Hyperkalaemia is a significant electrolyte imbalance in chronic kidney disease (CKD). Renin–angiotensin–aldosterone system inhibitors (RAASi) have beneficial cardio-renal properties, although they can often cause hyperkalaemia.
ObjectiveTo examine the prevalence of hyperkalaemia in CKD, identify factors associated with its appearance and the relationship between hyperkalaemia and mortality.
Patients and methodsRetrospective observational study on patients with CKD in the period 1971–2017. The population was categorised into 3 groups: Group 1, patients with CKD without renal replacement therapy; Group 2, patients on haemodialysis; and Group 3, patients on continuous ambulatory peritoneal dialysis.
ResultsA total of 2629 patients were evaluated. The prevalence observed in the different groups was: 9.6%, 16.4% and 10.6%, respectively. Risk factors related to the appearance of hyperkalaemia in the CKD group were glomerular filtration rate (GFR) (p<0.001), plasma creatinine (p<0.001), plasma sodium (p<0.001), haemoglobin (p=0.028), diastolic blood pressure (p=0.012), intake of ACE inhibitors and/or angiotensin ii receptor blockers (p=0.008), treatment with metformin (p<0.001) and diabetes (p=0.045). Treatment with RAASi significantly increased hyperkalaemia as GFR decreased, as well as in patients with diabetes or heart failure.
ConclusionsHyperkalaemia is a frequent metabolic alteration in CKD patients that increases in the presence of drugs with beneficial cardio-renal properties (RAASi), which means that patients often lose the benefit associated with these drugs. New, recently appearing non-absorbable compounds, which bind to potassium in the gastrointestinal tract, enhancing faecal excretion and thus maintaining the cardio-renal benefit of the RAASi, could be relevant in the progress of patients with CKD.
La hiperpotasemia constituye un importante desequilibrio electrolítico en la enfermedad renal crónica (ERC). Los inhibidores del sistema renina-angiotensina-aldosterona (iSRAA) tienen propiedades beneficiosas cardiorrenales, aunque son causa importante de hiperpotasemia.
ObjetivoExaminar la prevalencia de la hiperpotasemia en la ERC, identificar factores asociados a su aparición y la relación entre hiperpotasemia y mortalidad.
Pacientes y métodosEstudio observacional retrospectivo en pacientes con ERC en el período 1971-2017. La población se categorizó en 3grupos: grupo 1, pacientes con ERC sin tratamiento renal sustitutivo; grupo 2, pacientes en hemodiálisis, y grupo 3, pacientes en diálisis peritoneal continua ambulatoria.
ResultadosSe evaluó a 2.629 pacientes. La prevalencia observada en los distintos grupos fue del 9,6, el 16,4 y el 10,6%, respectivamente. Los factores de riesgo relacionados con la aparición de hiperpotasemia en el grupo de ERC fueron la tasa de filtrado glomerular (FG) (p < 0,001), la creatinina plasmática (p < 0,001), el sodio plasmático (p < 0,001), la hemoglobina (p = 0,028), la presión arterial diastólica (p = 0,012), la ingesta de inhibidores de la enzima de conversión de la angiotensina o antagonistas de receptores de angiotensina ii (p = 0,008), el tratamiento con metformina (p < 0,001) y la diabetes (p = 0,045). El tratamiento con iSRAA incrementó de forma relevante la hiperpotasemia a medida que disminuía el FG, así como en pacientes con diabetes o insuficiencia cardiaca.
ConclusionesLa hiperpotasemia es una alteración metabólica frecuente en pacientes con ERC que aumenta en presencia de fármacos con propiedades beneficiosas cardiorrenales (iSRAA), por lo que en muchos casos los pacientes pierden el beneficio asociado a estos fármacos. Nuevos compuestos no absorbibles de reciente aparición, que se unen al potasio en el tracto gastrointestinal potenciando su excreción fecal, manteniendo el beneficio cardiorrenal de los iSRAA, pudieran ser relevantes en la evolución de los pacientes con ERC.
