Congestion is a common complication in the critical care setting, these patients are at increased risk of developing acute kidney injury (AKI). Congestive nephropathy (CN) has recently been described as a mechanism of worsening renal function, and evaluation of renal venous flow by pulsed Doppler (PD) is a useful tool to assess the presence of renal vein congestion. We comprehensively explore the ability of the PD in the evaluation of the intrarenal venous flow (IRVF) to predict the development of AKI in critically ill patients.
We searched Pubmed-MEDLINE, Scopus, Embase, and Cochrane Library of Systematic Reviews (to 31th December 2021). We evaluated the association between Doppler-based Intrarenal venous flow demodulation and AKI. CN was defined as the presence of a pulsatile pattern (biphasic or monophasic) in the PD.
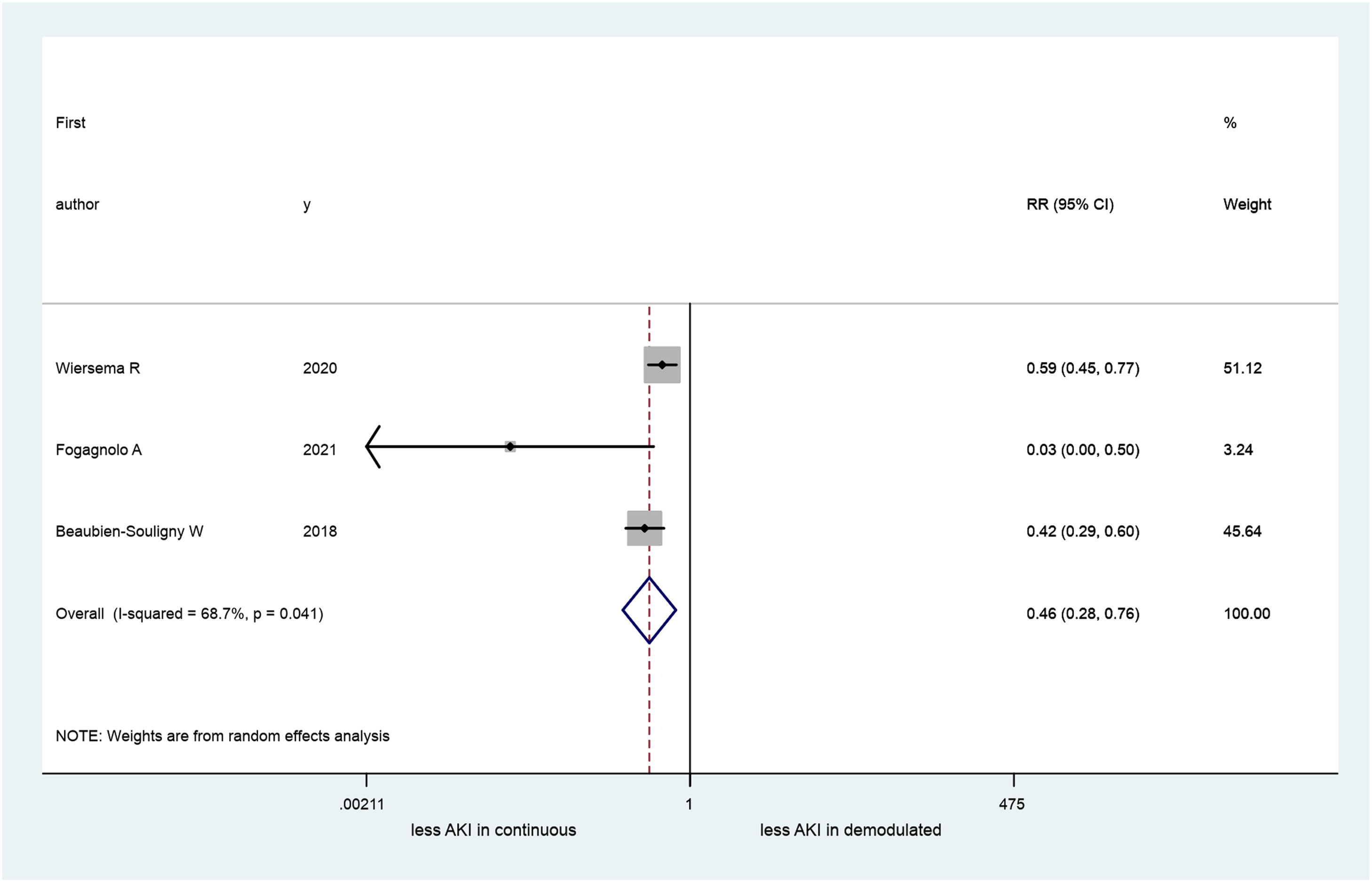
A total of 4 articles (660 patients) were included in our systematic review, three of these in the metanalysis (413 patients): one study was excluded because its data were inadequate for pooling. Two studies originated in Europe and the other two in the United States. AKI occurrence ranged between 34 and 68%. Patients who developed AKI had a significant difference in PD pattern (continuous vs. pulsatile) in the IRVF (RR=0.46; 95% CI 0.28–0.76). Nevertheless, a large heterogeneity was observed among the studies (I2=68.7%; p=0.04).
Albeit preliminary, these findings suggest that the presence of a pulsatile pattern in the PD of the IRVF may be involved in the development of AKI in the critically ill patient. The effect of alterations in the IRVF and renal function warrant further investigation.
