Lupus nephritis (LN) is a serious manifestation of systemic lupus erythematosus that can lead to end-stage renal disease. Many clinical and prognostic data on which our therapeutic decisions are based come from international cohorts, which have important ethnic and prognostic differences. To identify clinical and prognostic data from patients with LN in Spain, we undertook a bibliographic search of NL-related papers by Spanish authors and published in national and international journals between 2005 and 2022. According to the selected references, renal biopsy is not only essential for LN diagnosis but its repetition can be useful for the follow-up. Regarding LN treatment, standard strategy consists of an induction phase and a maintenance phase. However, as new drugs have been released, a new paradigm of treatment in a single, continuing and personalized phase has been proposed.
La nefritis lúpica (NL) es una manifestación grave del lupus eritematoso sistémico que puede llevar a una enfermedad renal terminal. La mayor parte de los datos clínicos y pronósticos que manejamos y sobre los que tomamos decisiones terapéuticas proceden de cohortes internacionales con importantes diferencias étnicas y relativas al pronóstico renal. Para conocer los datos clínicos y pronósticos de los pacientes con NL en España, se realizó una búsqueda bibliográfica de artículos relacionados con la NL publicados por autores españoles en revistas nacionales e internacionales entre 2005 y 2022. Las referencias seleccionadas mostraron que la biopsia no solo es clave en el diagnóstico de la NL, sino que su repetición puede ser útil en el seguimiento. En cuanto al tratamiento, el abordaje estándar de la NL consiste en una fase de inducción y una fase de mantenimiento. Sin embargo, la aparición de nuevos fármacos ha motivado que se postule un nuevo paradigma de tratamiento en una sola fase continuada y personalizada.
Lupus nephritis (LN) is a severe and frequent manifestation of systemic lupus erythematosus (SLE) which is caused by renal deposition of immunocomplexes.1 It affects a high percentage of SLE patients, from 35–50%2 to 75%,3 depending on the population studied and the diagnostic criteria and methods used.3 It usually manifested during the first decade of SLE evolution2 and is more frequent in women.4
The LN is associated with significant morbidity and mortality.2 Multiple demographic, clinical and serological factors influence the evolution of LN, in aspects such as response to treatment and the appearance of flares and progression to end-stage renal disease (ESRD).5 Factors associated with ESRD include male sex, younger age at onset, and African, Asian or Hispanic American ethnicity,4 and also the presence of immunological markers such as -anti-C1q antibodies.4 Several genes predisposing to the development of LN have been identified, as well as environmental, hormonal and epigenetic triggers.1 Early diagnosis and treatment are key to avoid progression to ESRD.6
According to data from the SLE patient registry of the Spanish Society of Rheumatology (RELESSER registry) in 2016, the efficacy of LN treatments was insufficient. A 31.7% of patients with LN did not present a complete response to standard treatment, a 17.9% of patients had active renal disease and a 10.35% developed of ESRD. Of the latter, 45% had renal transplantation.7 However, in recent years the diagnosis and especially the treatment of LN have undergone a number of changes thanks to the research performed on pathophysiology of LN and the development of new drugs.
The aim of of the present study is to find out the clinical and prognostic data of patients with LN in Spain, as well as the evolution of diagnosis and therapeutic strategies in LN, as reflected in publications by Spanish authors.
MethodsIn March 2022 we searched for LN-related articles published by Spanish authors between 2005 and 2022 and indexed in the PubMed, Science Direct, MEDES and SciELO databases. In general terms, the search strategy was based on the keywords "lupus" and "nephritis OR nephropathy" in the title or abstract, together with "Spain[Affiliation]". An example search in PubMed was "((lupus[Title/Abstract]) AND (nephritis[Title/Abstract] OR nephropathy[Title/Abstract])) AND (Spain[Affiliation]) Filters: from 2005−2022".
A total of 327 references were obtained with at least one Spanish author with affiliation in Spain. The references were entered in a bibliographic manager, which made it possible to detect and eliminate repeated references. The manuscripts were then tabulated and transferred to an Excel spreadsheet, including abstracts. Publications by Spanish authors with affiliation outside Spain, conference abstracts, case reports and articles dedicated exclusively to patients with pediatric SLE were excluded. Finally, 195 references were selected, of which only publications referring to the diagnosis and treatment of LN were included, as well as some epidemiological studies to determine the profile of patients with LN in Spain.