Chronic kidney disease (CKD) is associated with significant electrolyte imbalances. Hyperkalaemia is one of the most important due to the potential risk of arrhythmias associated with adverse cardiac outcomes and an increase in mortality rates.1–3 The long-term maintenance of potassium homeostasis by the kidneys despite deterioration in the glomerular filtration rate (GFR) related to CKD progression is achieved by the gradual adaptation of the serum potassium-concentration regulatory mechanisms.4 However, the ability to respond to an acute increase in potassium load is hampered, and this leads to episodes of hyperkalaemia. The adaptive mechanism means that until GFR levels reach ≤10–15ml/min. serum potassium levels are usually in the normal range, unless there are other contributing factors, such as certain drugs, or hyporeninaemic hypoaldosteronism, where hyperkalaemia can occur with lesser degrees of reduced GFR, as in diabetic nephropathy, interstitial nephropathies and obstructive uropathy. Common comorbidities during CKD, such as diabetes mellitus (DM) and cardiovascular disease, are associated with the development of hyperkalaemia through various mechanisms. Insulin deficiency and hypertonia caused by hyperglycaemia in patients with DM contribute to the inability to disperse high potassium loads in the intracellular space.5 DM is also associated with hyporeninaemic hypoaldosteronism and the consequent inability to positively regulate the tubular secretion of potassium.6 Cardiovascular diseases, such as left ventricular hypertrophy and heart failure, require various medical treatments which have been related to hyperkalaemia. In clinical practice, renal failure and drugs are the main predisposing factors for the development of hyperkalaemia. Treatment with certain medications, such as potassium-sparing diuretics, beta-blockers, non-steroidal anti-inflammatory drugs (NSAIDs) and, primarily, renin–angiotensin–aldosterone system inhibitors (RAASi), can affect the ability of the kidney to maintain an adequate balance between the intake, excretion and transcellular distribution of potassium, leading to a range of potassium disorders. RAASi have shown efficacy in slowing the progression of CKD, hypertension, proteinuria and heart failure. However, they are associated with an increased risk of hyperkalaemia,7 and this can limit their use and often creates an important dilemma when having to weigh up the risks/benefits of treatment. Considering the relationship between renal failure and hyperkalaemia, we examined the prevalence of high plasma potassium values and their association with mortality rates in a cohort of patients from our Nephrology Department.XXX
Patients and methodsRetrospective observational study in patients with CKD included in the Hospital Universitario Marqués de Valdecilla (HUMV) Nephrology Department registry from 30 November 1971 to 1 February 2017. Data for 3331 patients were analysed. The population was categorised into three groups according to the estimated GFR (eGFR) and whether or not they were on renal replacement therapy at the time of the study. A total of 198 patients who had been on haemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD) only on a temporary basis during the study period were excluded from the calculations. Another 504 patients were excluded because of the hyperkalaemic nature of the calcineurin inhibitors used in the immunosuppressive therapy for kidney transplant patients. The final population analysed consisted of 2629 patients. The groups analysed were:
- –
Group 1: patients with CKD (eGFR<60ml/min/1.73m2) not on renal replacement therapy (n=1088).
- –
Group 2: patients on treatment with HD at the time of carrying out the study (n=1097).
- –
Group 3: patients on treatment with CAPD at the time of carrying out the study (n=444).
Hyperkalaemia was defined as a serum potassium level >5.5mmol/l. In the period 30 November 1971 to 3 February 2013, renal function was estimated using the MDRD-4 equation. Once serum creatinine was standardised, renal function was determined by the CKD-EPI equation. As ethnic group was not available, the whole population was considered as white for the calculation of the eGFR. Following the recommendations of our laboratory, haemolysed samples were not taken into consideration and new samples were obtained. Only patients with an eGFR <60ml/min/1.73m2 were considered and they were categorised into the different stages of CKD according to the current KDIGO 2012 classification criteria.
The quantification of serum potassium in the different groups analysed was carried out according to the following criteria:
- –
In CKD group 1, the result on the day that CKD was confirmed (2nd estimation of eGFR showing <60ml/min/1.73m2, at least 90 days after the first).
- –
In groups 2 and 3 of patients on HD or CAPD the mean value of potassium measurements corresponding to the month they started renal replacement therapy.
The severity of hyperkalaemia was classified into the following four categories: mild (K: 5.5–5.9mmol/l), moderate (K: 6.0–6.4mmol/l), severe (K: 6.5–6.9mmol/l) and very severe (K≥7.0mmol/l). The demographic details were obtained from the HUMV Nephrology Department patient registry. DM, hypertension, coronary disease and other comorbidities were defined using criteria which were pre-specified and validated from the literature. Serum potassium levels and other relevant laboratory data were obtained from electronic laboratory records. The main outcomes of interest, causes of death and end-stage renal disease were verified and linked to our CKD registry. The patients were followed up from their date of inclusion in the registry (date of the second measurement of eGFR) until 1 February 2017.
