Editor’s note
This collaboration and consensus document between the Spanish Society of Internal Medicine and the Spanish Society of Nephrology has been published in the journals of both scientific societies: Revista clínica Española (DOI: 10.1016/j.rce.2012.01.001) and the current issue of Nefrología [Nefrologia 2012;32(Suppl.1):1-35; doi:10.3265/Nefrologia.pre2011.Dec.11298].
INTRODUCTION
Lupus nephritis affects over half of all patients with systemic lupus erythematosus (SLE). This condition increases mortality and morbidity rates among patients due to, among other reasons, the risk of chronic kidney disease with the need for renal replacement therapy in approximately 25% of cases. Lupus nephritis is diagnosed in our health area in women in their thirties and is the primary cause of systemic disease with secondary renal involvement.1 Although marked advances have been made in recent decades in the diagnosis and treatment of this condition, there are several aspects that require collaboration between different specialists.
With the objective of establishing a consensus on the primary subjects related to the diagnosis, treatment, and follow-up of patients with lupus nephritis, the auto-immune disease group (Grupo de Enfermedades Autoinmunes Sistémicas, GEAS) of the Spanish Society of Internal Medicine (Sociedad Española deMedicina Interna, SEMI) and the Spanish Society of Nephrology (S.E.N.) has formed a joint task force for the elaboration of a consensus document following a critical review of the available literature.
METHODS
Both scientific societies proposed five representatives that held their first meeting in Barcelona on 17 June 2010. At this meeting, the participants elaborated a list of concrete topics that should be covered, and each of the five topics was assigned to a group, headed by two authors, one from each scientific society. After a literature review a first