Fibroblast growth factor 23 (FGF23) is known to cause left ventricular hypertrophy (LVH), but controversy exists concerning its effect in dialysis. This study evaluated associations between FGF23 levels, echocardiography and prognosis in patients on hemodialysis (HD).
MethodsPatients >18 years on chronic HD were included in this cross-sectional study. Plasma C-terminal FGF23 concentration was measured with ELISA and transthoracic echocardiography was performed, both before and after HD treatment.
Results239 haemodialysis (HD) patients were included in the study. The FGF23 was median 3560RU/ml (IQR 1447–9952). The mean left ventricular mass index (LVMI) was 110.2±26.7g/m2 and the left ventricular ejection fraction (LVEF) was 52.7±9.9%. Defined by LVMI, LVH was found in 110 patients (46%), of which 92 (84%) had hypertension (p<0.01). Patients with LVH had FGF23 levels of 5319 RU/ml (IQR 1858–12,859) and those without 2496 RU/ml (IQR 1141–7028) (p<0.01). FGF23 was significant positive correlated with LVMI (p<0.01), and negatively to LVEF (p<0.01). In a multivariate analysis, FGF23 was correlated with LVEF (p<0.01), but only marginally to LVMI (p<0.01). Cardiovascular events in the follow up period was not correlated with FGF23. Furthermore, FGF23 was independently correlated with overall mortality (p<0.001).
ConclusionFGF23 was positively correlated with LVH and negatively to LVEF. FGF23 was an independent predictor for overall mortality.
Se sabe que el factor de crecimiento fibroblástico 23 (FGF23) provoca hipertrofia ventricular izquierda (HVI), pero existe controversia sobre su efecto en la diálisis. Este estudio evaluó las asociaciones entre los niveles del FGF23, la ecocardiografía y el pronóstico en pacientes en hemodiálisis (HD).
MétodosSe incluyeron pacientes >18 años con HD crónica en este estudio transversal. La concentración del FGF23 en el extremo C del plasma se midió con ELISA y se realizó una ecocardiografía transtorácica, antes y después del tratamiento de HD.
ResultadosSe incluyeron 239 pacientes en HD en el estudio. El FGF23 tenía una mediana de 3.560RU/ml (amplitud intercuartílica: 1.447-9.952). El índice de masa ventricular izquierdo (IMVI) medio fue de 110,2±26,7g/m2 y la fracción de eyección del ventrículo izquierdo (FEVI) fue del 52,7±9,9%. Definida por el IMVI, la HVI se localizó en 110 pacientes (46%), de los cuales 92 (84%) presentaban hipertensión (p<0,01). Los pacientes con HVI presentaron niveles del FGF23 de 5.319RU/ml (amplitud intercuartílica: 1.858-12.859) y aquellos sin 2.496RU/ml (amplitud intercuartílica: 1.141-7.028) (p<0,01). El FGF23 fue considerablemente positivo correlacionado con el IMVI (p<0,01) y negativo con la FEVI (p<0,01). En un análisis multivariante, el FGF23 se correlacionó con la FEVI (p<0,01), pero solo marginalmente con el IMVI (p<0,01). Los episodios cardiovasculares en el período de seguimiento no se correlacionaron con el FGF23. Además, el FGF23 se correlacionó independientemente con la mortalidad general (p<0,001).
ConclusiónEl FGF23 se correlacionó positivamente con la HVI y negativamente con la FEVI. El FGF23 fue un factor independiente para la mortalidad general.
Patients treated with chronic hemodialysis (HD) or peritoneal dialysis (PD) due to end stage renal disease (ESRD) have a massively increased risk of cardiovascular disease (CVD).1 The risk is increased by a factor of 100 in younger patients, compared to the general population.2 CVD in uremia is characterized by Mönckeberg arteriosclerosis with medial calcification,3 cardiac fibrosis, left ventricular hypertrophy (LVH), diastolic dysfunction and progressive heart failure (HF).2 Common causes of LVH are volume overload, hypertension and an increased pulse pressure secondary to arterial calcification, with consequent accelerated pulse wave velocity. A newly discovered hormone, fibroblast growth factor 23 (FGF23), has been shown to have an, apparently klotho-independent,4,5 direct effect on the heart. FGF23's primary function is to increase renal excretion of phosphate, with klotho acting as a co-factor. A proposed mechanism for FGF23's effect on the heart is the activation of FGF receptor-4 on cardiac myocytes to stimulate phospholipase Cγ/calcineurin-nuclear factor of activated T cell signaling.6
FGF23 is massively elevated in ESRD patients on HD,7 by the feedback mechanism of intractable hyperphosphatemia, and deficiency of the necessary co-factor klotho.8 FGF23 independently causes LVH in rodents5 and is associated with LVH, both in non-uremic subjects9–13 and in patients with chronic kidney disease (CKD).11,13–15 The relationship is less clear for patients with ESRD. While two studies, by Hsu et al. in HD patients16 and Sarmento-Dias et al. in PD patients,17 showed a positive relation, three other studies, failed to find a relationship.11,18,19 Systolic function has received less attention, but shows a similar picture. A relationship between FGF23 and prevalent or later heart failure (HF) is seen in non-uremic11,12,20,21 and CKD patients.11,22,23 Only two studies have addressed this question for ESRD patients11,16; neither found any relationship.
Other cardiovascular associations of FGF23 include increased arterial stiffness and atherosclerosis in rodents,24 non-uremic subjects25,26 and CKD,27 with a consequent increase in pulse pressure.24,28 FGF23 is associated with an increased risk of cardiovascular events and/or death in rodents,29 non-uremic patients,30,31 and CKD.22,23,31–34 Theoretical pathogenic mechanisms for this association exist. FGF23 is induced by renin-angiotensin-aldosterone system activation and promotes pro-fibrotic crosstalk between cardiac myocytes and fibroblasts in CKD patients.35 Calcitriol deficiency is almost universal in CKD; calcitriol treatment in uremic rats attenuates cardiac FGF23/FGFR4 and hypertrophy36 As previously mentioned, confusion exists concerning the effect of FGF23 on prognosis in ESRD patients. A relationship to death has7,37 and has not38 been found; a relationship to subsequent cardiovascular events was seen in one study,39 but not in another.33
The existence of detailed echocardiographic information for a large number of HD patients permits a definitive answer to the relationship between FGF23 and heart function and prognosis in ESRD. We hypothesized that FGF23 was related to LVH, a reduced left ventricular ejection fraction (LVEF), and an increased risk of death and cardiovascular events in HD patients.
MethodsPatientsThis cross-sectional study included patients from two dialysis centers between January and April 2014. Inclusion criteria were clinically stable patients aged 18 years or older on chronic maintenance HD for more than 2 weeks who were able to give their written informed consent. At inclusion all patients had blood samples collected and transthoracic echocardiography performed before and after a single routine HD treatment.
Patient demographics, comorbidity, treatment and biochemistry data were obtained from hospital medical files. Vital parameters (blood pressure (BP) and heart rate (HR)) were measured at the beginning of HD treatment. All-cause mortality, cause of death, cardiovascular events (de novo HFrEF, lung embolus, acute myocardial infarction, endocarditis, arrhythmias and sudden cardiac death) and time to first admission was assessed from medical files (OPUS Arbejdsplads, version 2.5.0.0 ©2010 Computer Sciences Corporation (CSC) and Epic, Sundhedsplatformen ©2016 Epic Systems Corporation). Data for drug treatment for chronic kidney disease – mineral bone disorder (CKD-MBD) were incomplete, and could not be supplemented due to changes in the electronic patient files.
