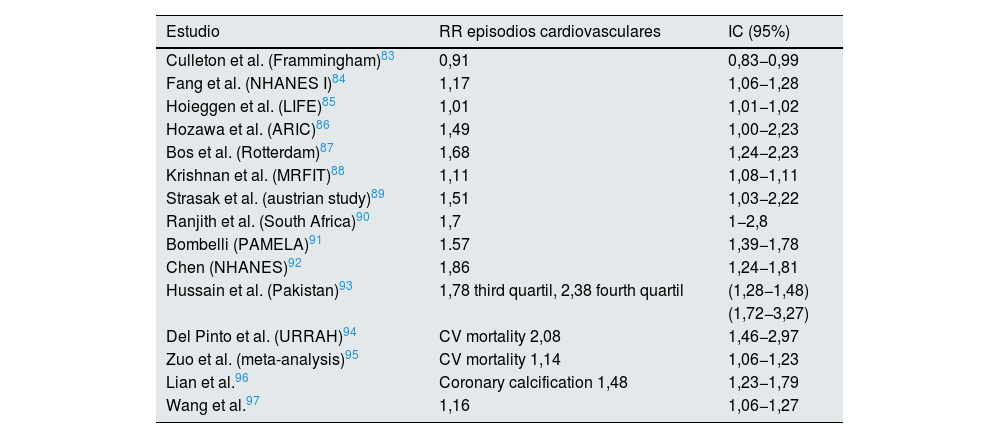
Association between hyperuricemia and cardiovascular risk in the general population.
| Estudio | RR episodios cardiovasculares | IC (95%) |
|---|---|---|
| Culleton et al. (Frammingham)83 | 0,91 | 0,83−0,99 |
| Fang et al. (NHANES I)84 | 1,17 | 1,06−1,28 |
| Hoieggen et al. (LIFE)85 | 1,01 | 1,01−1,02 |
| Hozawa et al. (ARIC)86 | 1,49 | 1,00−2,23 |
| Bos et al. (Rotterdam)87 | 1,68 | 1,24−2,23 |
| Krishnan et al. (MRFIT)88 | 1,11 | 1,08−1,11 |
| Strasak et al. (austrian study)89 | 1,51 | 1,03−2,22 |
| Ranjith et al. (South Africa)90 | 1,7 | 1−2,8 |
| Bombelli (PAMELA)91 | 1.57 | 1,39−1,78 |
| Chen (NHANES)92 | 1,86 | 1,24−1,81 |
| Hussain et al. (Pakistan)93 | 1,78 third quartil, 2,38 fourth quartil | (1,28−1,48) |
| (1,72−3,27) | ||
| Del Pinto et al. (URRAH)94 | CV mortality 2,08 | 1,46−2,97 |
| Zuo et al. (meta-analysis)95 | CV mortality 1,14 | 1,06−1,23 |
| Lian et al.96 | Coronary calcification 1,48 | 1,23−1,79 |
| Wang et al.97 | 1,16 | 1,06−1,27 |
Note: NHANES (National Health and Nutrition Examination Survey). MRFIT (Multiple Risk intervention factor trial). ARIC (Atherosclerosis Risk communities), LIFE (The Losartan Intervention For Endpoint reduction in hypertension study).
URRAH: Uric Acid Right for Heart Health, PAMELA: Pressioni Arteriose Monitorate E Loro Associazioni, CV: Cardiovascular.