Statistical analysisCategorical variables were expressed as number and percentage and continuous variables as the mean±the standard deviation, or median and interquartile range (IQR) according to whether they had normal distribution or not. The comparison of mean values between the different groups of patients was carried out using the analysis of variance (ANOVA), with the Bonferroni correction. The association between continuous and categorical variables was carried out using Student's t-test and the Chi-square test, respectively. With the results obtained, a multivariate logistic regression analysis was carried out to identify the independent predictors which act as risk factors for the development of hyperkalaemia. The survival analysis and the statistical significance of the different curves were carried out using the Kaplan–Meier method and Log Rank tests, respectively. A p value <0.05 was considered statistically significant.
ResultsData from 2629 patients were analysed; 66.8% men with a mean age of 64.7 (56.4–75.6) and 33.2% women with a mean age of 64.8 (54.9–76.9). Mean follow-up time was 3.2 (1.4–6.1) years.
Mean serum K values were: 4.6mmol/l, 4.7mmol/l, 4.8mmol/l and 4.8mmol/l for stages 3a, 3b, 4 and 5 respectively in the CKD group; 4.7mmol/l in the HD group; and 4.5mmol/l in the CAPD group.
PrevalenceThe overall prevalence of hyperkalaemia (K>5.5mmol/l) in the whole population analysed was 12.6%. By subgroups, the prevalence was 9.6% in the CKD group, 16.4% in the HD group and 10.6% in the CAPD group. In terms of severity, the prevalence of hyperkalaemia in the population analysed overall was 7.5% for mild, 3.2% for moderate, 1.0% for severe and 0.9% for very severe. Table 1 shows the distribution of the prevalence of hyperkalaemia by population groups, stages of CKD and degree of hyperkalaemia severity.
Prevalence of hyperkalaemia in population with CKD.
| Group/CKD stage | Overall hyperkalaemia K>5.5mmol/l | Mild hyperkalaemia K>5.5–6.0mmol/l | Moderate hyperkalaemia K>6.0–6.5mmol/l | Severe hyperkalaemia K>6.5–7.0mmol/l | Very severe hyperkalaemia K>7mmol/l |
|---|---|---|---|---|---|
| Total population | 12.6% | 7.5% | 3.2% | 1.0% | 0.9% |
| Group 1. CKD | 9.6% | 6.5% | 2.3% | 0.2% | 0.6% |
| G3 | 7.2% | 5.5% | 1.3% | – | 0.4% |
| G3a | 5.7% | 3.9% | 1.4% | – | 0.4% |
| G3b | 8.6% | 7.1% | 1.1% | – | 0.4% |
| G4 | 9.3% | 7.0% | 2.3% | – | – |
| G5 | 17.3% | 8.6% | 5.4% | – | 2.2% |
| Group 2. HD | 16.4% | 8.7% | 4.4% | 2.0% | 1.4% |
| Group 3 CAPD | 10.6% | 7.0% | 2.7% | 0.5% | 0.5% |
In the univariate analysis, in the population analysed overall, the following risk factors were associated with the development of hyperkalaemia: eGFR, plasma creatinine, sodium and haemoglobin levels, diastolic blood pressure (DBP), statin, furosemide and metformin therapy, and congestive heart failure (CHF). In the group of patients with CKD, risk factors were GFR, creatinine, sodium and haemoglobin levels, DBP, angiotensin-converting enzyme (ACE) inhibitor, angiotensin ii receptor blocker (ARB) and metformin therapy and DM. Risk factors in the HD group were plasma sodium levels, erythropoietin, iron and furosemide therapy, a history of CHF and previous stroke. Last of all, in the group of patients on CAPD, age and, statin and erythropoietin therapy were significant factors. Table 2 shows the detail of the different factors analysed by group of population.
Main factors related to the development of hyperkalaemia in the different population groups.