La congestión es una complicación frecuente en el entorno de las unidades de cuidado crítico, y estos pacientes tienen un mayor riesgo de desarrollar una lesión renal aguda (LRA). La nefropatía congestiva (NC) se ha descrito recientemente como un mecanismo de deterioro de la función renal, y la evaluación del flujo venoso renal mediante Doppler pulsado (DP) es una herramienta útil para valorar la presencia de congestión venosa renal. Exploramos de forma exhaustiva la capacidad del DP en la evaluación del flujo venoso intrarrenal (FVIR) para predecir el desarrollo de LRA en pacientes en estado crítico.
Se realizaron búsquedas en Pubmed-MEDLINE, Scopus, Embase y la Biblioteca Cochrane de Revisiones Sistemáticas (hasta el 31 de diciembre de 2021). Se evaluó la asociación entre la demodulación del flujo venoso intrarrenal basada en Doppler y la LRA. La NC se definió como la presencia de un patrón pulsátil (bifásico o monofásico) en el DP.
Se incluyeron un total de 4 artículos (660 pacientes) en nuestra revisión sistemática, tres de ellos en el metaanálisis (413 pacientes): se excluyó un estudio porque sus datos eran inadecuados para el análisis. Dos estudios procedían de Europa y los otros dos de Estados Unidos. La aparición de LRA osciló entre el 34 y el 68%. Los pacientes que desarrollaron LRA presentaban una diferencia significativa en el patrón de DP (continua frente a pulsátil) en el FVIR (RR=0,46; IC del 95%: 0,28-0,76). No obstante, se observó una gran heterogeneidad entre los estudios (I2=68,7%; p=0,04).
Aunque preliminares, estos hallazgos sugieren que la presencia de un patrón pulsátil en el DP del FVIR puede estar asociado al desarrollo de la LRA en el paciente crítico. El efecto de las alteraciones en el FVIR y la función renal obligan a desarrollar futuras investigaciones.
The occurrence of acute kidney injury (AKI) in patients admitted to the intensive care unit (ICU) varies between 30% and 60%.1,2 AKI is defined as a decrease in kidney function based on elevated serum creatinine (SCr) levels, a decrease in urinary output, or the need for renal replacement therapy.3 Different pathophysiological pathways can lead to the development of AKI in critically ill patients.4 Renal hypo-perfusion has long been considered as one of the main predisposing factors for AKI in critically ill patients.5 However, already in 1931,6 the role of venous congestion in worsening renal function came to light, and this historical study demonstrated that glomerular intra-capillary pressure is about two-thirds the renal artery pressure. Reduction in glomerular intra-capillary pressure results in a decrease in renal perfusion pressure and consequent diminution of the glomerular filtrate rate (GFR).7 On the other hand, the effect of increased central venous pressure transmitted through low-resistance renal vessels in encapsulated organs, such as the kidneys, increases renal afterload and intrarenal pressure, driving renal dysfunction and resulting in a condition known as congestive nephropathy, which was recently described as a mechanism of AKI.8 The increase in pressure decreases renal perfusion and intratubular flow, leading to a decrease in GFR, an increase in sodium and water retention mediated by activation of the renin-angiotensin aldosterone system, and tubular damage mediated by the activation of inflammatory mechanisms.9
In the ICU setting, many factors could lead to venous congestion, such as fluid overload,9-11 positive pressure mechanical ventilation,12 right ventricular failure and pulmonary hypertension.13 However, there is no validated diagnostic test to assess whether venous congestion and/or fluid overload play a direct role in impairing renal perfusion. Recently, Doppler-based evaluation of intrarenal vessels has been used for assessing renal haemodynamics. With Doppler imaging, increase in renal venous congestion can be detected through the analysis of renal venous waveforms. Discontinuous intrarenal venous flow (IRVF) patterns suggest an increase in compliance of the renal parenchyma and its venous vessels secondary to increase in venous pressure within the encapsulated kidney.13–18 Doppler-based ultrasonography could have high clinical feasibility and acceptable reproducibility for the evaluation of IRVF in a non-invasive way.19,20 Therefore, the main objective of this systematic review and meta-analysis is to assess the ability of renal pulsed Doppler (RPD) evaluation of IRVF to predict the development of AKI in critically ill patients.
MethodsSearch strategy and eligibility assessmentThe present systematic review and meta-analysis were performed according to the general principles of the MOOSE statement21,22 (Supplementary Information 2).
We reviewed public-domain databases including Pubmed-MEDLINE, Scopus, Embase and the Cochrane Library. For the search, medical subject heading (MeSH) terms and text words were used with the Boolean strategy, and cross-searches were performed with the following three categories: (1) disease (“acute kidney injury” OR “kidney injury” OR “acute renal injury” OR “renal injury” OR “renal failure” OR “acute renal failure” OR “acute kidney failure” OR “kidney failure” OR “acute renal damage” OR “renal damage” OR “acute kidney damage” OR “kidney damage”) AND (2) tools (“renal Doppler” OR “renal vascular Doppler” OR “intrarenal Doppler” OR “intrarenal venous Doppler” OR “intrarenal vascular Doppler” OR “intrarenal arterial Doppler” OR “renal arterial Doppler” OR “renal venous Doppler” OR “renal ultrasound” OR “renal echography” OR “kidney ultrasound” OR “kidney echography” OR “kidney Doppler” OR “kidney vascular Doppler” OR “kidney arterial Doppler” OR “kidney venous Doppler” OR “renal resistive index” OR “renal venous flow” OR “Intrarenal venous flow”) AND (3) setting (“intensive care unit” OR “critical care” OR “critically ill patients” OR “critically ill patient” OR “critical illness” OR “critically ill” OR “critical care unit” OR “intensive care”). The search was restricted to articles published in English and studies on humans published between the 1st of January 2000 and the 31st of December 2021. We considered as type of study for inclusion, the randomised trial and the observational studies.