Until June 2020, there were many studies published on the association between hyperuricemia, cardiovascular and renal risk. However, interventional studies were few and with limited sample size. Kanbay et al.36 demonstrate in 59 healthy subjects, that the treatment with allopurinol of patients with asymptomatic hyperuricemia results in an increase in the GFR. Talaat et al.37 in 52 patients with CKD stage 3 and 4 shows that withdrawal of allopurinol worsens hypertension and the renal function. Siu et al.,38 randomized 54 CKD stage 3 and 4 patients to receive allopurinol at doses of 100–300 mg/day for 12 months or continue with their usual therapy; the treatment with allopurinol delayed the progression of renal disease. Kao et al. treated with allopurinol (300 mg/day) 53 patients with CKD stage 3 with left ventricular hypertrophy improving endothelial function and LVH.39 Goicoechea et al. conducted a study in 113 patients with CKD40 that were randomized to continue with their usual medication or receive treatment with 100 mg/day of allopurinol for an average of 2 years. Allopurinol decreased the risk of hospitalization and it also reduced the risk of cardiovascular events by 71% and reduced the progression of renal disease at 2 years. In a post hoc analysis, after a mean follow-up period of 84 months, treatment with allopurinol reduced renal and cardiovascular events by 68% and 57% respectively.41 Subsequently, Kanbay et al.42 performed a study in 105 subjects: 72 hyperuricemic and 33 controls with normal UA levels and with normal renal function. The 72 hyperuricemic patients were randomized to receive either 300 mg allopurinol for 4 months or no treatment. The administration of allopurinol produced a decrease in uric acid level that was associated with an improvement in endothelial function (p < 0.003), in estimated glomerular filtration rate (p = 0.001) and systolic blood pressure (p = 0.001).
Shi et al.,43 in an open randomized controlled study evaluated treatment with allopurinol in 40 patients with IgA nephropathy, and they found no effect on proteinuria or in renal function, although it improved the blood pressure significantly.
In June/2020, the NEJM published two randomized clinical trials on the treatment of hyperuricemia in patients with CKD stage 3 and 4 (CKD-FIX trial)44 and in type 1 diabetic patients with albuminuria (PERL trial).45 In both populations of patients, treatment with allopurinol showed no benefit on renal function. Two considerations about these two trials: (1) the CKD-FIX included patients with rapid progression of renal disease and proteinuria whereas, in the previously published small trials, the degree of renal dysfunction and albuminuria were lower, (2) the mean UA level in the PERL clinical trial was 6.1 mg/dl, so a large percentage of patients did not have hyperuricemia and (3) in both studies the rate of dropout was high, greater than 20%. Therefore, there are still many questions to be resolved, such as what are the ideal plasma levels of UA, whether the greater reduction of UA increases the benefit or whether the beneficial effect demonstrated by xanthine oxidase inhibitors is due to their antioxidant effect or endothelial effect and which population would benefit most from a reduction of UA: early stages of CKD, interstitial or vascular and non-glomerular nephropathies, etc. Regarding a cardiovascular benefit, a randomized trial (the ALL-HEART study) found no benefit of allopurinol in patients with a history of coronary artery disease, but patients with gout were excluded and had a high dropout rate (>55%).
Therefore, some of these findings would corroborate that UA may be a modifiable factor in the progression of chronic kidney disease, without sufficient data to state whether the beneficial effect of allopurinol is due to the reduction of UA or to the antioxidant effect produced by inhibiting the xanthine oxidase enzyme, however, the two large randomized clinical trials do not offer positive results so there is insufficient evidence to recommend allopurinol treatment in patients with asymptomatic hyperuricemia.
Treatment with febuxostat in patients with asymptomatic hyperuricemiaAs observed with Allopurinol, small clinical trials have shown that febuxostat, can also offer renal protection. In an experimental study performed in 5/6 nephrectomized normo- and hyperuricemic rats, febuxostat protected against renal damage, and prevented proteinuria, preserving vessel morphology and glomerular pressure, thus it prevents progression of CKD independently of its effect on UA.46 In the FOCUS47 extension study, 116 patients treated with febuxostat were followed for 5 years. Those patients with a reduction of UA to levels below 6 mg/dL had the lowest drop in glomerular filtration rate throughout follow-up. The FEATHER48 clinical trial included 467 Japanese patients with CKD stage 3 and asymptomatic hyperuricemia who were randomized to receive febuxostat or placebo. Patients were followed for 108 weeks. Treatment with febuxostat did not reduce significantly the progression renal disease, with a fall in eGFR in the placebo group of (-0.47 ± 4.48 ml/min/1.73 m2) vs 0.23 ± 5.26 ml/min/1.73 m2 in the febuxostat group. However, febuxostat was beneficial in the group of patients without proteinuria and with lower creatinine levels, suggesting that it is more effective in patients with a less renal damage. Important limitations of this study are that it was conducted only in a Japanese population, with a short follow-up time of 2 years and in a group of patients with very little progression of renal disease, since the drop of eGFR in the placebo group was less than 1 ml/min/year.