| CKD | HD | CAPD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| K≤5.5mmol/l | K>5.5mmol/l (n=104) | p | K≤5.5mmol/l (n=917) | K>5.5mmol/l (n=180) | p | K≤5.5mmol/l (n=397) | K>5.5mmol/l (n=47) | p | |
| Gender (% male) | 65.9 | 64.4 | 0.792 | 68.3 | 67.6 | 0.784 | 66.7 | 61.7 | 0.512 |
| Age (years) | 69.9 (59.2–78.2) | 68.8 (58.5–77.8) | 0.948 | 66.8 (54.8–74.5) | 64.9 (55–74.3) | 0.262 | 66.1 (50.8–74.1) | 54.7 (45.8–64.7) | <0.001 |
| GFR (ml/min./1.73m2) | 31.7 (19.2–46.2) | 23.2 (14.5–38.4) | <0.001 | – | – | – | – | – | – |
| Creatinine (ml/min.) | 2.0 (1.5–3.2) | 2.8 (1.7–4.1) | <0.001 | – | – | – | – | – | – |
| Glucose (mg/dl) | 101.0 (87.0–126.0) | 104.0 (88.0–141.0) | 0.116 | 110.0 (94.0–148.0) | 105.0 (88.0–132.0) | 0.921 | 112.0 (97.0–140.0) | 101.0 (94.0–127.0) | 0.115 |
| Sodium (mmol/l) | 141.0 (139.0–143.0) | 140.0 (137.0–143.0) | <0.001 | 139.0 (136.0–141.0) | 138.0 (135.0–141.0) | 0.011 | 139.0 (136.0–141.0) | 137.0 (136.0–140.0) | 0.152 |
| Albumin (g/dl) | 4.2 (3.9–4.4) | 4.1 (3.8–4.4) | 0.419 | 3.8 (3.4–4.1) | 3.9 (3.6–4.1) | 0.157 | 3.8 (3.4–4.0) | 3.7 (3.3–4.0) | 0.698 |
| Haemoglobin (g/dl) | 12.2 (11.1–13.6) | 11.8 (10.7–12.9) | 0.028 | 10.6 (9.5–11.7) | 10.9 (9.4–11.9) | 0.632 | 11.8 (10.6–13) | 10.9 (8.9–13) | 0.073 |
| SBP (mmHg) | 140 (128–153) | 140.0 (129.0–152.0) | 0.762 | 140.0 (130.0–151.5) | 140.0 (130.0–160.0) | 0.742 | 140.0 (130.0–160.0) | 142.0 (131.5–155.0) | 0.567 |
| DBP (mmHg) | 77.0 (70.0–84.8) | 73.0 (67.0–84.0) | 0.012 | 80.0 (70.0–85.0) | 80.0 (70.0–82.0) | 0.597 | 80.0 (70.0–90.0) | 90.0 (78.0–91.5) | 0.122 |
| BMI (kg/m2) | 29.3 (26.2–32.8) | 29.7 (25.8–32.9) | 0.888 | 26.8 (23.7–29.9) | 26.8 (22.3–29.6) | 0.252 | 27.2 (24.5–29.6) | 26.2 (23.8–28.5) | 0.414 |
| ACE inhib.+ARB (%) | 38.4 | 52.9 | 0.008 | 17.0 | 12.8 | 0.160 | 16.9 | 25.5 | 0.142 |
| Statins (%) | 45.2 | 42.3 | 0.570 | 24.6 | 18.9 | 0.097 | 22.9 | 6.4 | 0.009 |
| EPO (%) | 20.1 | 26.9 | 0.104 | 61.6 | 53.3 | 0.038 | 55.7 | 40.4 | 0.047 |
| Iron (%) | 13.4 | 15.4 | 0.577 | 24.3 | 16.7 | 0.026 | 18.1 | 14.9 | 0.583 |
| Thiazide (%) | 2.8 | 1.0 | 0.257 | 0.2 | 0.0 | 0.531 | 1.5 | 0.0 | 0.396 |
| Furosemide (%) | 39.5 | 40.4 | 0.866 | 42.2 | 23.9 | <0.001 | 36.5 | 27.7 | 0.230 |
| Distal diuretics (%) | 15.3 | 14.4 | 0.804 | 1.3 | 0.0 | 0.123 | 1.8 | 2.1 | 0.859 |
| Digoxin (%) | 2.0 | 3.8 | 0.231 | 1.3 | 1.7 | 0.705 | 1.3 | 0.0 | 0.439 |
| Insulin (%) | 16.9 | 23.1 | 0.113 | 16.5 | 13.3 | 0.294 | 13.4 | 4.2 | 0.074 |
| Metformin (%) | 1.9 | 7.7 | <0.001 | – | – | – | – | – | – |
| Diabetes (%) | 36.2 | 46.2 | 0.045 | 33.7 | 31.7 | 0.597 | 29.0 | 19.1 | 0.156 |
| Coronary heart disease (%) | 13.4 | 8.7 | 0.169 | 16.4 | 13.9 | 0.408 | 15.4 | 8.5 | 0.209 |
| CHF (%) | 8.0 | 4.8 | 0.242 | 10.1 | 5.0 | 0.030 | 8.1 | 4.3 | 0.354 |
| Stroke (%) | 10.5 | 7.7 | 0.374 | 10.1 | 5.0 | 0.030 | 5.0 | 2.1 | 0.374 |
| Cancer (%) | 7.6 | 8.7 | 0.708 | 11.9 | 11.7 | 0.933 | 8.1 | 4.3 | 0.354 |
In the multivariate logistic regression analysis, the independent risk factors for hyperkalaemia (K>5.5mmol/l) in the different groups of patients were as follows:
- –
In the group of patients with CKD, GFR (OR: 0.962; 95% CI: 0.944–0.982; p<0.001), plasma sodium (OR: 0.906; 95% CI: 0.847–0.968; p=0.004), the use of ACE inhibitors or ARB (OR: 2.040; 95% CI: 1.223–3.402; p=0.006) and the use of metformin (OR: 6.027; 95% CI: 2.070–17.547; p=0.001).