All references were downloaded for consolidation, elimination of duplicates and further analysis. Three authors, two intensivist and one nephrologist, (NSMBB, GRG and FF) independently determined the eligibility of all the studies identified in the initial research.
Data extractionTwo authors (NSMBB and GRG) carried out data extraction independently. Disagreements between the two were resolved by a third investigator (FF).
We framed the search around a PICOS (participants, intervention, comparison, outcomes, study design) model to determine the eligibility criteria of the studies to be included in this systematic review. The participants of interest were critically ill adult patients >18 years old admitted to any kind of ICU. Patients with end-stage chronic kidney disease and those who had undergone renal transplant were excluded. “Intervention” was considered as the execution of the RPD with the venous pattern at the time of ICU admission, and the comparison was IRVF pattern related to AKI occurrence.
The outcome of interest was the association between Doppler-based intrarenal venous flow demodulation (any IRVF pattern that is not continuous) and AKI. We included studies that diagnosed AKI based on the AKIN,23 RIFLE24 diagnosed AKI based and KDIGO3 criteria.
The study design included prospective and retrospective observational studies and randomised clinical trials.
Statistical analysisWe conducted all the analyses using the statistical package STATA 13. We statistically analysed the dichotomous outcomes using risk ratios (RRs) as the summary statistic. Data were pooled only for studies that reported sufficiently similar clinical and methodological variables. A pooled estimate of the RR was computed using the DerSimonian and Laird random-effects model.25 This calculation provides an appropriate estimate of the average treatment effect when studies are statistically heterogeneous. Heterogeneity among the studies was assessed by the I2 test and a null hypothesis test, in which p<0.1 was considered to indicate significant outcome heterogeneity.
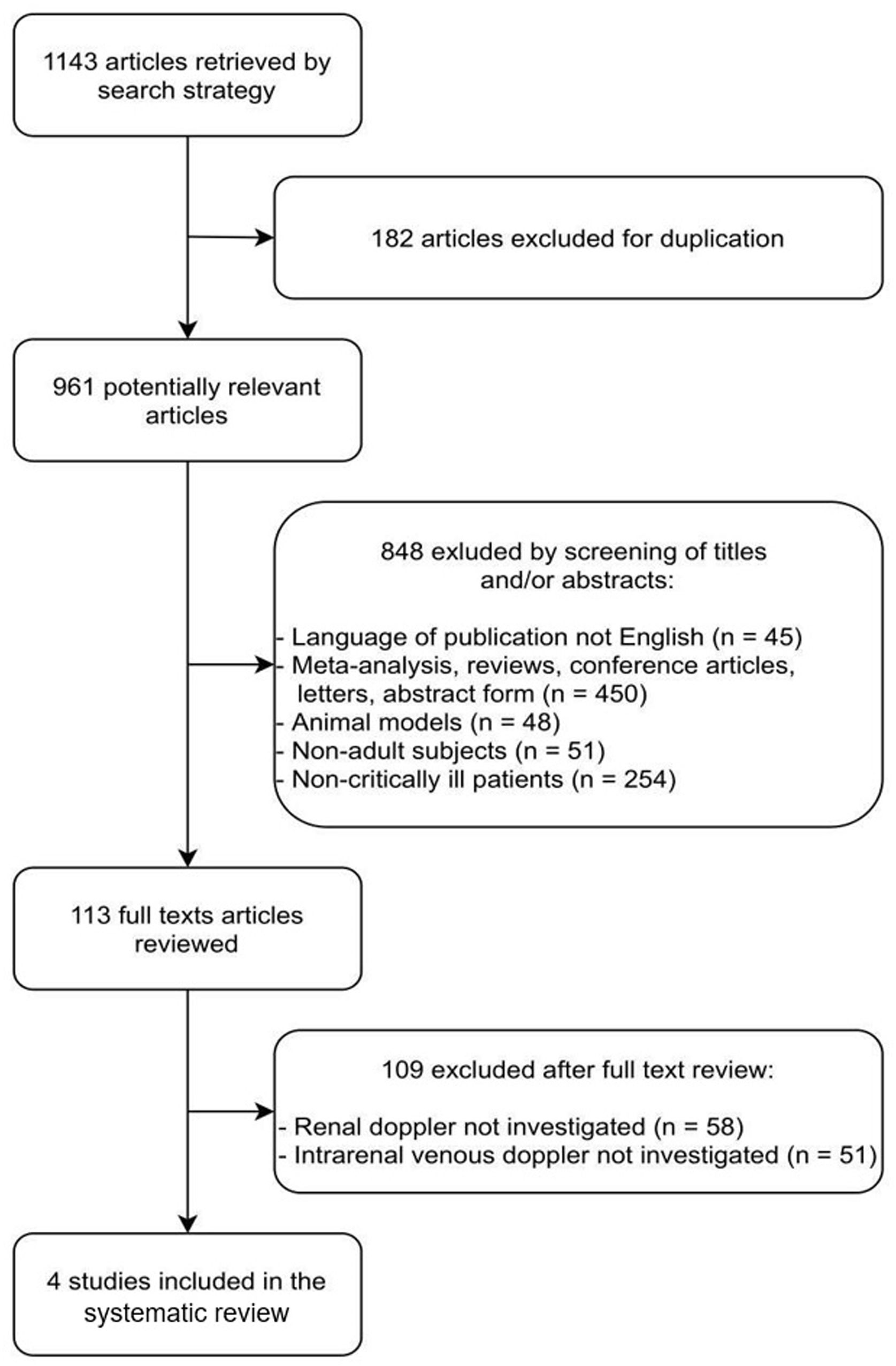
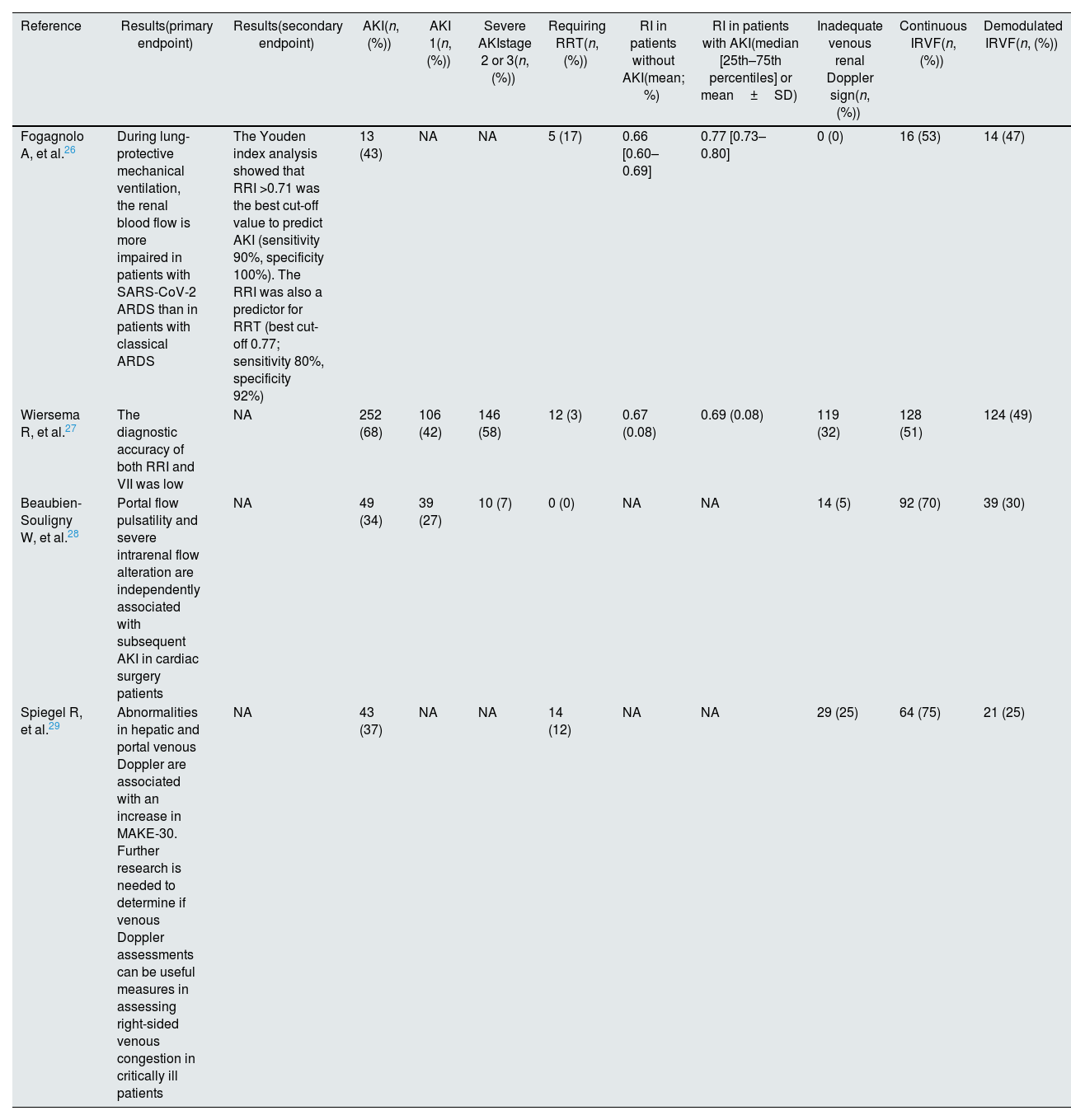
ResultsThe literature search yielded 1143 potentially relevant articles, of which 182 were excluded because they were duplicates. Among the 961 remaining articles, 848 were excluded because they were irrelevant based on the scope of this review (45 were not published in English; 450 were abstracts, letters, conference articles, reviews, or meta-analyses; 48 were conducted on non-human subjects; 51 included children; and 254 included non-critically ill patients).
The full texts of the remaining 113 articles were reviewed independently by two investigators (NSMBB and GRG) for further evaluation. Of these 113 articles, 109 were excluded because they did not include Doppler-based intrarenal venous flow evaluation (58 did not include any kind of RPD measurements, and 51 reported only arterial RPD measurements). Finally, four studies24–27 fulfilled our eligibility criteria and were included (Fig. 1 and Table 1).