A meta-analysis has been recently published (reference?) on the effect of UA reduction with febuxostat on the renal events and CKD progression. The meta-analysis included 16 studies including patients with asymptomatic hyperuricemia and/or gout treated with febuxostat and were compared with placebo/allopurinol, demonstrating a significant reduction in renal events (doubling of serum creatinine concentration and/or entry into renal replacement therapy): HR 0.56, 95% CI 0.37–0.84, p = 0.006 and a slight decrease in the fall of glomerular filtration rate (0.90 ml/min/1.73 m2) and in albuminuria.49 Like the observed with allopurinol, there is currently insufficient evidence to suggest treatment with febuxostat for renal or cardiovascular protection in patients with asymptomatic hyperuricemia.
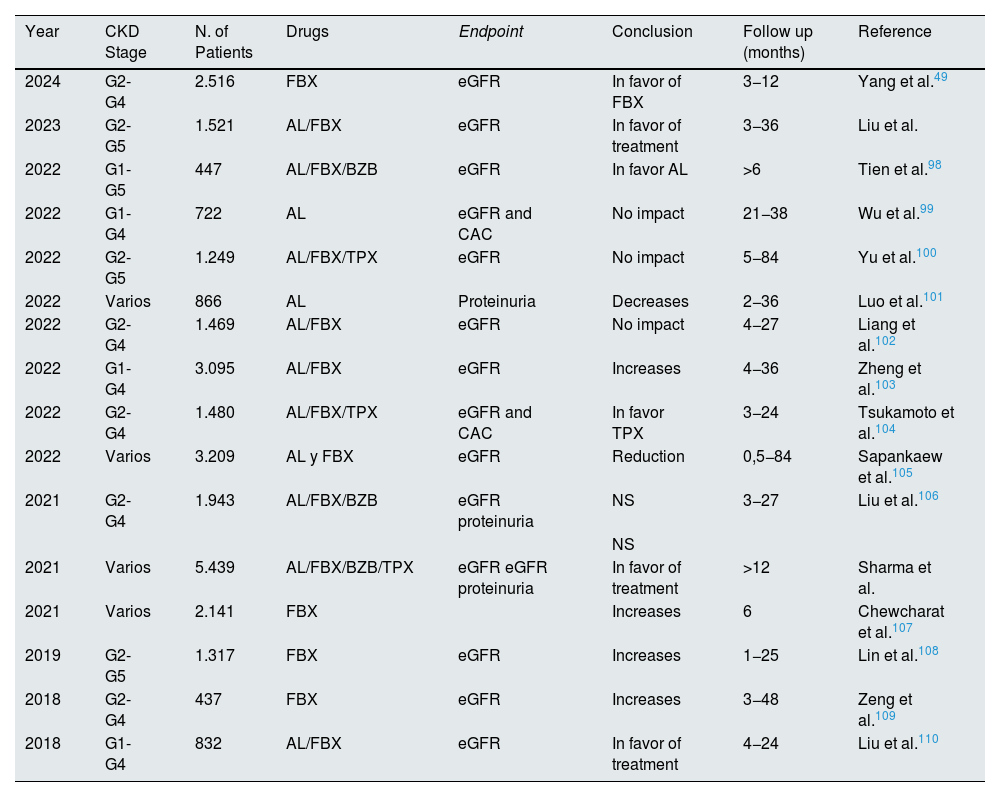
Table 5 summarizes the main meta-analyses published on the effect of hyperuricemia treatment on renal progression. The results are controversial, with considerable heterogeneity in the population evaluated, short follow-up times, and using eGFR and proteinuria as the final endpoints, rather than robust renal events. These data, together with the three published clinical trials with negative results, do not support treatment with uric acid-lowering drugs in patients with CKD to reduce progression of renal function deterioration. These meta-analyses did not include clinical trials of patients with gout. A post-hoc analysis of the CARES clinical trial was recently published, showing a reduction of CKD progression (a drop in GFR of 0.5 ml/min/year) in patients with gout treated with allopurinol or febuxostat who maintained a UA level of less than 6 mg/dL for a median of 2.5 years.50 These data could suggest that the deposits of monosodium urate crystals in the kidneys may be responsible for the progression of kidney disease. It is likely that the center of the causal association between hyperuricemia and kidney disease progression should not be on patients with asymptomatic hyperuricemia, but rather on the population with gout.51
Meta-analyses published in recent years on the effect of different urate-lowering drugs on kidney function.