ResultsClinical profile of adult patients with lupus nephritis in SpainThe major source of information on the characteristics of patients with LN in Spain is the RELESSER registry. Of 3575 patients with SLE, 1092 (30.5%) had LN confirmed by renal biopsy. The most frequent histological class was proliferative (iii and iv) (67.3%). Regarding patient characteristics, 90.2% were of Caucasian ethnicity and 85.7% were women. However, 48% of the male patients included in the RELESSER registry presented with LN compared to 31% of the women, so that the risk of LN in men was almost 3 times higher than in women (OR 2.57 [95% CI: 2.023−0.29], p < 0.001).7 In the cohort studied by the Systemic Lupus International Collaborating Clinics (SLICC) group, with Spanish participation, LN was also more frequent in men (44.3%) than in women (29.3%).4 In the RELESSER registry, the risk of LN was also increased in patients with early-onset SLE, before the age of 16 years, being more than 6 times higher than in patients with onset after the age of 50 years (OR 6.06 [95% CI: 4.298−0.56], p < 0.001). Likewise, the risk og LN in Hispanic-American patients almost doubled that of Caucasian patients (OR 1.85 [95% CI: 1.372−0.51], p < 0.001).7
Regarding renal prognosis, 68% of patients with LN in the RELESSER registry had a complete response to treatment, defined as normalization of urinalysis and serum creatinine. However, 10.35% of patients developed ESRD, defined according to one or more of the following criteria: the SLICC and American College of Rheumatology damage index, the need for dialysis and the need for renal transplantation. The group of patients with ESRD had a high morbidity and mortality. Compared with patients without renal involvement, patients with LN had a more than 2-fold increased risk of cardiovascular and cerebrovascular events (OR 2.69 [95% CI: 1.–43.44], p < 0.001) and more than 3-fold increased risk of mortality (OR 3.7 [95 CI: % 2.584−0.95], p < 0.001).7 Renal prognosis in LN was also studied with data from 1648 patients with a histological diagnosis of LN included in the Spanish Registry of Glomerulonephritis of the Spanish Society of Nephrology (S.E.N.). It was found that the main risk factors for ESRD were, age (p < 0.001), male sex (p = 0.005), arterial hypertension (p < 0.001) and histological subtypes of LN III and IV (p = 0.009), in addition to elevated proteinuria (p < 0.001). In patients with an estimated glomerular filtration rate (eGFR) <0.60 ml/min/1.73 m2, the mean proteinuria was 4.3 ± 3.1 g/d, whereas in those with an eGFR ≥ 60 ml/min/1,73 m2 proteinuria was 3.2 ± 2.7 g/d (p < 0.001).8 Furthermore, a poor prognosis of renal function has been related to data obtained from the renal biopsy such as the degree of tubulointerstitial inflammation9 and tubulointerstitial fibrosis.1 By contrast, a proteinuria <0.7−0.8 g/d at 12 months of treatment indicates a good prognosis, as occurred in the Euro-Lupus10 and MAINTAIN11 studies, both with Spanish participation.
It should be taken into account that some genetic factors increase the risk of LN,12 especially in those with African-American, Asian and Hispanic-American ethnicity as compared to patients of Caucasian ethnicity.12 Other factors, such as cultural and socioeconomic factors,13 also influence the risk of LN. Therefore, the heterogeneity of the Spanish population with respect to ethnicity and other demographic factors may condition clinical practice in our country. In Spain, since 1996, there has been a progressive and statistically significant (p < 0.001) increase in patients of Hispanic American descent,7 which in 2016 accounted for 7.5% of patients with LN in the RELESSER registry.7 The LN population that was neither Caucasian nor of Hispanic American origin accounted for only 2.4% of all patients.7 However, the increase in Hispanic-American ethnicity could lead to a higher incidence of LN in Spain in the coming years.