Patients were followed until death or 1.1.2017. Time to first cardiovascular event (cardiovascular death, myocardial infarction, heart failure, cardiovascular operation) was registered.
Some of the data presented in this paper has been used to address another question, and has been published previously.40
Patients were receiving routine HD treatment on Gambro machines (Artis™ Dialysis System) using synthetic high flux filters >1.6m2, Polyamix® (210H or 170H Gambro Polyflux®) and Polysulfone (Fresenius FX 100, FX 80 or FX 50) filters.
FGF23 measurementBlood samples were collected from all patients from the arteriovenous fistula or the dialysis catheter before a single routine dialysis session and directly after centrifuged for 10min at 3000rpm at 20°C. Plasma was stored at −80°C in cryotubes until analysis. Plasma levels of standard biochemistry such as CRP, hemoglobin and creatinine were measured by the local clinical laboratory using standard methods (Siemens Dimension Vista 1500). Plasma concentrations of FGF23 (C-Term) were measured with a commercial available 2nd generation two-site immunosorbent assay (ELISA; Immutopics Inc., Santa Clemente, CA, USA) in January 2017. The assay used streptavidin-coated microplate wells, a biotinylated human FGF23 antibody and a horseradish peroxidase conjugated human FGF23 antibody. The bound enzyme activity was detected with tetramethylbenzidine (TMB) as substrate. The detection limit was 1.5RU/ml (assay sensitivity), and the intra-assay coefficients of variation were 2.4% (at 33.7RU/ml) and 1.4% (at 302RU/ml). The inter-assay coefficient of variation was 4.7% (at 33.6RU/ml) and 2.4% (at 293RU/ml). FGF23 is stable for 40 months in plasma samples stored at −80°C.41 The reference range of FGF23 used was mean±SD, 129±33RU/ml.
EchocardiographyTransthoracic echocardiography was performed on GE machines (Vivid S6) by three skilled echocardiographers before and after HD treatment. The examinations were then analyzed in GE software (GE Healthcare EchoPAC v113.1.3 R3) and revised by consultants in cardiology. LVH was defined as left ventricular mass index (LVMI) >115g/m2 for men and >95g/m2 for women after the Devereux formula indexed for Body Surface Area (BSA).42,43 Simpson's biplane method was used for calculating LVEF biplane.44,45 HFrEF was defined as LVEF <40%.
StatisticsNormally distributed variables were compared using student's t-test and MANOVA. Where relevant, logarithmic transformation was performed. Non-normally distributed variables were compared using Mann–Whitney U test and categorical analysis using Chi-square test. FGF23 values were divided into approximate quartiles for analysis. Pearson regression analysis was used for correlation. A probability of p<0.05 was considered significant. Significance values were classified as p<0.05, p<0.01, p<0.001and as non-significant (NS) at p>0.05. Kaplan Meier and Cox proportional hazards backward stepwise analysis was performed on follow-up data in order to identify risk factors for death and cardiac events.
EthicsThe study was performed in accordance with the Helsinki Declaration II and was approved by the The Danish National Committee on Research Ethics (H-3-2013-098) and the Danish Data Protection Agency, Copenhagen (HIH2013-027).
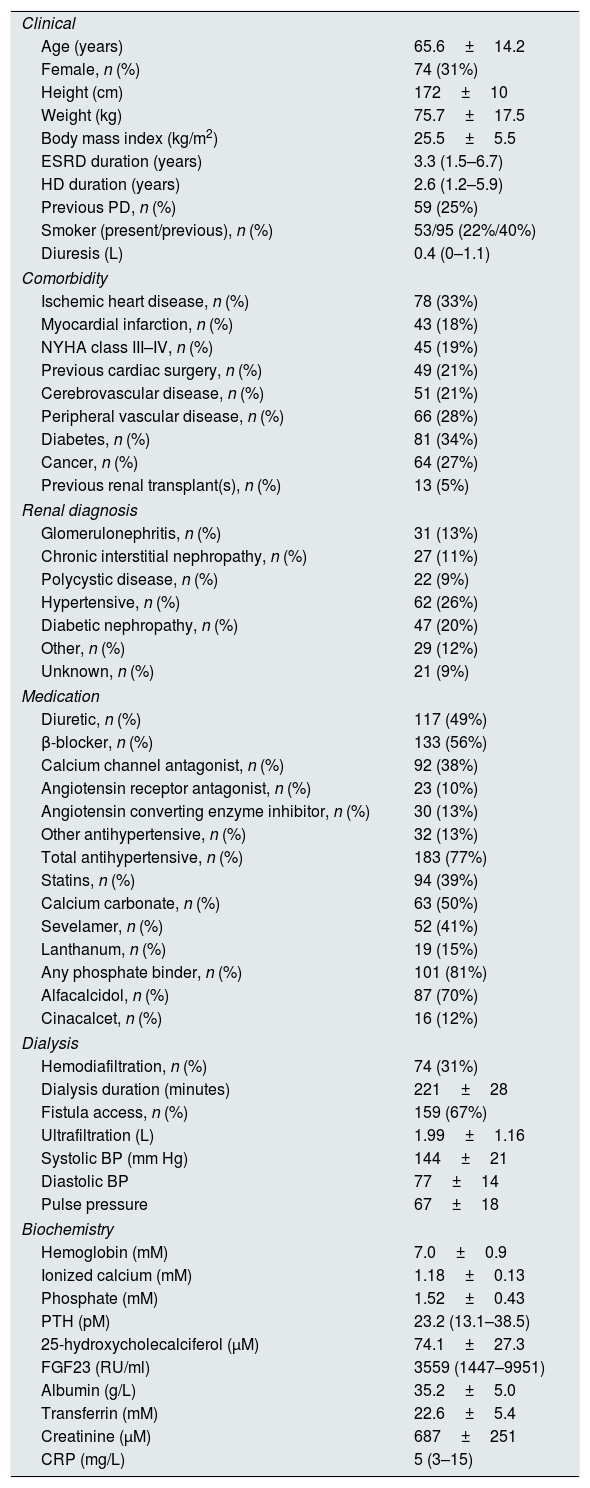
Results239 HD patients were included in the study. Patient details are shown in Table 1. Patients had median 3560RU/ml FGF23 plasma concentrations (IQR 1447-9952). Mean LVMI was 110.2±26.7g/m2. Mean septal and posterior wall thickness were 1.12±0.17cm and 1.09±0.15cm respectively, and mean LVIDd was 2.64±0.40cm/m2. The LVEF was 52.7±9.9%. 27 (12%) had echocardiographic HFrEF. Defined by Devereux formula for LVMI, LVH was found in 110 patients (46%), where 92 (84%) had hypertension (p<0.05). Patients with LVH had a median FGF23 plasma concentrations 5319RU/ml FGF23 (IQR 1858–12,859) and those without 2496RU/ml (IQR 1141–7028) (p<0.01).