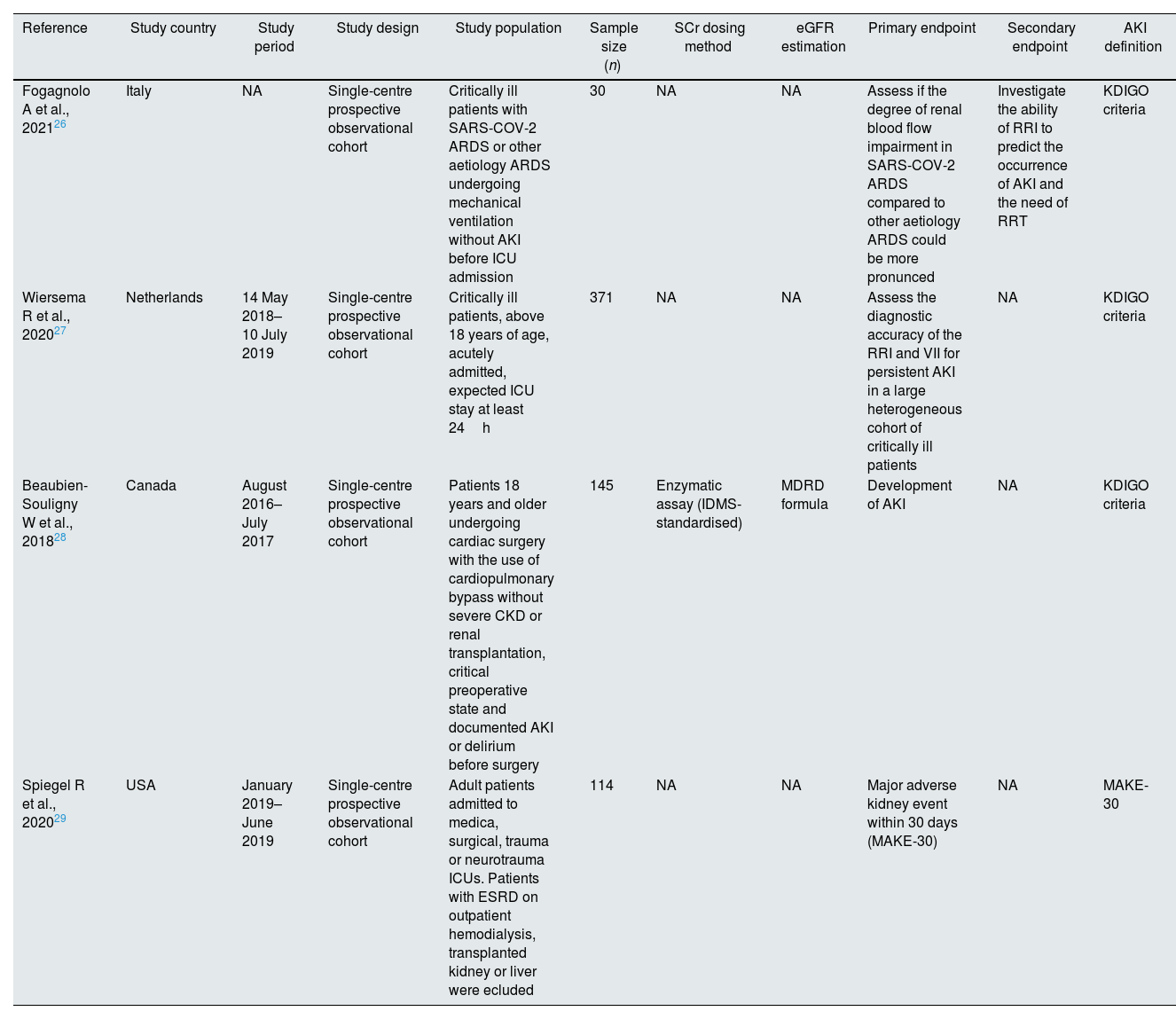
Characteristics of the selected studies.
| Reference | Study country | Study period | Study design | Study population | Sample size (n) | SCr dosing method | eGFR estimation | Primary endpoint | Secondary endpoint | AKI definition |
|---|---|---|---|---|---|---|---|---|---|---|
| Fogagnolo A et al., 202126 | Italy | NA | Single-centre prospective observational cohort | Critically ill patients with SARS-COV-2 ARDS or other aetiology ARDS undergoing mechanical ventilation without AKI before ICU admission | 30 | NA | NA | Assess if the degree of renal blood flow impairment in SARS-COV-2 ARDS compared to other aetiology ARDS could be more pronunced | Investigate the ability of RRI to predict the occurrence of AKI and the need of RRT | KDIGO criteria |
| Wiersema R et al., 202027 | Netherlands | 14 May 2018–10 July 2019 | Single-centre prospective observational cohort | Critically ill patients, above 18 years of age, acutely admitted, expected ICU stay at least 24h | 371 | NA | NA | Assess the diagnostic accuracy of the RRI and VII for persistent AKI in a large heterogeneous cohort of critically ill patients | NA | KDIGO criteria |
| Beaubien-Souligny W et al., 201828 | Canada | August 2016–July 2017 | Single-centre prospective observational cohort | Patients 18 years and older undergoing cardiac surgery with the use of cardiopulmonary bypass without severe CKD or renal transplantation, critical preoperative state and documented AKI or delirium before surgery | 145 | Enzymatic assay (IDMS-standardised) | MDRD formula | Development of AKI | NA | KDIGO criteria |
| Spiegel R et al., 202029 | USA | January 2019–June 2019 | Single-centre prospective observational cohort | Adult patients admitted to medica, surgical, trauma or neurotrauma ICUs. Patients with ESRD on outpatient hemodialysis, transplanted kidney or liver were ecluded | 114 | NA | NA | Major adverse kidney event within 30 days (MAKE-30) | NA | MAKE-30 |
The included studies were conducted between 2016 and 2020 and enrolled a total of 660 patients. All of them were single-centre prospective cohort studies (Table 1).