Diagnosis of lupus nephritisDue to the variability of the clinical presentation of SLE and LN it is essential the participation different medical specialists in the care of these patients.2 This holistic and multidisciplinary approach is recommended in the 2019 European guideline14 and it is used in Spain, where the authors of publications on LN come mainly from nephrology, rheumatology, systemic autoimmune diseases, internal medicine, pathological anatomy and immunology units. The participation of different specialists is also reflected in the consensus document on diagnosis and treatment of LN published by the Systemic Autoimmune Diseases Group of the Spanish Society of Internal Medicine (SEMI) and the S.E.N. in 2012,15 as well as in the protocol for diagnosis and treatment of LN of the Hospital Clínic de Barcelona.16
In the joint guideline of the European League Against Rheumatism, the European Renal Association and the European Transplant and Dialysis Association (EULAR/ERA-EDTA), in which Spanish authors participated, it is recommended to suspect LN if a patient with SLE who presents signs of renal involvement such as glomerular hematuria (casts, dysmorphic red blood cells), proteinuria >0.5 g/d, decreased eGFR or increased serum creatinine level.14 In addition, it should be monitored the signs of renal involvement in patients with SLE with risk factors mentioned above.17 The recently published consensus document of the S.E.N. Glomerular Disease Study Group (GLOSEN) recommends periodic urinalysis in all patients with SLE, although more frequently (at least every 6–12 months) in patients who also present risk factors of LN.18
An essential element to improve the diagnosis of LN is the search for new biomarkers that assess LN activity and detect subclinical flares and be able to better assesss the response to treatment. These biomarkers could replace renal biopsy.19 Traditional biomarkers such as serum creatinine, hematuria and proteinuria are not specific enuogh and have been used for more than 30 years.20
Numerous molecules have been investigated and results of the studies have ruled out several Th1, Th2 and Th17 cytokines and growth factors as possible biomarkers in LN.21 The Spanish contribution to the field of potential urinary biomarkers in LN includes monocyte chemotactic protein 1 (MCP1-),1,21,22 TWEAK23–25 and urinary exosomal microRNAs,26 including -miR146a.27,28 Also several types of autoantibodies, free light chains and complement components as other examples of potential biomarkers in urine.29 Immunological markers in serum are also being investigated.20