Baseline characteristics, n=239.
| Clinical | |
| Age (years) | 65.6±14.2 |
| Female, n (%) | 74 (31%) |
| Height (cm) | 172±10 |
| Weight (kg) | 75.7±17.5 |
| Body mass index (kg/m2) | 25.5±5.5 |
| ESRD duration (years) | 3.3 (1.5–6.7) |
| HD duration (years) | 2.6 (1.2–5.9) |
| Previous PD, n (%) | 59 (25%) |
| Smoker (present/previous), n (%) | 53/95 (22%/40%) |
| Diuresis (L) | 0.4 (0–1.1) |
| Comorbidity | |
| Ischemic heart disease, n (%) | 78 (33%) |
| Myocardial infarction, n (%) | 43 (18%) |
| NYHA class III–IV, n (%) | 45 (19%) |
| Previous cardiac surgery, n (%) | 49 (21%) |
| Cerebrovascular disease, n (%) | 51 (21%) |
| Peripheral vascular disease, n (%) | 66 (28%) |
| Diabetes, n (%) | 81 (34%) |
| Cancer, n (%) | 64 (27%) |
| Previous renal transplant(s), n (%) | 13 (5%) |
| Renal diagnosis | |
| Glomerulonephritis, n (%) | 31 (13%) |
| Chronic interstitial nephropathy, n (%) | 27 (11%) |
| Polycystic disease, n (%) | 22 (9%) |
| Hypertensive, n (%) | 62 (26%) |
| Diabetic nephropathy, n (%) | 47 (20%) |
| Other, n (%) | 29 (12%) |
| Unknown, n (%) | 21 (9%) |
| Medication | |
| Diuretic, n (%) | 117 (49%) |
| β-blocker, n (%) | 133 (56%) |
| Calcium channel antagonist, n (%) | 92 (38%) |
| Angiotensin receptor antagonist, n (%) | 23 (10%) |
| Angiotensin converting enzyme inhibitor, n (%) | 30 (13%) |
| Other antihypertensive, n (%) | 32 (13%) |
| Total antihypertensive, n (%) | 183 (77%) |
| Statins, n (%) | 94 (39%) |
| Calcium carbonate, n (%) | 63 (50%) |
| Sevelamer, n (%) | 52 (41%) |
| Lanthanum, n (%) | 19 (15%) |
| Any phosphate binder, n (%) | 101 (81%) |
| Alfacalcidol, n (%) | 87 (70%) |
| Cinacalcet, n (%) | 16 (12%) |
| Dialysis | |
| Hemodiafiltration, n (%) | 74 (31%) |