- –
In the HD group, plasma sodium (OR: 0.953; 95% CI: 0.916–0.992; p=0.017) and the use of furosemide (OR: 0.443; 95% CI: 0.306–0.640; p<0.001).
- –
In the group of patients on CAPD, age (OR: 0.969; 95% CI: 0.951–0.987; p=0.001) and taking statins (OR: 0.232; 95% CI: 0.070–0.768; p=0.017).
The prevalence of hyperkalaemia in CKD group 1 among patients on RAASi therapy was 12.5%, and 7.7% among those not taking RAASi. Comparing patients with or without RAASi therapy, by gender, the prevalence among females was 13.2% vs 7.7% and, among males, 12.1% vs 7.7%. By stages of CKD, among women, the largest differences in prevalence were observed in stage 4 (2.9% vs 17.4%) and stage 5 (13.0% vs 25.0%), and, among males, stage 3b (3.0% vs 10.1%) and stage 4 (7.0% vs 15.3%). Table 3 shows the distribution of prevalences of hyperkalaemia by gender and according to RAASi therapy for CKD group 1 patients in the different stages of CKD.
Distribution of hyperkalaemia by gender and treatment with RAASi in CKD group 1.
| Overall | Female | Male | ||||
|---|---|---|---|---|---|---|
| Group/CKD stage | No Tx with RAASi | Tx with RAASi | No Tx with RAASi | Tx with RAASi | No Tx with RAASi | Tx with RAASi |
| Group 1. CKD | 7.7 | 12.5 | 7.7 | 13.2 | 7.7 | 12.1 |
| G3 | 5.2 | 9.3 | 8.1 | 9.6 | 3.6 | 9.2 |
| G3a | 4.3 | 7.2 | 4.2 | 4.7 | 4.3 | 8.4 |
| G3b | 6.0 | 11.5 | 11.8 | 13.7 | 3.0 | 10.1 |
| G4 | 5.8 | 16.1 | 2.9 | 17.4 | 7.0 | 15.3 |
| G5 | 15.6 | 23.7 | 13.0 | 25.0 | 17.2 | 23.1 |
Within CKD group 1, the prevalence of hyperkalaemia among diabetic patients was 17.3% for those on RAASi therapy and 7.6% for those not on RAASi therapy. Among the patients with heart failure in this same group, the prevalence was 11.5% for those on RAASi therapy and 3.4% for those not on RAASi therapy.
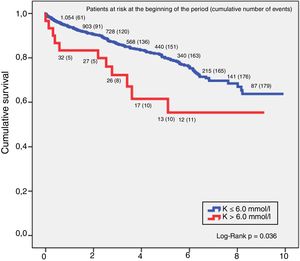
SurvivalThe relationship between hyperkalaemia and death from any cause in the CKD group showed that potassium values above 6.0mmol/l had a higher mortality risk. Analysis of survival using the Kaplan–Meier technique in this group of patients found that for a K>6.0mmol/l, the 5-year survival rate was 64.5% and 10-year, 59.1%, and for K≤6.0mmol/l, 5-year survival was 80.6% and 10-year 69.1%, with the difference between the two curves being statistically significant (log-rank p=0.036). These results are shown in Fig. 1.