The usual clinical and analytical parameters are generally not sufficient to determine renal histological alterations.18 For this reason, confirmation of the diagnosis of LN is obtained by renal biopsy, as recommended in Spanish15,18 and international14 publications. The practice of a second biopsy or rebiopsy in patients under active treatment could be useful in some cases.18
Therapeutic approach in lupus nephritisTreatment objectivesTreatment of LN aims at stabilization or improvement of renal function and reduction of proteinuria by at least 25% at 3 months and 50% at 6 months, in addition to a complete clinical response, defined as a 24 h urine protein/creatinine ratio (UPCR) <500−700 mg/g at 12 months after initiating the treatment. If the initial proteinuria is within the nephrotic range, treatment may be necessary for an additional period of 6–12 months.14 Spanish publications include similar criteria although with slight variations. The GLOSEN group consensus document defines "complete remission" as the presence of a proteinuria ≤0.5 g/24 h or UPCR ≤ 0.5 g/g, an inactive urinary sediment (≤5 hematocytes/field), a serum albumin ≥3.5 g/dl and a normal eGFR or ≤10% lower than the measured before the flare.18 In addition, the Hospital Clínic de Barcelona protocol establishes the following criteria for complete renal response: normal renal function (or a GFR with a variation ±15% from baseline in case of previous renal dysfunction), a proteinuria ≤0.5 g/d, an inactive sediment and a serum albumin >3 g/d.18
Furthermore, the treatment of LN should increase patient survival, prevent or delay ESRD, avoid flares and improve patients' quality of life. Treatment should have the best possible tolerability and should include management of comorbidities.14 However, less than 50% of patients have a complete response after 12 months of treatment with immunosuppressants and corticosteroids.1
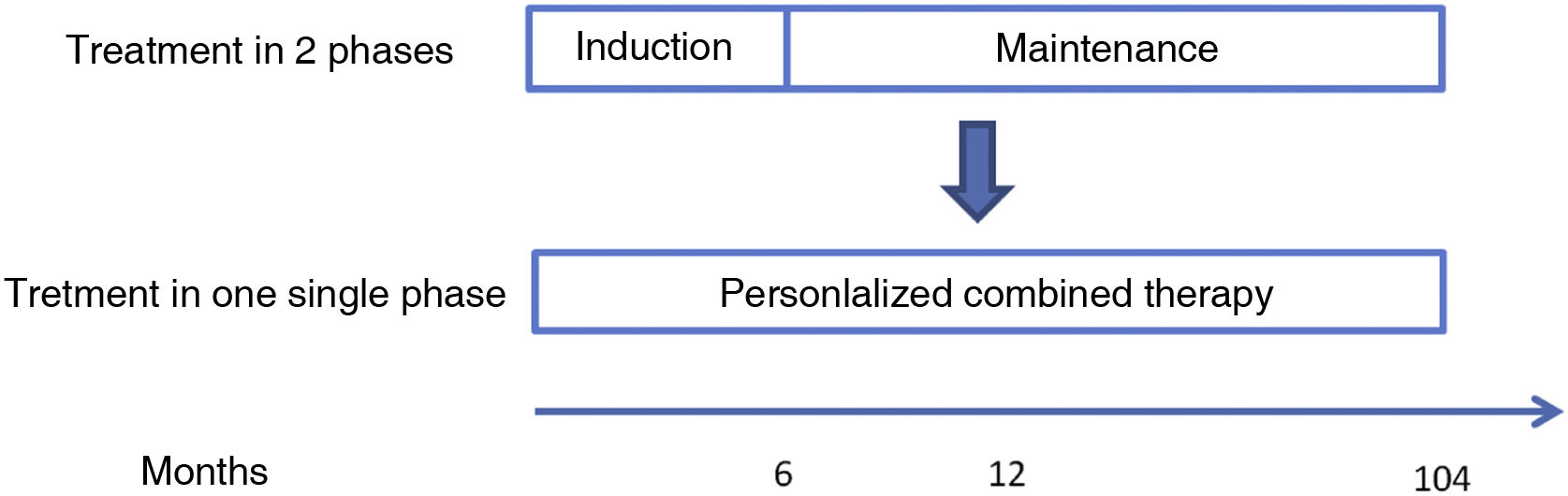
Evolution of the treatment of lupus nephritisUntil 2021 the treatment of LN was sequential, with an induction phase and a maintenance phase. The induction phase lasts 3–6 months and its objectives are to reverse renal inflammation and control the autoimmune response. The objectives of the maintenance phase, which is of long duration, are to control autoimmunity, maintain remission and prevent flares. A summary of the evolution of the treatment of class iii and iv LN between 2005 and 2021, including the drugs used and their doses, is presented in Fig. 1.30
Summary of the evolution of the treatment of class -iiiiv (± class v) LN: from a 2-phase approach to a personalized triple therapy.
AZA: azathioprine; CYC: cyclophosphamide; CYC: cyclophosphamide; MMF: mycophenolate mofetil (for all doses of MMF, an equivalent dose of mycophenolate sodium is also considered); MP: methylprednisolone; P: prednisone; eGFR: estimated glomerular filtration rate.