| Dialysis duration (minutes) | 221±28 |
| Fistula access, n (%) | 159 (67%) |
| Ultrafiltration (L) | 1.99±1.16 |
| Systolic BP (mm Hg) | 144±21 |
| Diastolic BP | 77±14 |
| Pulse pressure | 67±18 |
| Biochemistry | |
| Hemoglobin (mM) | 7.0±0.9 |
| Ionized calcium (mM) | 1.18±0.13 |
| Phosphate (mM) | 1.52±0.43 |
| PTH (pM) | 23.2 (13.1–38.5) |
| 25-hydroxycholecalciferol (μM) | 74.1±27.3 |
| FGF23 (RU/ml) | 3559 (1447–9951) |
| Albumin (g/L) | 35.2±5.0 |
| Transferrin (mM) | 22.6±5.4 |
| Creatinine (μM) | 687±251 |
| CRP (mg/L) | 5 (3–15) |
Values expressed in numbers (percentage), mean±standard deviation (SD) or median (interquartile range (IQR)). ESRD=end stage renal disease; HD=hemodialysis; PD=peritoneal dialysis; NYHA=New York Heart Association; BP=blood pressure; PTH=parathyroid hormone; FGF23=fibroblast growth factor 23; CRP=C-reactive protein.
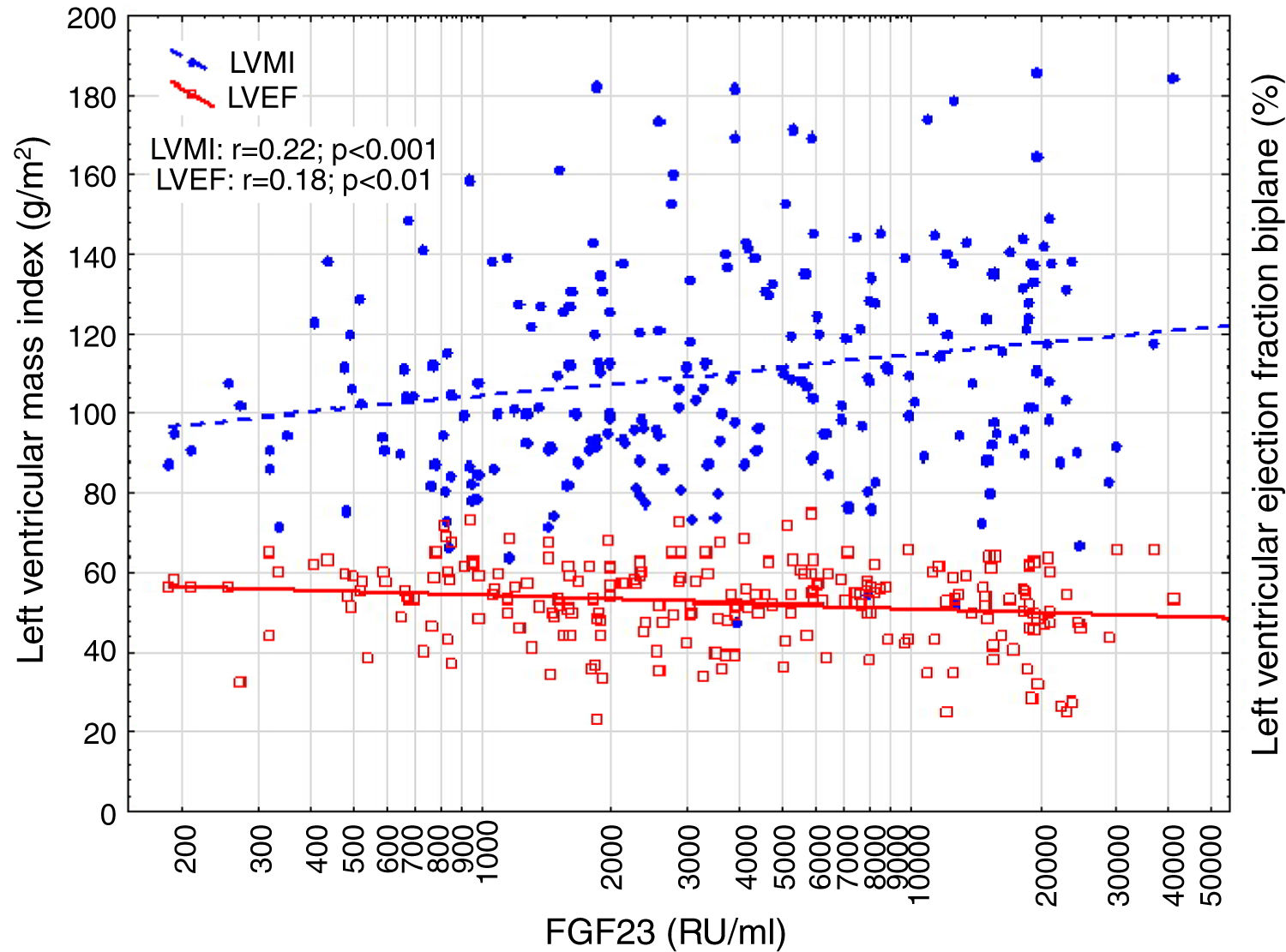
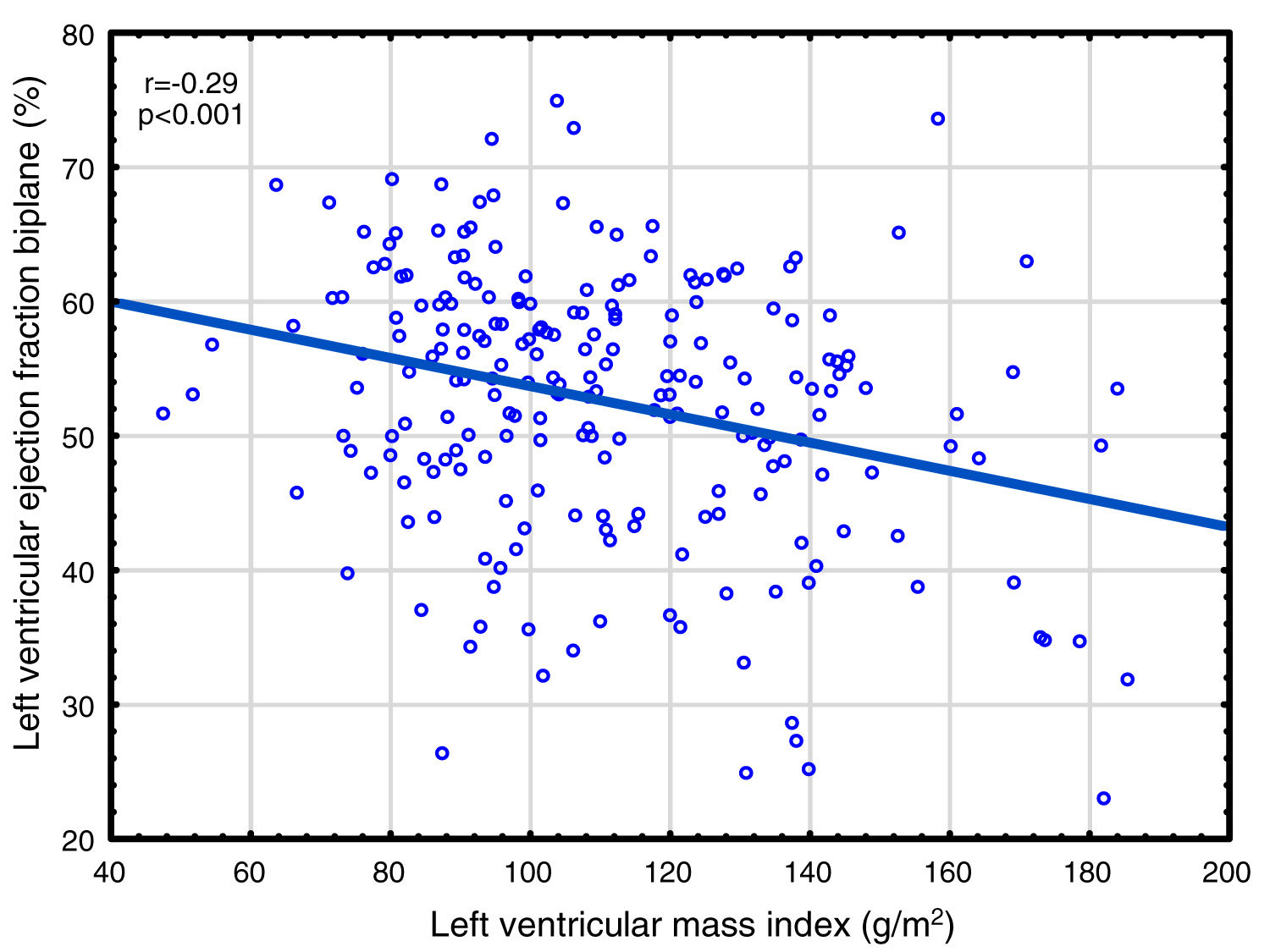
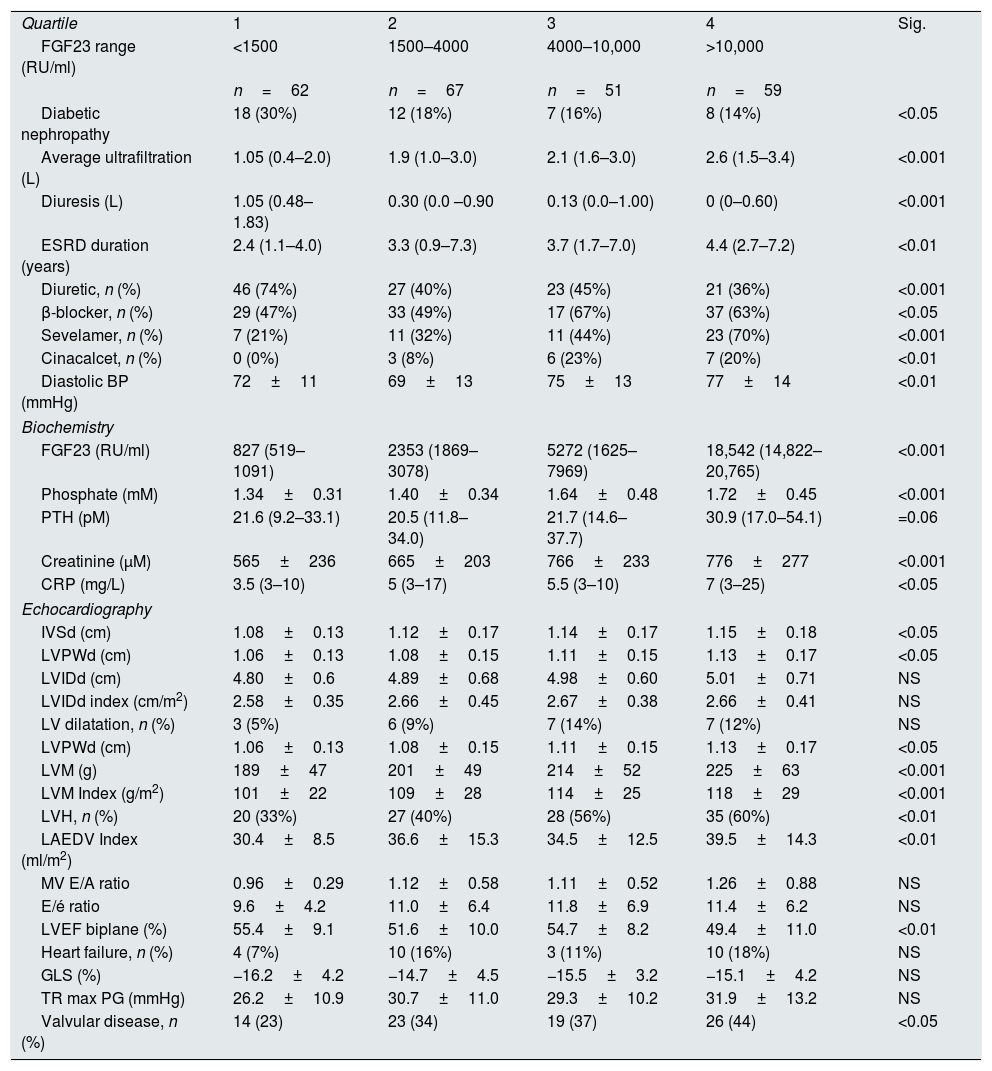
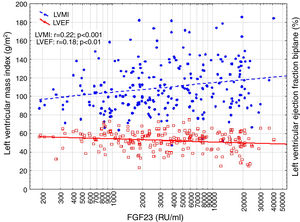
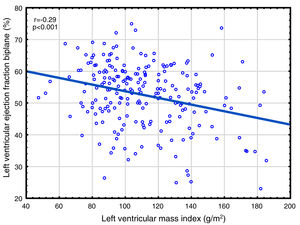
Correlations to FGF23 are shown in Table 2 and Fig. 1. Where LVMI was positively and LVEF was negatively correlated with FGF23 (Fig. 1). High FGF23 levels were associated with markers of increasing uremia (dialysis ultrafiltration, ESRD duration, p-creatinine, low diuresis and low diuretic use). They were also correlated with use of β-blockers, sevelamer and cinacalcet, diastolic blood pressure, p-phosphate, p-PTH, and C-reactive protein (CRP). They were negatively associated with presence of diabetic nephropathy. No correlation to prevalent cardiovascular disease was seen. LVMI and LVEF were negatively correlated (Fig. 2).