DiscussionIn our study, the overall prevalence of hyperkalaemia in the population with CKD was 12.6%. This prevalence increased in CKD stage 5, not on dialysis, to 25.0% in females and 23.1% in males on RAASi therapy. Extrapolating these results to the Spanish population as a whole, we can quantify that around 500,000 people with CKD will have hyperkalaemia, the magnitude of which should make us reflect on the measures that need to be adopted. The prevalence of hyperkalaemia in the general population is low (2–3%).8 In CKD, prevalence data are mixed, with rates in the range 40–50%, depending on the size and structure of the population analysed, the CKD stage, the cut-off point for the definition of hyperkalaemia (>5 or >5.5mmol/l) and concomitant comorbidities, with the highest prevalence rates for advanced stages of CKD, patients with diabetes, kidney transplant recipients and patients on RAASi therapy.8 In a recent study, Latts et al.9 assessed the prevalence of hyperkalaemia in approximately 1.7 million patients with CKD stages 3 and 4 and concomitant heart failure. They found, on the one hand, that at least 47.6% of the patients had had an episode of hyperkalaemia and, on the other, that the concurrence with CKD of DM or heart failure increased the risk of hyperkalaemia 2.5–5.6 times.
As has been reported in numerous studies, our results confirm an inverse relationship between GFR and the degree of hyperkalaemia, such that as kidney disease progresses and GFR decreases, serum potassium levels increase. The overall prevalence of hyperkalaemia in the CKD group was 9.6% (7.2% in stage 3, 9.3% in stage 4, and 17.3% in stage 5). Even within stage 3 we observed this same pattern of an inverse relationship between serum eGFR and K, such that the prevalence in stage 3a was 5.7%, increasing to 8.6% in stage 3b. Among those on renal replacement therapy, the prevalences were 16.4% in the HD group and 10.6% in the CAPD group. This same relationship of dependence between eGFR and the serum potassium level is observed between the different degrees of severity of hyperkalaemia (mild, moderate, severe and very severe).
The most commonly reported demographic factors associated with the development of hyperkalaemia are gender and age. With regard to gender, several studies point to being male as an independent risk factor.10–12 Our results are consistent with these data; in the overall population, 65.6% of males vs 34.4% of females (ratio 1.9:1) had hyperkalaemia. By population subgroups, the proportions remained very similar: 64.4% vs 35.6% in the CKD group; 67.2% vs 32.8% in the HD group and 61.7% vs 38.3% in the CAPD group.
Despite this imbalance towards males, a number of studies show contradictory figures for the prevalence of hyperkalaemia by gender, but this is probably due to differences in the methodology followed, eating habits, comorbidities, etc. In a large study by Epstein et al.,13 out of 195,000 patients treated with RAASi, the prevalence of hyperkalaemia was 52.6% in females and 47.2% in males. Another study by Einhorn et al.,3 with 245,808 patients, on the frequency of hyperkalaemia and its importance in CKD reported a prevalence of 14.0% in males and 7.3% in females. Our study found the prevalence of hyperkalaemia to be similar in males and females: 9.9% in females vs 9.4% in males in CKD group 1; 16.9% in females vs 16.2% in males in HD group 2; and 11.9% in females vs 9.9% in males in CAPD group 3. However, unlike the above studies, our results did not show statistical significance for gender as a factor associated with the development of hyperkalaemia. With regard to age, the above study by Latts et al.9 reported that the prevalence of hyperkalaemia in patients with similar comorbidities practically doubled in patients aged ≥65 compared to the under-65s. We did not find essential age differences between the different population groups in our study. As an independent risk factor, we found no significant statistical association between age and the development of hyperkalaemia, except in the case of the CAPD group, where, in contrast to that reported in the literature, we found an inverse relationship between hyperkalaemia and age (OR: 0.969; 95% CI: 0.951–0.987, p=0.001), which could be attributed to a more liberal diet in younger patients. The older population is more susceptible to the development of hyperkalaemia due to the decrease in the secretion of renin and aldosterone which leads to hyporeninaemic hypoaldosteronism and, to the greater number of comorbidities in this population, the treatment of which very often involves drugs with hyperkalaemic effects, such as NSAIDs and RAASi primarily.14,15 In our study, we found no significant differences in the prevalence of hyperkalaemia in patients aged ≥70, with or without DM (10.4% vs 10.8%, p=0.382).