| Year | CKD Stage | N. of Patients | Drugs | Endpoint | Conclusion | Follow up (months) | Reference |
|---|---|---|---|---|---|---|---|
| 2024 | G2-G4 | 2.516 | FBX | eGFR | In favor of FBX | 3−12 | Yang et al.49 |
| 2023 | G2-G5 | 1.521 | AL/FBX | eGFR | In favor of treatment | 3−36 | Liu et al. |
| 2022 | G1-G5 | 447 | AL/FBX/BZB | eGFR | In favor AL | >6 | Tien et al.98 |
| 2022 | G1-G4 | 722 | AL | eGFR and CAC | No impact | 21−38 | Wu et al.99 |
| 2022 | G2-G5 | 1.249 | AL/FBX/TPX | eGFR | No impact | 5−84 | Yu et al.100 |
| 2022 | Varios | 866 | AL | Proteinuria | Decreases | 2−36 | Luo et al.101 |
| 2022 | G2-G4 | 1.469 | AL/FBX | eGFR | No impact | 4−27 | Liang et al.102 |
| 2022 | G1-G4 | 3.095 | AL/FBX | eGFR | Increases | 4−36 | Zheng et al.103 |
| 2022 | G2-G4 | 1.480 | AL/FBX/TPX | eGFR and CAC | In favor TPX | 3−24 | Tsukamoto et al.104 |
| 2022 | Varios | 3.209 | AL y FBX | eGFR | Reduction | 0,5−84 | Sapankaew et al.105 |
| 2021 | G2-G4 | 1.943 | AL/FBX/BZB | eGFR proteinuria | NS | 3−27 | Liu et al.106 |
| NS | |||||||
| 2021 | Varios | 5.439 | AL/FBX/BZB/TPX | eGFR eGFR proteinuria | In favor of treatment | >12 | Sharma et al. |
| 2021 | Varios | 2.141 | FBX | Increases | 6 | Chewcharat et al.107 | |
| 2019 | G2-G5 | 1.317 | FBX | eGFR | Increases | 1−25 | Lin et al.108 |
| 2018 | G2-G4 | 437 | FBX | eGFR | Increases | 3−48 | Zeng et al.109 |
| 2018 | G1-G4 | 832 | AL/FBX | eGFR | In favor of treatment | 4−24 | Liu et al.110 |
Note: FBX: febuxostat, AL: Allopurinol, BZB: benzbromarone, TPX: topiroxostat (a urate-lowering drug marketed only in Japan), treatment: any urate-lowering treatment.
- none-
Hyperuricemia increases the risk of developing hypertension, cardiovascular disease, and chronic kidney disease in the general population.
- none-
In the acute treatment of gout, nonsteroidal anti-inflammatory drugs are contraindicated and colchicine should be used with caution. The most effective treatment is the use of low-dose of corticosteroids of 20−30 mg for 3−5 days.
- none-
All patients with gout and CKD should receive chronic treatment aiming to achieve UA levels below 6 mg/dL (5 mg/dL in the case of tophaceous gout). This strategy is likely to be associated with a reduction of kidney disease progression at the long term.
- none-
In the chronic treatment of hyperuricemia, the drugs of choice are xanthine oxidase inhibitors, always starting with low doses and monitoring uric acid levels until the goal is achieved.
- none-
Currently, based on published clinical trials, there is no evidence to treatment of asymptomatic hyperuricemia in patients with CKD improve renal and cardiovascular prognosis.
MG has received lecture fees from Menarini, RGM has no conflict of interest.