Relationship of FGF23 to clinical, biochemical and echocardiographic variables. Data for non-significant clinical (32 variables)* and biochemical variables (5 variables)* not shown.
| Quartile | 1 | 2 | 3 | 4 | Sig. |
| FGF23 range (RU/ml) | <1500 | 1500–4000 | 4000–10,000 | >10,000 | |
| n=62 | n=67 | n=51 | n=59 | ||
| Diabetic nephropathy | 18 (30%) | 12 (18%) | 7 (16%) | 8 (14%) | <0.05 |
| Average ultrafiltration (L) | 1.05 (0.4–2.0) | 1.9 (1.0–3.0) | 2.1 (1.6–3.0) | 2.6 (1.5–3.4) | <0.001 |
| Diuresis (L) | 1.05 (0.48–1.83) | 0.30 (0.0 –0.90 | 0.13 (0.0–1.00) | 0 (0–0.60) | <0.001 |
| ESRD duration (years) | 2.4 (1.1–4.0) | 3.3 (0.9–7.3) | 3.7 (1.7–7.0) | 4.4 (2.7–7.2) | <0.01 |
| Diuretic, n (%) | 46 (74%) | 27 (40%) | 23 (45%) | 21 (36%) | <0.001 |
| β-blocker, n (%) | 29 (47%) | 33 (49%) | 17 (67%) | 37 (63%) | <0.05 |
| Sevelamer, n (%) | 7 (21%) | 11 (32%) | 11 (44%) | 23 (70%) | <0.001 |
| Cinacalcet, n (%) | 0 (0%) | 3 (8%) | 6 (23%) | 7 (20%) | <0.01 |
| Diastolic BP (mmHg) | 72±11 | 69±13 | 75±13 | 77±14 | <0.01 |
| Biochemistry | |||||
| FGF23 (RU/ml) | 827 (519–1091) | 2353 (1869–3078) | 5272 (1625–7969) | 18,542 (14,822–20,765) | <0.001 |
| Phosphate (mM) | 1.34±0.31 | 1.40±0.34 | 1.64±0.48 | 1.72±0.45 | <0.001 |
| PTH (pM) | 21.6 (9.2–33.1) | 20.5 (11.8–34.0) | 21.7 (14.6–37.7) | 30.9 (17.0–54.1) | =0.06 |
| Creatinine (μM) | 565±236 | 665±203 | 766±233 | 776±277 | <0.001 |
| CRP (mg/L) | 3.5 (3–10) | 5 (3–17) | 5.5 (3–10) | 7 (3–25) | <0.05 |
| Echocardiography | |||||
| IVSd (cm) | 1.08±0.13 | 1.12±0.17 | 1.14±0.17 | 1.15±0.18 | <0.05 |
| LVPWd (cm) | 1.06±0.13 | 1.08±0.15 | 1.11±0.15 | 1.13±0.17 | <0.05 |
| LVIDd (cm) | 4.80±0.6 | 4.89±0.68 | 4.98±0.60 | 5.01±0.71 | NS |
| LVIDd index (cm/m2) | 2.58±0.35 | 2.66±0.45 | 2.67±0.38 | 2.66±0.41 | NS |
| LV dilatation, n (%) | 3 (5%) | 6 (9%) | 7 (14%) | 7 (12%) | NS |
| LVPWd (cm) | 1.06±0.13 | 1.08±0.15 | 1.11±0.15 | 1.13±0.17 | <0.05 |
| LVM (g) | 189±47 | 201±49 | 214±52 | 225±63 | <0.001 |
| LVM Index (g/m2) | 101±22 | 109±28 | 114±25 | 118±29 | <0.001 |
| LVH, n (%) | 20 (33%) | 27 (40%) | 28 (56%) | 35 (60%) | <0.01 |
| LAEDV Index (ml/m2) | 30.4±8.5 | 36.6±15.3 | 34.5±12.5 | 39.5±14.3 | <0.01 |
| MV E/A ratio | 0.96±0.29 | 1.12±0.58 | 1.11±0.52 | 1.26±0.88 | NS |
| E/é ratio | 9.6±4.2 | 11.0±6.4 | 11.8±6.9 | 11.4±6.2 | NS |
| LVEF biplane (%) | 55.4±9.1 | 51.6±10.0 | 54.7±8.2 | 49.4±11.0 | <0.01 |
| Heart failure, n (%) | 4 (7%) | 10 (16%) | 3 (11%) | 10 (18%) | NS |
| GLS (%) | −16.2±4.2 | −14.7±4.5 | −15.5±3.2 | −15.1±4.2 | NS |
| TR max PG (mmHg) | 26.2±10.9 | 30.7±11.0 | 29.3±10.2 | 31.9±13.2 | NS |
| Valvular disease, n (%) | 14 (23) | 23 (34) | 19 (37) | 26 (44) | <0.05 |
Values expressed in numbers (percentage), mean±standard deviation (SD) or median (interquartile range (IQR)). FGF23=fibroblast growth factor 23; ESRD=end stage renal disease; BP=blood pressure; PTH=parathyroid hormone; CRP=C-reactive protein; IVSd=interventricular septum end diastolic diameter; LVPWd=left ventricular posterior wall end diastolic diameter; LVIDd=left ventricular internal end diastolic diameter; LV=left ventricle; LVPWd=left ventricular posterior wall end diastolic diameter; LVH=left ventricular hypertrophy, LAEDV=left atrium end diastolic volume; MV=mitral valve; LVEF=left ventricular ejection fraction; GLS=global longitudinal strain; TR=tricuspid regurgitation; PG=peak gradient. *See Table 1
FGF23 was positively correlated with interventricular septum end diastolic diameter (IVSd), left ventricular posterior wall end diastolic diameter (LVPWd), LVMI, left atrium end diastolic volume (LAEDV), and negatively to LVEF. There were no significant correlations to LVIDd, mitral valve E/A ratio (MV E/A), mitral valve E/é ratio (MV E/é), global longitudinal strain (GLS) or tricuspid regurgitation peak gradient (TR maxPG). Patients with an FGF23 >10,000RU/ml had a LVMI 17g/m2 (17%) greater than patients <1500RU/ml and a LVEF that was 6 percent points (11%) lower.