Our study shows an association between plasma sodium and hyperkalaemia in the CKD and HD patient groups, which we attribute in part to hyperglycaemia, as well as to the progression of the CKD itself. DM is associated with alterations in electrolyte metabolism, with the decrease in serum sodium being an osmoregulatory response associated with hyperglycaemia, the cause of which may lie in diabetic dysregulation, or in other words, insulin deficiency.16,17 In patients with advanced stages of CKD, the kidney's adaptive mechanisms for maintaining adequate sodium homeostasis are insufficient, and this can lead to volume depletion or overload due to loss or retention of sodium by the kidneys.18 In our case, there were 339 cases of hyponatraemia, 305 (90.0%) of whom were in CKD stage 5 and 104 (30.7%) in CKD stage 5 with diabetes. The incidence of hyperkalaemia is also higher in patients with diabetes than in the general population, with hyporeninaemic hypoaldosteronism being more prevalent in tubulointerstitial nephropathy and diabetic nephropathy, responsible for a reduction in tubular K secretion, the most common chronic, usually asymptomatic, hyperkalaemia factor in diabetic patients with mild to moderate CKD.19–21
In clinical practice, drugs are considered the biggest cause of hyperkalaemia, interfering with potassium homeostasis through various mechanisms, such as the decrease in production or secretion of aldosterone, inhibiting the renal secretion of potassium or altering its intra/extracellular distribution. Of all of the above, the reduction in renal excretion of potassium due to the inhibition of RAAS represents the most important mechanism by which drugs can cause hyperkalaemia. In the first clinical trials with RAASi the incidence of hyperkalaemia was generally low (<1–2%). However, subsequent trials with more heterogeneous patients found incidences of hyperkalaemia from 1.9% to 38.4%. Hyperkalaemia was more common in patients with CKD and its incidence increased with the number of RAASi received.7,8 At the same time, in an adjusted model, it was found that all-cause mortality associated with hyperkalaemia increased significantly for every 0.1mEq/l increase in serum potassium, pointing to RAASi as the main drugs related to the hyperkalaemia. The evidence suggests the existence of a U-shaped relationship between serum potassium and death, where both low and high levels of serum potassium are associated with an increase in cardiovascular and all-cause mortality. In this U-shaped association, for death associated with hyperkalaemia, a point of inflection was found for K levels ≥6.0mmol/l, from which there is a significant increase in the risk of death; we therefore carried out a survival analysis, comparing higher and lower values.11,22,23
Our results pointed exclusively to two drugs as independent risk factors for the development of hyperkalaemia: metformin (OR: 5.269; 95% CI: 1.768–15.708; p=0.003); and ACE inhibitors or ARB (OR: 2.206; 95% CI: 1.311–3.711; p=0.003), in both cases in CKD group 1.
The incidence of metformin-induced lactic acidosis, at 3–10 cases per 100,000 patient-years in the CKD population, is very low.24,25 Therefore, in view of the follow-up period of our study (11,699.8 patient-years), there will have been very few cases of lactic acidosis and its influence on potassium is irrelevant. In inorganic acidosis (hyperchloraemic or with normal anion gap), the hydrogen ions of the extracellular medium enter the cell and a passive exit of potassium to the extracellular medium occurs to maintain the electroneutrality. This phenomenon is less pronounced in the acidosis with increased anion gap produced by organic acids (as is the case of lactic acidosis due to metformin), as these organic anions are more permeable and penetrate the cells more easily, thereby reducing the electrical gradient which prompts the exit of potassium from the cell. Hyperkalaemia associated with DM includes acidosis (for each 0.1 drop in pH, potassium increases approximately 0.4–0.6mmol/l).21,26 Although in our study, in the CKD group, we found a significant difference in serum K levels between patients treated with metformin and those not (5.1±0.6mmol/l vs 4.7±0.7mmol/l, p=0.001), we consider it unlikely that the hyperkalaemia was induced by metformin, but rather a consequence of hyperkalaemia associated with DM itself.