The treatment in two phases, with an induction phase with corticosteroids and mycophenolate mofetil (MMF) or cyclophosphamide (CYC) and a maintenance phase with corticosteroids and MMF or azathioprine (AZA), has been maintained over the years. However, the recommended doses have been varying and the doses of corticosteroids used have been significantly reduced (Fig. 1).30
Current guidelines and protocols recommend initiating treatment with MMF or mycophenolic acid (MPA) or low-dose CYC (Euro-Lupus guideline) in patients with grades iii and iv LN. In case of nephrotic range proteinuria, it can be used a combination of MMF (or MPA) and a calcineurin inhibitor, preferably tacrolimus. All patients should receive hydroxychloroquine unless contraindicated.16,18,30
In relation to corticosteroids, both in Spain and in other countries have been used in different administration patterns.31,32 Currently, one of the key points in the treatment of LN is to minimize their dose as much as possible to avoid toxicity, which depends on the dose administered and the accumulated dose.33 The 2012 SEMI and S.E.N. consensus recommended initiating corticosteroid therapy with prednisone at doses ≤1 mg/kg/d, with a maximum dose of 60 mg/d, although it has been considered to use lower doses (≤0.5 mg/kg/d) together with pulses of methylprednisolone.15 Current management of patients with LN focuses on using the lowest possible dose of corticosteroids and some therapeutic schemes have been suggested such as methylprednisolone pulses in moderate and severe flares, followed by oral prednisone and rapid dose reduction in about 24 weeks down to ≤5 mg/d as maintenance dose from the fifth month onwards.34 Another alternative could be the administration of 0.5 mg/kg prednisone without prior corticosteroid pulses, with similar efficacy to methylprednisolone pulses and 1 mg/kg/d prednisone, but with less adverse effects.35 A minimimal dose of corticosteroid is also recommended in the GLOSEN group consensus document, where it is suggested to administer IV methylprednisolone pulses (250−500 mg/day) for 3 consecutive days, followed by a maintenance regimen with reduced dose ≤5 mg/d,18 as had already been recommended in the 2012 Spanish consensus.15 In the Hospital Clínic de Barcelona protocol, initial boluses of methylprednisolone are 125−500 mg/d for 3 days followed by oral prednisone at decreasing doses up to 5 mg/d from week 14 onwards. However, this tapering guideline may vary according to the extra-renal symptoms of SLE.16
New therapiesNew treatments are needed to achieve complete renal responses, slow the progression of renal involvement to ESRD and increase patient survival.1,5,20 It has been described that almost 30% of patients with LN do not respond to standard treatment or present safety and toxicity problems.36 In an international study with Spanish participation, published in 2015, LN was still associated with ESRD and death.4
Consequently, new therapies have been developed that could change the approach to the disease. The differentiation between induction treatment and maintenance treatment could even disappear with the use of these new drugs.30 One of them is belimumab, approved in 2021 by the European Medicines Agency for the treatment of active LN.37 Belimumab is a human IgG1 monoclonal antibodyλ that specifically binds to the soluble form of human B-lymphocyte stimulatory protein (BLyS), a B-lymphocyte activator whose level is increased in SLE patients. BLISSLN -was an international phase iii study of belimumab in which 3 Spanish centers participated. It included 448 patients with LN who received 10 mg/kg belimumab intravenously or placebo in addition to standard treatment with corticosteroids and CYC or MMF in the induction phase followed by AZA or MMF, respectively, in the maintenance treatment. After 2 years of follow-up the addition of belimumab to standard treatment significantly improved outcomes, with higher percentages of patients with renal response (p = 0.03) and complete renal response (p = 0.02) compared to placebo. In addition, patients treated with belimumab had a lower risk of developing a renal event or death relative to those treated with standard therapy alone (p = 0.001). In the 2019 EULAR/ERA-EDTA European guideline, prior to the publication of the BLISSLN study-, belimumab is mentioned as a potential strategy in patients with LN and insufficient response to standard therapy.14 Belimumab is already included in current Spanish recommendations from induction for some patient profiles.16,18,30
Another new drug is voclosporin, a new generation calcineurin inhibitor that does not require monitoring of trough concentrations.38 Its efficacy was demonstrated in the phase iii study with Spanish participation AURORA, where voclosporin 23.9 mg/12 h or placebo was added to standard treatment with corticosteroids and MMF to 358 patients with active LN. After 52 weeks the percentage of patients with complete renal response was higher (p < 0.001) in the group treated with voclosporin.39 Current Spanish recommendations contemplate the use of this calcineurin inhibitor.16,18,30
Numerous molecules are under study with the involvement of Spanish researchers. Among them, obinutuzumab (an anti-CD20 monoclonal antibody)40 and bortezomib (a proteasome inhibitor).41