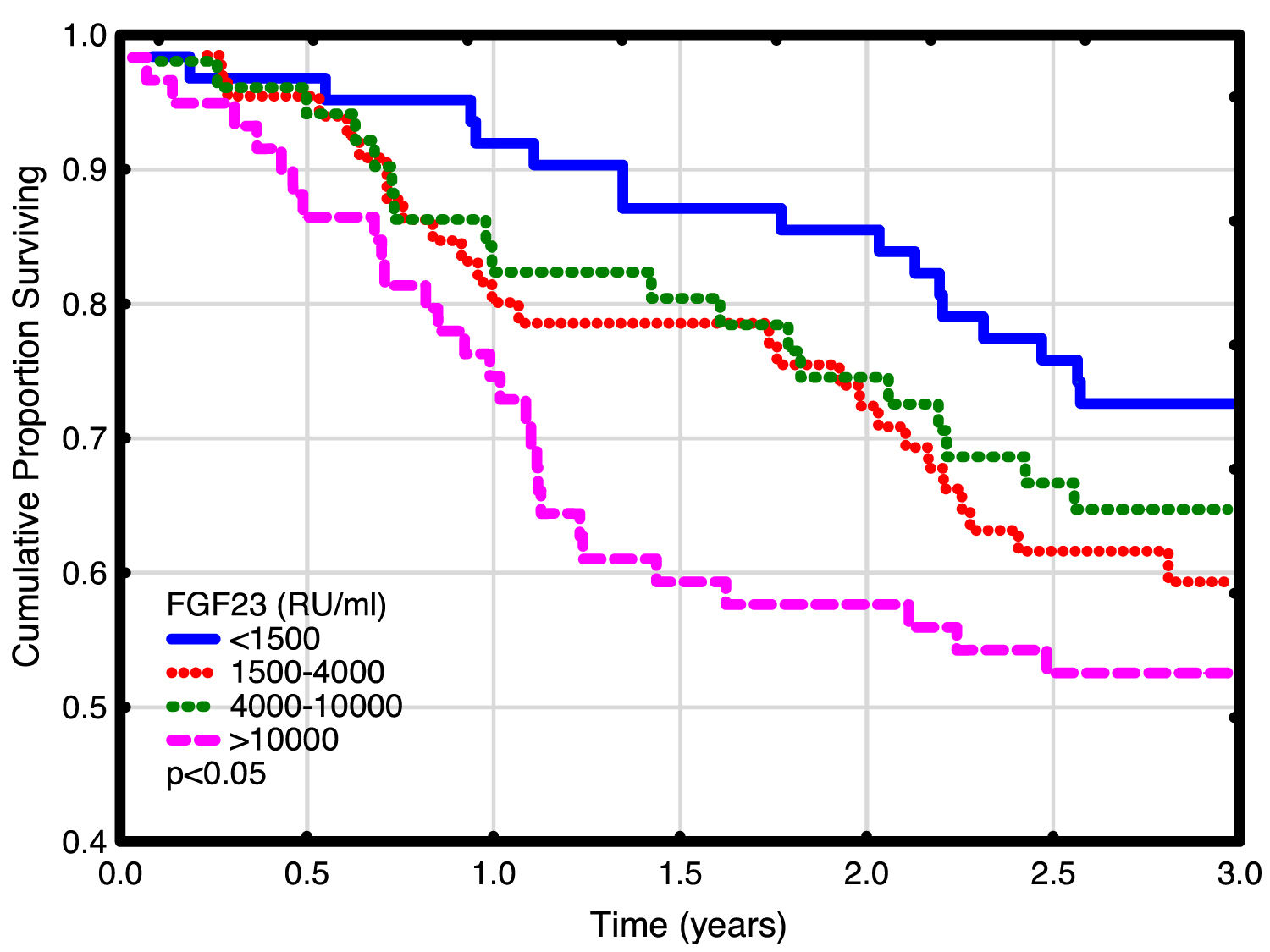
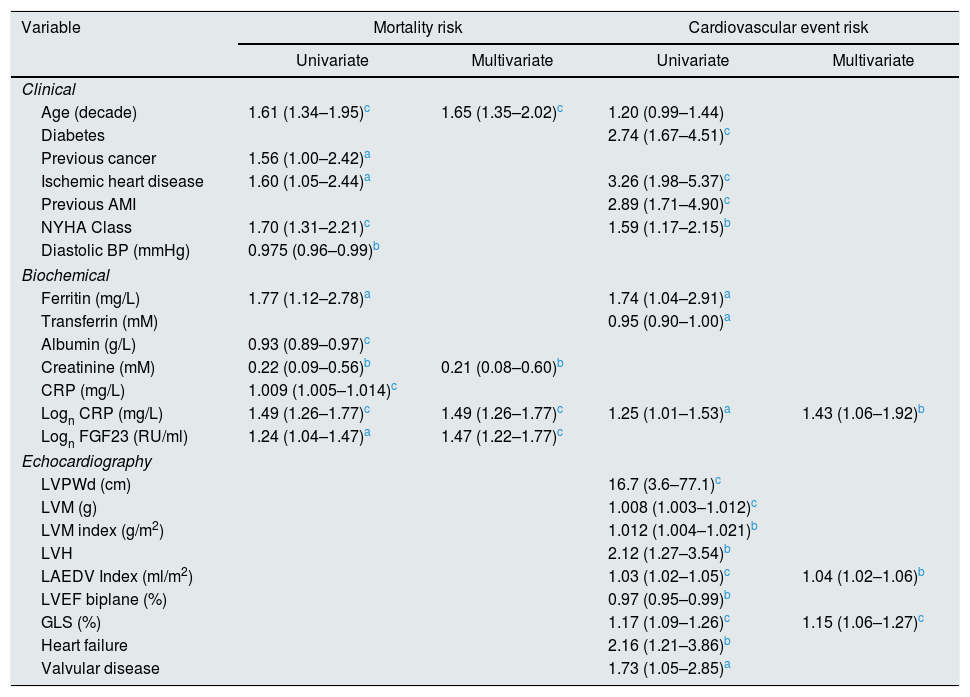
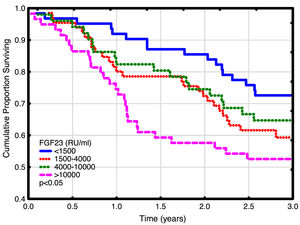
Univariate and multivariate correlations to patient death and cardiovascular event risk are shown in Table 3. There were 89 deaths (37%) and 63 (26%) cardiovascular events: endocarditis 7, cardiac valve operation 2, peripheral vascular operation 12, cardiac vascular operation 6, myocardial infarction 6, de novo HFrEF (LVEF <40% described in the patient records) 16 and cardiovascular death 14. FGF23, but not LVMI was significantly correlated with patient survival (Fig. 3). While patients without echocardiographic HFrEF had similar patient survival rates regardless of LVEF (2-year survival 76%), but patients with HFrEF had significantly lower survival (48%, p<0.05). Adjusted mortality risk ratios for FGF23 groups compared to FGF23 <1500 RU/ml were: FGF23 1500–4000RU/ml 1.85 (95% CI 0.99–3.45, NS); 4000–10,000RU/ml 3.00 (1.46–6.13, p<0.01); >10,000RU/ml 3.76 (1.98–7.14, p<0.001). Independent risk factors for mortality were age, p-creatinine (protective), CRP and FGF23. Independent risk factors for cardiovascular events were CRP, LAEDV index and global longitudinal strain (GLS). Differences between the correlations to patient mortality and cardiovascular even risk were noted. Patient mortality was primarily related to clinical and biochemical variables, whereas cardiovascular risk was primarily related to echocardiographic findings, previous cardiovascular disease, and markers of inflammation (CRP, ferritin) and malnutrition (low transferrin). FGF23 was not associated to cardiovascular event risk. 37 (15%) patients developed arrhythmias, primarily atrial fibrillation. There was no relationship between FGF23 and prevalent or incident arrhythmia.