RAASi, such as ACE inhibitors, aldosterone receptor blockers, ARB, and direct renin inhibitors, have proven effective in the treatment of heart failure and DM, and in reducing blood pressure and proteinuria, slowing the progression of CKD. However, their use is also associated with various adverse events, including hyperkalaemia, which can occur at any level of renal function, although the greater risk is thought to be in patients with CKD and heart failure.27 This creates a difficult dilemma in terms of weighing up the risks/benefits of treatment, and can restrict the use of this type of drug or lead to underuse, precisely in the patients for whom it is hoped to obtain greatest benefit.7,12,19,28–30 Epstein et al. found that in patients on maximum doses of RAASi, after an episode of moderate to severe hyperkalaemia, their dose was reduced or treatment discontinued in almost 50% of cases. Dose reduction or treatment discontinuation in patients with stages 3 and 4 CKD led to an increase in adverse effects of 5–7%, and a doubling of mortality rates.13
Various studies indicate that hyperkalaemia secondary to treatment with RAASi occurs in 10–38% of hospitalised patients and approximately 10% of outpatients within a year after starting treatment.19 It has also been reported that adding a mineralocorticoid receptor antagonist to an ACE inhibitor/ARB doubles the risk of hyperkalaemia.31 Other studies suggest that the risk of hyperkalaemia with RAASi monotherapy is low (<2%) in patients without predisposing factors, although it increases when several drugs are combined (<5%), and even more so if they are used in patients with additional risk factors, such as advanced age, DM, CKD, especially with eGFR <30ml per min., hypertension or heart failure (from 5 to 10%). There is also a suggestion that this risk may be slightly lower in ARB compared to ACE inhibitors.32
Some studies, such as CHARM-Added33 and EPHESUS,34 have not only shown the benefits of treatment with combinations of RAASi, but also that these benefits are maintained even in patients at higher risk of developing hyperkalaemia.35–37 It has therefore been concluded that the risk of developing hyperkalaemia is not sufficient reason to deny patients the benefits they are likely to obtain from the use of these agents. In view of these discrepancies and to minimise the risks associated with taking RAASi and to prevent hyperkalaemia, the current guidelines for the assessment and management of kidney disease recommend measuring GFR and serum K within the week following the start of treatment with ACE inhibitors/ARB, regardless of the baseline potassium level, and assessing whether the patient's diet includes excessive intake of potassium or they are on treatment with other hyperkalaemic drugs.38
Our results show that treatment with RAASi substantially increases the prevalence of hyperkalaemia. In group 1 of patients with CKD, the prevalence of patients not on treatment with RAASi was 7.7%, while that of patients who were taking RAASi was 12.5%. Within this same group of patients, comparing patients not on RAASi therapy with those taking RAASi, we found an increase in hyperkalaemia as renal function declined (5.2% vs 9.3% in stage 3, 5.8% vs 16.1% in stage 4 and 15.6% vs 23.7% in stage 5). Analysing differences in prevalence by gender, unlike other studies which report higher prevalences of hyperkalaemia in males treated with RAASi than in females,39 our results show the prevalence to be practically equal, even somewhat higher in females (7.7% vs 12.1% in males and 7.7% vs 13.2% in females), but we attribute this to the small number of patients.
The treatment of hyperkalaemia currently tends to consist of the adoption of hygiene/dietary measures, the withdrawal or restriction of hyperkalaemic drugs such as RAASi13 and the use of exchange resins and diuretics. Cation exchange resins are the predominant treatment at present, although they have significant gastrointestinal adverse effects.40 In addition, the oral forms of presentation of these resins tend to have an unpleasant taste which does not encourage adherence to treatment.41,42
Two new potassium chelators, patiromer43 and ZS-944 have recently been developed which, in the trial phase, showed good efficacy and safety profiles in the treatment of hyperkalaemia, and for maintaining normokalaemia, without dose reduction or withdrawal of RAASi. This could benefit patients with mild hyperkalaemia who have had to stop their treatment with RAASi, or who have not been prescribed RAASi for fear of developing hyperkalaemia.45 However, patients with hyperkalaemia >6.5mmol/l and patients on dialysis were excluded from the trials that support these drugs, and their efficacy will therefore have to wait to be endorsed through long-term clinical practice.
The main weakness of our study lies in its observational and retrospective nature. Other limitations, although we do not believe they substantially affect the study's objective, may be the lack of determination of metabolic acidosis, the failure to record the use of mineralocorticoid receptor antagonists, the fact of being based on a single determination of serum potassium and measurement of serum potassium in patients on renal replacement therapy a month after starting the therapy, and the lack of adjustment for possible confounding factors in the survival analysis. As main advantages, in addition to the long period analysed, it should be noted that we examined a broad and representative cohort of the entire CKD spectrum in a single centre.
We conclude that hyperkalaemia is a common metabolic disorder in patients with CKD, it is dependent on GFR and associated with higher mortality rates. This complication increases in the presence of drugs with beneficial cardiorenal properties (ACE inhibitors/ARB), so in many cases patients lose the benefit associated with these drugs. New nonabsorbable compounds have recently emerged which produce a selective cation exchange between sodium/calcium and potassium in the gastrointestinal tract. This limits resorption and increases faecal excretion of potassium, maintaining the cardiorenal benefit of the RAASi. These new compounds could therefore play an important role in the progression of patients with CKD. In any event, further randomised control trials are required, in addition to verification of their efficacy in clinical practice.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Belmar Vega L, Rodrigo Galabia E, Bada da Silva J, Bentanachs González M, Fernández Fresnedo G, Piñera Haces C, et al. Epidemiología de la hiperpotasemia en la enfermedad renal crónica. Nefrologia. 2019;39:277–286.