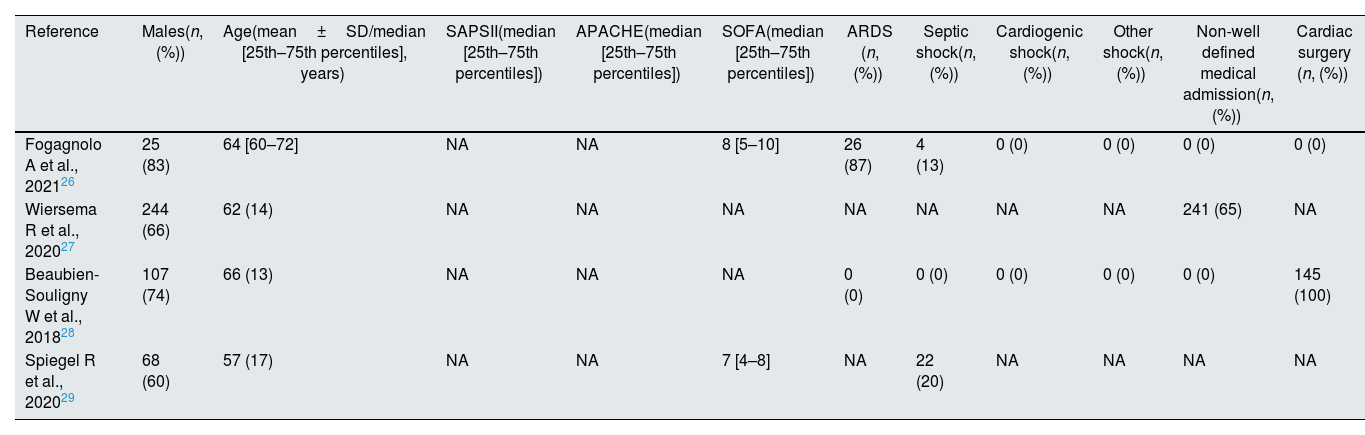
Two of the studies were conducted in Europe and included a total of 401 (60.75%) patients (of whom 371 were part of a Netherlands study27 and 30 were part of an Italian study26), and two were conducted in North America on a total of 259 (39.25%) patients (145 were recruited in a USA study29 and 114 were recruited in a Canadian study28). Of the 660 participants, 444 (67.27%) were males with an age range of 57–66 years (Table 2). The race of the participants was only specified by Spiegel et al.29: 58 (50.90%) were Caucasians, 49 (42.90%) were Africans and 2 (1.8%) were Asians. The reported diagnosis of the patients varied across studies: 275 (41.67%) were scheduled for surgery25,26 (of whom 145 underwent elective cardiac surgery28), 26 (3.94%) had septic shock24–27 and 26 (3.94%) had non-categorised Adults Respiratory Distress Syndrome (ARDS).26 However, the diagnosis at admission was not defined clearly in 241 cases (36.52%),27 and a diagnosis was lacking at the time of admission in 92 (13.94%) patients.29 The most recent study included 15 (2.27%) COVID-19 patients.26 Three of the selected studies24,25,27 were performed in a multidisciplinary ICU (these three studies included 515 patients or 78.03% of the total participants included in this review), while the fourth one28 was conducted in a specific post-cardiac surgery ICU (this study included 145 patients or 21.97% of the total participants). Severity score was determined in two studies24,27; both studies reported the SOFA score to range from 7 to 8 (Table 2).
Description of the target population of the studies.
| Reference | Males(n, (%)) | Age(mean±SD/median [25th–75th percentiles], years) | SAPSII(median [25th–75th percentiles]) | APACHE(median [25th–75th percentiles]) | SOFA(median [25th–75th percentiles]) | ARDS (n, (%)) | Septic shock(n, (%)) | Cardiogenic shock(n, (%)) | Other shock(n, (%)) | Non-well defined medical admission(n, (%)) | Cardiac surgery (n, (%)) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fogagnolo A et al., 202126 | 25 (83) | 64 [60–72] | NA | NA | 8 [5–10] | 26 (87) | 4 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Wiersema R et al., 202027 | 244 (66) | 62 (14) | NA | NA | NA | NA | NA | NA | NA | 241 (65) | NA |
| Beaubien-Souligny W et al., 201828 | 107 (74) | 66 (13) | NA | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 145 (100) |
| Spiegel R et al., 202029 | 68 (60) | 57 (17) | NA | NA | 7 [4–8] | NA | 22 (20) | NA | NA | NA | NA |
| Other major surgery(n, (%)) | Politrauma (n, (%)) | Spontaneous breathing(n, (%)) | Non-invasive ventilation(n, (%)) | PEEP during NIV(mean±SD; cmH20) | Invasive ventilation(n, (%)) | PEEP during IV(±SD/median [25th–75th percentiles]; cmH20) | Admission SCr(±SD/median [25th–75th percentiles]; mg/dl) | Admission BUN(±SD/median [25th–75th percentiles]; mg/dl) | Admission eGFR (mean±SD; ml/min/1.73m2) | CKD admission(n, (%)) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | 30 (100) | 12 [10–14] | 1.0 [0.7–2.5] | NA | NA | 7 (23) |
| 130 (35) | NA | NA | NA | NA | 234 (63) | NA | NA | NA | NA | 21 (6) |
| 0 (0) | 0 (0) | NA | NA | NA | NA | NA | NA | NA | 75.9 (±20) | NA |
| NA | NA | NA | NA | NA | 85 (75) | NA | NA | NA | NA | 5 (4) |
The quality of studies was assessed using the risk-of-bias method recommended by the Newcastle–Ottawa quality assessment scale for cohort studies.30 FF, NSMBB and GRG individually assessed the scores, which are presented in Supplementary Information 1.