Correlations to mortality and cardiovascular events. Relative risk (confidence interval). Significant variables only. Pearson correlation analysis.
| Variable | Mortality risk | Cardiovascular event risk | ||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Clinical | ||||
| Age (decade) | 1.61 (1.34–1.95)c | 1.65 (1.35–2.02)c | 1.20 (0.99–1.44) | |
| Diabetes | 2.74 (1.67–4.51)c | |||
| Previous cancer | 1.56 (1.00–2.42)a | |||
| Ischemic heart disease | 1.60 (1.05–2.44)a | 3.26 (1.98–5.37)c | ||
| Previous AMI | 2.89 (1.71–4.90)c | |||
| NYHA Class | 1.70 (1.31–2.21)c | 1.59 (1.17–2.15)b | ||
| Diastolic BP (mmHg) | 0.975 (0.96–0.99)b | |||
| Biochemical | ||||
| Ferritin (mg/L) | 1.77 (1.12–2.78)a | 1.74 (1.04–2.91)a | ||
| Transferrin (mM) | 0.95 (0.90–1.00)a | |||
| Albumin (g/L) | 0.93 (0.89–0.97)c | |||
| Creatinine (mM) | 0.22 (0.09–0.56)b | 0.21 (0.08–0.60)b | ||
| CRP (mg/L) | 1.009 (1.005–1.014)c | |||
| Logn CRP (mg/L) | 1.49 (1.26–1.77)c | 1.49 (1.26–1.77)c | 1.25 (1.01–1.53)a | 1.43 (1.06–1.92)b |
| Logn FGF23 (RU/ml) | 1.24 (1.04–1.47)a | 1.47 (1.22–1.77)c | ||
| Echocardiography | ||||
| LVPWd (cm) | 16.7 (3.6–77.1)c | |||
| LVM (g) | 1.008 (1.003–1.012)c | |||
| LVM index (g/m2) | 1.012 (1.004–1.021)b | |||
| LVH | 2.12 (1.27–3.54)b | |||
| LAEDV Index (ml/m2) | 1.03 (1.02–1.05)c | 1.04 (1.02–1.06)b | ||
| LVEF biplane (%) | 0.97 (0.95–0.99)b | |||
| GLS (%) | 1.17 (1.09–1.26)c | 1.15 (1.06–1.27)c | ||
| Heart failure | 2.16 (1.21–3.86)b | |||
| Valvular disease | 1.73 (1.05–2.85)a | |||
p<0.001. AMI=acute myocardial infarction; NYHA=New York Heart Association; BP=blood pressure; CRP=C-reactive protein; FGF23=fibroblast growth factor 23; LVPWd=left ventricular posterior wall end diastolic diameter; LVIDd=left ventricular internal end diastolic diameter; LV=left ventricle; LVPWd=left ventricular posterior wall end diastolic diameter; LVM=left ventricular mass; LVH=left ventricular hypertrophy, LAEDV=left atrium end diastolic volume; LVEF=left ventricular ejection fraction; GLS=global longitudinal strain.
Correlations to LVMI and LVEF are shown in Table 4. Independent correlations to LVMI were presence of cardiac arrhythmia, peripheral vascular disease and p-phosphate. While log FGF23 was highly correlated with LVMI on a univariate analysis, it was only marginally significant on the multivariate analysis (r=0.13, p=0.05), and only if arrhythmia and peripheral vascular disease were forced out of the model. A post hoc multivariate analysis of LVM however showed an independent effect of FGF23. (r=0.16, p<0.05). In contrast, phosphate was not correlated with LVEF on univariate and multivariate analyses. The independent correlates were age, log FGF23 and β-blocker treatment.
Regression correlations to left ventricular mass index (LVMI) and left ventricular ejection fraction (LVEF). Pearson correlation R values. Significant variables only.
| Variable | LVMI | LVEF | ||
|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate* | |
| Clinical | ||||
| Age | −0.16b | −0.16b | ||
| Diabetes | 0.13a | |||
| Diuresis | −0.21b | |||
| Peripheral vascular disease | 0.16a | 0.17b | ||
| Hypertension | 0.20b | 0.15a | ||
| Arrhythmia | 0.19b | 0.21c | −0.28c | |
| Pulse (bpm) | −0.22c | |||
| O2 Saturation (%) | 0.19b | |||
| Heart failure | 0.25c | −0.27c | ||
| NYHA Class | −0.20b | |||
| Dialysis ultrafiltration | 0.27c | −0.18b | ||
| Medication | ||||
| Diuretic | 0.16a | |||
| β-blocker | 0.18b | 0.18b | 0.20b | |
| RAAS blockade | 0.18b | |||
| Calcium antagonist | 0.19b | |||
| α-blocker | 0.14a | |||
| Minoxidil | 0.16a | |||
| Biochemical | ||||
| Creatinine (μM) | 0.15a | |||
| Logn CRP (mg/L) | −0.14a | |||
| Phosphate (mM) | 0.25c | 0.20c | ||
| Logn FGF23 (RU/ml) | 0.21c | −0.17b | −0.21c | |
| Echocardiography | ||||
| IVSd (cm) | 0.44c | |||
| LVIDd (cm) | 0.66c | −0.33c | ||
| LVIDd index (cm/m2) | 0.66c | −0.27c | ||
| LV dilatation | 0.40c | −0.15a | ||
| LVPWd (cm) | 0.49c | −0.26c | ||
| LVM (g) | NI | −0.29c | ||
| LVM Index (g/m2) | NI | −0.29c | ||
| LVH | NI | −0.27c | ||
| LAEDV Index (ml/m2) | 0.43c | −0.37c | ||
| LVEF biplane (%) | −0.29c | NI | ||
| MV E/A | 0.28c | −0.27c | ||
| MV E/é | 0.14a | −0.17a | ||
| GLS (%) | 0.29c | −0.51c | ||
| TR maxPG (mmHg) | 0.21b | −0.22b | ||
Echocardiography variables not included. NI=not included; NYHA=New York Heart Association; RAAS=renin-angiotensin-aldosterone system; CRP=C-reactive protein; FGF23=fibroblast growth factor 23; IVSd=interventricular septum end diastolic diameter; LVIDd=left ventricular internal end diastolic diameter index; LV=left ventricle; LVPWd=left ventricular posterior wall end diastolic diameter; LVM=left ventricular mass; LVH=left ventricular hypertrophy; LAEDV=left atrium end diastolic volume; LVEF=left ventricular ejection fraction; MV E/A=mitral valve E/A ratio; MV E/é=mitral valve E/é ratio; GLS=global longitudinal strain; TR max PG=tricuspid regurgitation max peak gradient.