New paradigm in the treatment of lupus nephritisThe availability of these new drugs has made it possible to postulate treatment of class iii and iv (± class v) LN in a single phase continued with combination and personalized therapy from baseline20,30 (Fig. 2). Triple therapy, consisting of adding belimumab or a calcineurin inhibitor such as voclosporin to the usual standard therapy of LN (a corticosteroid and an immunosuppressant), could offer better results.16,18,30
Other strategies to increase survivalTo reduce the risk of complications of LN and mortality, lifestyle modifications are recommended, such as increasing physical activity, reducing body mass index, quitting smoking and regulating sodium intake.16,18 In addition, it is essential to control comorbidities such as hypertension and dyslipidemias, follow the vaccination schedule and prevent osteoporosis.16,18
An emerging strategy that may increase survival in patients with LN is to repeat renal biopsy. This test is useful for the evaluation of refractory LN and for the diagnosis of LN flares.42 It can also provide information on the evolution of the disease,43 such as the progression of renal fibrosis or tubulointerstitial inflammation.44 Rebiopsy can also help to make therapeutic decision,45 such as a possible withdrawal of treatment upon remission of LN.46 It is also used in clinical trials to evaluate the efficacy of study drugs.47
The usefulness of renal rebiopsy in patients with LN has been evaluated in 2 studies performed in Spain in recent years. Piñeiro et al. described how a renal rebiopsy was able to differentiate between clinical remission and histological remission in 35 patients with LN. Proliferative lesions were observed in the renal rebiopsy of 5 of the 11 patients with partial response and of all patients who did not respond to treatment. In contrast, no proliferative lesions were found in the biopsies of the 21 patients with complete response.45 In the work of Morales et al. a renal biopsy was performed in 107 patients with LN and, after a mean of almost 6 years, the procedure was repeated in 27 of them due to clinical indication. Due to the results of the rebiopsy, the classification of LN was changed in 73.1% of the patients, so that 38.4% were reclassified as higher class LN, and 34.6% as lower grade. By classes, a 75% of class ii LN at first biopsy were upgraded to class iii/iv + v at rebiopsy, whereas only 16.6% of proliferative LN at first biopsy were reclassified as mixed or without proliferative lesions at rebiopsy. Therefore, rebiopsy had a major impact on the classification of LN in these patients. In addition, a statistically significant increase in glomerulosclerosis, interstitial fibrosis, tubular atrophy, and chronicity index was observed, especially in patients who progressed to ESRD. In these patients the rate of glomerulosclerosis at first biopsy was higher and the rate of complete remission at 12 months of treatment was lower compared to the other patients.43 In both studies renal rebiopsy was useful at follow-up and to adjust treatment. This strategy made it possible to intensify or reduce treatment in some cases and to maintain the therapeutic approach in only 17% of patients in the study by Piñeiro et al.45 and in 19% of those in the study by Morales et al.43 The results of the European study with Spanish participation, ReBioLup (acronym for "repeat renal biopsy per protocol in new cases of LN") (NCT04449991), are expected to be conclusive about the real value of rebiopsy.
A group of Spanish researchers has proposed an algorithm that includes clinical, biochemical and immunological biomarkers together with the results of the rebiopsy to decide on the continuation of long-term immunosuppressive treatment. At 36 months after initiation of immunosuppressive therapy, with complete response at 12 months, a second biopsy is performed. Then, if the activity index is equal to 0, immunosuppression is discontinued, but if it is ≥1, it is maintained.20
However, a biopsy is an invasive technique, so alternative methods for the assessment of renal fibrosis and risk of CKD are being explored, for example MRI or ex vivo confocal microscopy, together with computer and digital pathology techniques44 or the use of new biomarkers.48
ConclusionsThe research activity developed in Spain on LN has provided us with data characteristic of the population we serve, which will allow us to improve both the diagnostic process and therapeutic decisions. Early diagnosis and treatment of LN are important to prevent the progression of renal disease to end-stage renal disease. The implementation of personalized combination therapy, instead of the classical induction and maintenance phases, seems to be the future of LN management. The development of new drugs in SLE is key to modify the therapeutic strategy in LN and improve the prognosis of these patients.
FinancingThis work was funded by GSK. The authors declare that they received no remuneration.
Conflict of interestCSR is an employee of GSK. ER, CL and DB declare no conflict of interest.
To Content Ed Net, which provided editorial assistance in the writing of this article.