Definition of renal function and AKIFogagnolo et al.26 defined renal function based on SCr level, and Beaubien-Souligny et al.28 defined renal function based on eGFR with the MDRD formula.31 However, renal function was not defined in the other two studies25,27 (Table 1).
Three studies24–26 defined AKI according to the KDIGO guidelines,3 without introducing any definition of the baseline SCr level. Spiegel et al.29 adopted major adverse kidney event (MAKE-30)32 as the primary outcome (Table 1).
Renal pulsed Doppler examination imaging quality, investigator, and timingAlthough assessment of both kidneys is preferable, the right kidney is generally more accessible. Therefore, all selected studies have limited the investigation to this side. The RPD examination was performed by investigators trained in RPD,25–27 under the supervision of a radiologist,25,26 a single well-trained anaesthesiologist with certified experience in examination of the right kidney,26 or a critical care fellow with 1-month clinical training in POCUS and transthoracic echocardiography. The inter-rater reliability between examiners or supervisors was evaluated only in two studies,26,27 and the method used was described in only one of these studies.28 In two studies,25,26 the investigators were blinded to the clinical and laboratory data. In the Spiegel et al. study, the images, interpretations and recommendations for care were not discussed with or made available to the treating clinicians, and Doppler waveforms were reviewed by two members of the research team who were blinded to the clinical outcomes.29 In one study,27 it was not disclosed whether the examiner was blinded to the patient data. Three studies25–27 reported that the venous renal RPD signal was inadequate. This meant that 162 (24.54%) of the patients did not have an IRVF evaluation (Table 3). Wieserma et al.26 performed RPD upon inclusion (mean time to inclusion was 13±7h); Spiegel et al.28 and Fogagnolo et al.25 within 24h from ICU admission and from the invasive mechanical ventilation start, respectively. Souligny et al. planned a preoperative ultrasound assessment27 and only they repeated the exam at Day #1, #2, #3 after the surgery.
Results of the studies.
| Reference | Results(primary endpoint) | Results(secondary endpoint) | AKI(n, (%)) | AKI 1(n, (%)) | Severe AKIstage 2 or 3(n, (%)) | Requiring RRT(n, (%)) | RI in patients without AKI(mean; %) | RI in patients with AKI(median [25th–75th percentiles] or mean±SD) | Inadequate venous renal Doppler sign(n, (%)) | Continuous IRVF(n, (%)) | Demodulated IRVF(n, (%)) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fogagnolo A, et al.26 | During lung-protective mechanical ventilation, the renal blood flow is more impaired in patients with SARS-CoV-2 ARDS than in patients with classical ARDS | The Youden index analysis showed that RRI >0.71 was the best cut-off value to predict AKI (sensitivity 90%, specificity 100%). The RRI was also a predictor for RRT (best cut-off 0.77; sensitivity 80%, specificity 92%) | 13 (43) | NA | NA | 5 (17) | 0.66 [0.60–0.69] | 0.77 [0.73–0.80] | 0 (0) | 16 (53) | 14 (47) |
| Wiersema R, et al.27 | The diagnostic accuracy of both RRI and VII was low | NA | 252 (68) | 106 (42) | 146 (58) | 12 (3) | 0.67 (0.08) | 0.69 (0.08) | 119 (32) | 128 (51) | 124 (49) |
| Beaubien-Souligny W, et al.28 | Portal flow pulsatility and severe intrarenal flow alteration are independently associated with subsequent AKI in cardiac surgery patients | NA | 49 (34) | 39 (27) | 10 (7) | 0 (0) | NA | NA | 14 (5) | 92 (70) | 39 (30) |
| Spiegel R, et al.29 | Abnormalities in hepatic and portal venous Doppler are associated with an increase in MAKE-30. Further research is needed to determine if venous Doppler assessments can be useful measures in assessing right-sided venous congestion in critically ill patients | NA | 43 (37) | NA | NA | 14 (12) | NA | NA | 29 (25) | 64 (75) | 21 (25) |
| Biphasic IRVF(n, (%)) | Monophasic IRVF(n, (%)) | AKI in continuous IRVF(n, (%)) | AKI in demodulated IRVF(n, (%)) | RRT dep (n, (%)) | follow up (n, (%)) |
|---|---|---|---|---|---|
| 7 (23) | 7 (23) | 0 (0) | 13 (100) | NA | NA |
| 108 (43) | 16 (6) | 44 (55) | 36 (45) | NA | NA |
| 22 (17) | 17 (13) | 27 (27) | 22 (51) | 0 (0) | NA |
| 19 (25) | 2 (2) | NA | NA | NA | NA |
AKI: acute kidney injury; IRVF: intra renal venous flow; NA: not available; RI: resistive index; RRT: renal replacement therapy; RRT dep: renal replacement therapy dependence.
AKI occurrence ranged between 34% and 68%. Severe AKI (KDIGO stage 2 and 3) occurrence was only found in two studies25,26: it was reported in 58%27 and 7%28 of the AKI patients (Table 3). Beaubien-Souligny demonstrated an association between monophasic venous flow and any AKI stage (HR=2.81; 95% CI 1.42–5.56; p=0.003). Fogagnolo et al.25 described a higher percentage of an impaired IRVF (13/13 in AKI group vs 1/17 in no AKI group; p<0.001) in the patients who developed AKI. On the other hand, Spiegel et al.28 demonstrated that the biphasic or monophasic patterns were not associated with an increase in the rate of MAKE-30 events in their cohort, as well as no difference was observed in IRVF patterns between patients with and without AKI by Wiersema et al.26
No study analysed the association between the IRVF patterns and the ethiology of AKI or with the renal recovery.