Changes in variables during dialysis were correlated with FGF23. Only two echocardiographic variables were significantly correlated with FGF23 during dialysis: change in LVM (r=0.24, p<0.05) and LVEF (r=−0.15, p<0.05).
Parathyroid hormone (PTH) was negatively related to LVIDd (r=−0.14, p<0.05) and GLS (r=−0.18, p<0.05), but not to any other echocardiographic variable.
DiscussionAs previously reported,7 we found extremely elevated FGF23 levels in HD patients compared to levels in healthy subjects. The prevalence of LVH in HD patients based on transthoracic echocardiography findings was 46% and FGF23 was associated to the presence and degree of LVH.
Hyperphosphataemia due to reduced renal clearance is almost universal in ESRD. Hyperphosphatemia is an important component of the disease complex called Chronic Kidney Disease – Mineral Bone Disorder (CKD-MBD), characterized by biochemical, bone and vascular disorders. Elements of this disease include hypercalcemia, hypocalcaemia, hyperparathyroidism, osteitis fibrosa, adynamic bone disease, low 25-hydroxycholecalciferol (25-OHD), low 1,25-dihydroxycolecalciferol (1,25-OHD), reduction of calcification inhibitors, and vascular calcification. Hyperphosphatemia can itself cause vascular deposition of calcium phosphate, but more importantly causes a phenotypic conversion of smooth muscle cells to osteoblasts.46 Plasma phosphate is positively correlated with LVH.47–49
LVH and increased myocardial fibrosis is primarily caused by hypertension, chronic secondary hyperaldosteronism and damage by unidentified uremic toxins. FGF23 also causes LVH5 independently of phosphate effects. FGF23 antagonist treatment reduces LVH in rodents.50 The effect is both direct and secondary to volume overload secondary to sodium retention18,51 and activation of the renin-angiotensin system (RAS).35,52
FGF23 is an element of CKD-MBD, and interacts with 1,25-OHD and parathyroid hormone (PTH). FGF23 suppresses PTH production,53 which in turn stimulates FGF23.54 FGF23 suppresses renal production of 1,25-OHD,55 while 1,25-OHD increases FGF23 production,56 attenuates FGF23/FGFR4 signaling and LVH in uremic rats.36 Overall, FGF23 is positively correlated with PTH in ESRD, presumably due to its suppressive effects on 1,25-OHD and PTHs stimulatory effects. PTH is also correlated with LVH in non-uremic subjects57–59 and ESRD patients in one study, but not another.47,60 Vitamin D appears to have a protective effect against LVH.61,62 Thus, it is not clear which of these four factors are most important in the development of LVH: phosphate, low 1,25-OHD or FGF23.
As discussed in the Introduction, controversy exists concerning the relationship of FGF23 to cardiovascular disease in ESRD. The present paper presents the largest study on this subject, almost doubling the available data. We found significant relationships between FGF23 and LVMI, LVEF, mortality and cardiovascular events. This is the first study reporting a negative relationship between LVEF and FGF23 in ESRD patients. This is perhaps surprising, since continuing LVH leads to maladaptive cardiomyocytes changes, cardiomyocytes death and myocardial fibrosis,63 and eventually to systolic dysfunction, as illustrated in Fig. 2. However, when employing multivariate analysis, the correlation to cardiovascular events disappeared. LVMI was mainly correlated with phosphate; after correction for this, the effect of FGF23 was marginal. LVMI and LVEF were not correlated with dialysis duration, suggesting that the correlations are not due to temporal factors. As the study was a cross-sectional study, any discussion of causation must be speculative and there is risk of confounding regarding the interpretation of the findings in the study. However, the lack of correlation of PTH to most of these variables suggest that this is not an important factor, and that previous correlations are mainly due to indirect effects, e.g. via FGF23 stimulation. Similarly, phosphate does not appear to have a pathogenic role in LVEF, but seems to be important to LVMI. Vitamin D was not evaluated in this study.
Other variables were also included in the investigation. As previously discussed, FGF23 is implicated in the development of vascular calcification. In this study, FGF23 was not related to pulse pressure, used as a marker of vascular calcification, but was associated with presence of valvular abnormalities (as valvular sclerosis), another marker of vascular calcification. Not surprisingly, echocardiographic variables were highly correlated with cardiovascular events, and LAEDV index and GLS, an advanced Doppler measure of LV's systolic function, remained significant in the multivariate analysis. The contractile function of the left atrium is important for cardiac function, in particular in patients with reduced LVEF, and reduced LAEDV is predictive of atrial fibrillation, cerebrovascular events, acute coronary syndromes, heart failure and mortality in non-uremic subjects.64 Increased LAEDV is correlated with death both in HD65 and PD66 patients. This study confirms these findings. Since FGF23 was correlated with LAEDV, it may also play a role in this problem. Finally FGF23 is predictive of atrial fibrillation in non-uremic,12 CKD,67 and ESRD37 patients; we were unable to confirm this finding.
Strengths and limitationsThis study is a cross-sectional study, with all the limitations that such a study entails. In particular, any discussion of possible causative mechanisms must be speculative. Our result showed a significant correlation between reduced LVEF and FGF23 which is a new finding but a pathological mechanism is not yet clear. Therefore further investigations between systolic function of left ventricle and FGF23 and its receptors in myocardial cells should clarify possible mechanisms. Our study was designed to evaluate LVH, ejection fraction and FGF23 in a large hemodialysis population, but for minimizing possible confounders a prospective cohort study could be a better option for a future study design, thereby looking at the level of FGF23 and the process in developing LVH in HD patients.
For acquisition and interpretation of the transthoracic echocardiography there were used three echocardiographers, which had the same amount of training and were trained at the same echocardiography laboratory to minimize any bias. Both acquisition and interpretation were done blinded for the study results.
Our study did consider the effect of dialysis duration on our findings, and showed no correlation between our echocardiography results and the duration of HD. But for a better understanding of fluid removal and volume overload on echocardiography findings it could have an impact on these results if corrected for accumulated time in hemodialysis per week or Kt/V, which we did not investigate in our study. It would also be of interest to investigate the effect of sodium and volume overload, but unfortunately sodium was not sufficient for our study. Almost all included patients had three days in HD per week and mostly during weekdays, with weekends without undergoing HD therapy. In the study almost all patients were included on weekdays.
Due to removal of patient files, some information concerning CKD-MBD therapy was deficient and could therefore not be investigated further. It is possible that drugs in CKD-MBD could have an effect on the results presented in the study.
ConclusionFGF23 levels were extremely high in HD patients compared to levels in healthy subjects. The prevalence of LVH in HD patients was 46% and echocardiographic HFrEF, defined by LVEF <40%, was 12%. FGF23 was associated to LVH as well as reduced LVEF. FGF23 predicted overall mortality but not cardiovascular events.
ContributionsTN wrote the manuscript, performed the statistical analyses and participated in the acquisition of data. LP, PW, KI participated in the acquisition of data and participated in the interpretation of data. OHM performed the blood analyses on FGF23. KI and JH had the original idea for the study and wrote the protocol. All authors critically revised the manuscript and approved the final version. TN is the guarantor of the paper.
Conflict of interestsThe authors declare no conflicts of interests.