All the studies we reviewed had investigated the RPD venous pattern. We used data obtained from three26–28 of these in our metanalysis (in total 413 patients, because an inadequate venous RPD sign was found in 113 patients. One study29 was excluded because its data were inadequate for pooling. We found a significant difference between patients who had RPD continuous flow and demodulated IRVF (RR=0.46; 95% CI=0.28–0.76; Fig. 2). Further, our results revealed significant heterogeneity across the studies (I2=68.7%; 95% CI 0–90, p=0.04).
DiscussionAdequate preventive and curative strategies for AKI are still lacking. Indeed, SCr, the most widely used biomarker for diagnosing AKI that is available worldwide, has poor specificity, results in delayed diagnosis, and does not provide prompt detection of tubular injury. In addition, SCr kinetics and other factors (such as catabolism, fluid overload, nutrition and lean tissue status) can postpone or confound the diagnosis in critically ill patients.3 Other diagnostic methods, such as detection of new biomarkers, have been studied with regard to their ability for prediction, timely diagnosis and risk stratification of AKI.33 However, the diffusion of these diagnostic tools is still limited, and their cost cannot be borne in low- and middle-income countries. As a result of this situation, ultrasound scanning has become a reliable tool for clinicians in the emergency and critical care setting,34 but in the ICU setting, RPD still has limited use compared with lung ultrasonography and echocardiography.
Renal resistive index (RRI) and IRVF are the parameters used to assess renal perfusion and congestion. In the kidneys, which are capsulated organs, interstitial oedema resulting from renal insult translates into increased subcapsular pressure that ultimately causes a decrease in renal perfusion35 and is likely to cause a decrease in renal vascular compliance while increasing RRI. Additionally, kidneys are also affected by increased intrabdominal pressure that could be related to abnormalities in the abdomen itself or transmission of raised airway pressure in a stiffened lung through the diaphragm.34,35 In such situations, IRVF could be beneficial as it can suggest increased pressure in a close compartment, such as Gerota's capsule. Waveforms detected by ultrasound can not only reflect kidney injury, but also indicate the severity of congestion. Therefore, waveforms have prognostic significance and can be used to monitor the efficacy of a therapy. Moreover, as point-of-care ultrasonography can be performed by experts at the patient's bedside, immediate clinical integration of the imaging data is possible.
In the present systematic review and meta-analysis, we explored the ability of Doppler-based evaluation of IRVF demodulation in predicting the development of AKI in critically ill patients. Notably, our findings also provide several insights that go beyond the primary objective of the study.
Our results indicated that patients with continuous IRVF had less than half the risk of developing AKI than patients with demodulated IRVF. All selected studies contributed to this result (Fig. 2).
However, despite the high quality of the included studies, our metanalysis showed a significant heterogeneity.
This heterogeneity may be partially explained by the small number of studies included in the metanalysis.36 On the other hand, based on the PICOS criteria, we identified adult critically ill patients, but we could not find a uniform target population. Many factors differentiate the population of the selected studies. The age of the population ranged from 57 to 66 years, and male sex was prevalent in all the studies. Severity scores were reported in only two studies, both of which exclusively calculated the SOFA scores,24,27 then we could not compare the severity of the illness of each study population. The diagnosis for which the patient was admitted to the ICU was not clearly defined in 241 cases (36.52%) and there was no diagnosis at admission in 92 (13.94%) patients. Furthermore, the multidisciplinary nature of some TI studies suggests a varied melting pot of diagnoses and degrees of severity.
Bedsides, RPD examination was performed in critically ill patients with various diagnoses at the time of ICU admission and in only one study28 RPD has been revaluated during ICU stay. Finally, AKI was defined based on the KDIGO guidelines in three of the studies, but only some of the data described renal function at the time of admission or baseline kidney function.
RPD examination was performed by investigators trained under the supervision of a single well-trained anaesthesiologist with certified experience or a critical care fellow with 1-month clinical training in POCUS and transthoracic echocardiography. The inter-rater reliability between examinators or supervisors was evaluated in two studies, but the method used was described in only one. RPD examination could not be performed in 24.54% of the patients, but the authors did not clearly explain the reason why it could not be performed or the limits of its feasibility and reliability.
However, the evaluation of RRI and IRVF is fast, non-invasive and repeatable, and can be performed in most patients by inexperienced operators after a half-day course.39 In critically ill patients, the interobserver reproducibility between a senior and inexperienced operator is good and the measures seem accurate (as indicated by the absence of systematic bias in the reviewed studies), although a lack of precision was found (based on an interobserver 95% confidence interval of ±0.1).37
ConclusionsDespite its limitations, IRVF is useful for detecting venous congestion and congestive nephropathy, which are associated with impaired renal function. Although the preliminary results in this field are promising, nowadays controversial results have been published in this setting. The heterogeneity of our metanalyses demonstrates once again that confirmatory studies are still needed to assess the feasibility of using IRVF measured with RPD as a predictor of AKI in critically ill patients.
Authors’ contributionsNSMBB conceived the study and participated in study design, acquisition of data, interpretation of data, drafting of the manuscript.
GAR participated in acquisition of data and participated in critical revision of the manuscript.
ADS performed the statistical analysis.
FF participated in study concept, design and coordination, statistical analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript.
FHS, participated in critical revision of the manuscript.
FF, NSMBB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors read and approved the final manuscript.
FundingNone declared.
Conflict of interestThe authors have declared that no conflict of interest exists.