draft was composed by each of the task forces, then the committee proceeded with a general discussion on each of the sections during two plenary sessions held in Madrid on 16 September 2010 and 9 February 2011, in which the final recommendations were decided upon between the 10 group members. After another round of review, the final manuscript was discussed electronically and agreed upon by all authors.
The format chosen to create the document was that of recommendations based on published evidence, evaluated using the GRADE system (Table 1).2-4 Each of the recommendations is followed by a summary of the literature they were based on.
1. DIAGNOSIS, DEFINITIONS, AND CRITERIA
1.1.Classification
Proposals
• Lupus nephritis should be classified according to the histological classes defined in 2003 by the International Society of Nephrology (ISN) and the Renal Pathology Society (RPS) (NG).
• The histological analysis requires optical microscope and immunofluorescence techniques, and an electron microscope analysis is also recommended (NG).
• Quantified data on the activity and chronicity of the disease should be included, along with a description of vascular and interstitial lesions (NG).
Justification
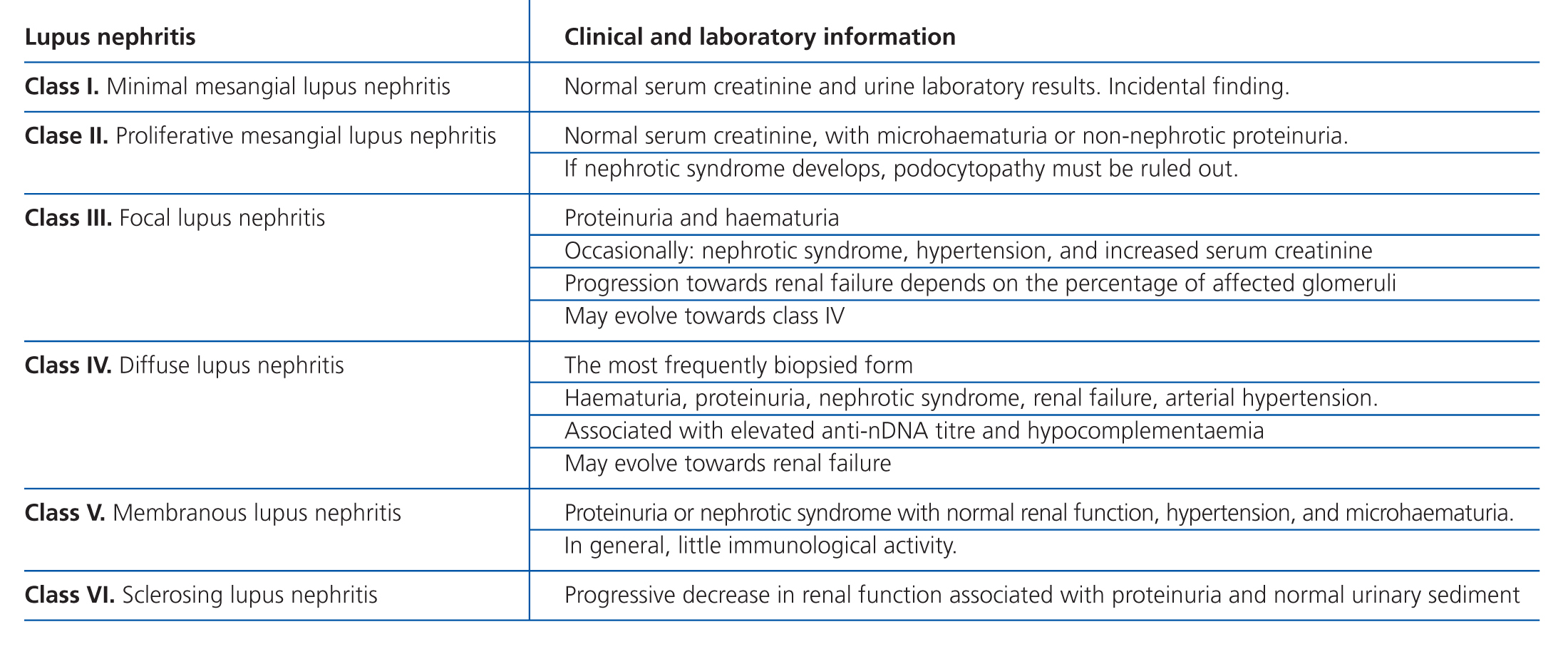
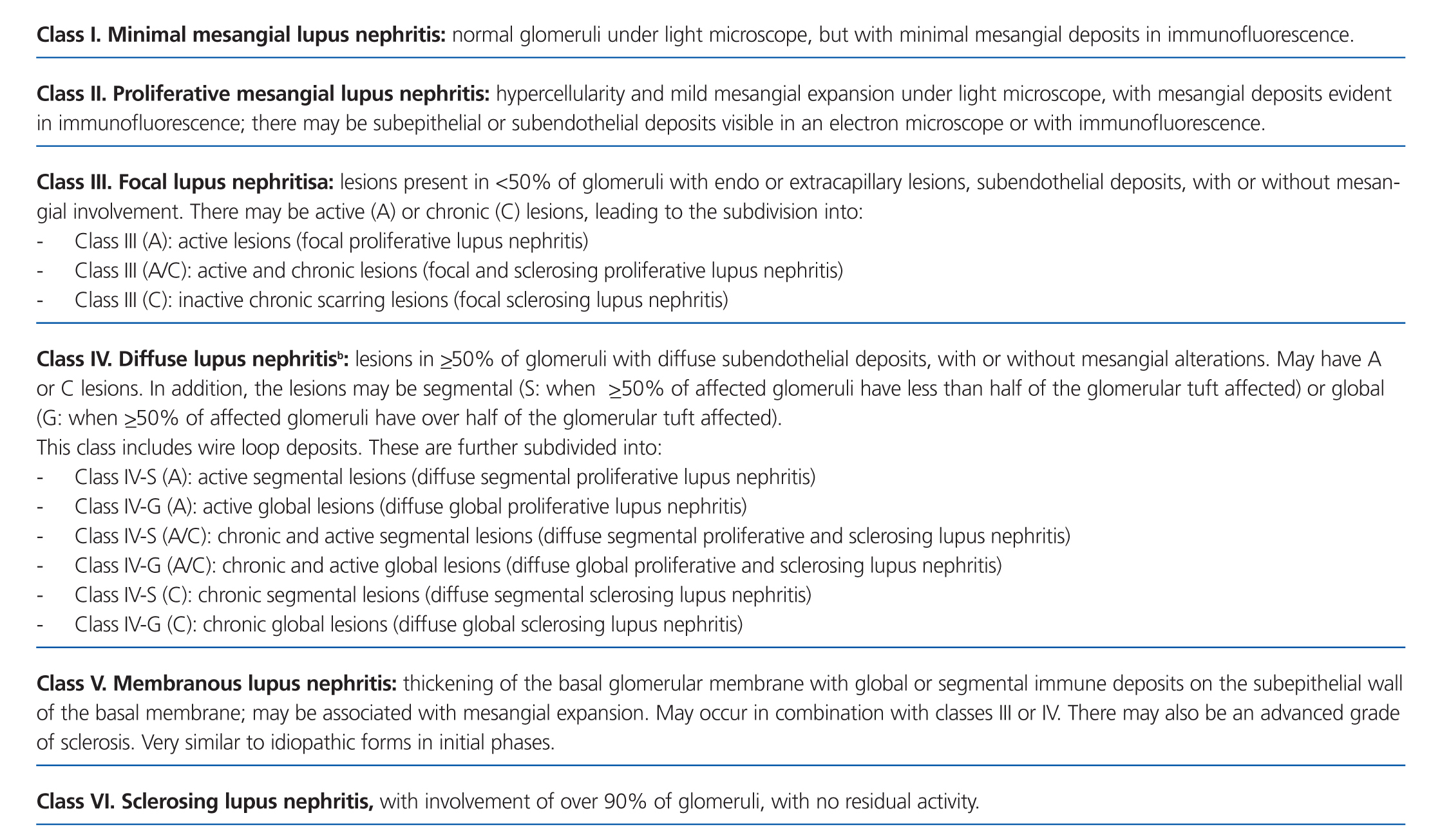
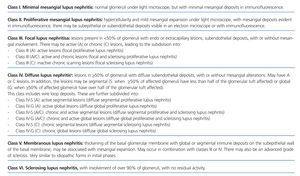
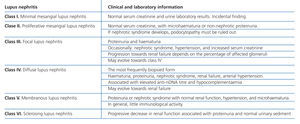
Lupus nephritis should be classified according to the results from a renal biopsy. Normal clinical and laboratory analyses cannot predict the histological findings in a high percentage of cases. The histopathological diagnosis plays a leading role in establishing a prognosis and treatment. The current classification system was proposed jointly by the ISN and RPS in 2003.5 This system established 6 different classes of disease based on analysis with optical microscope, immunofluorescence, and electron microscope (Table 2).
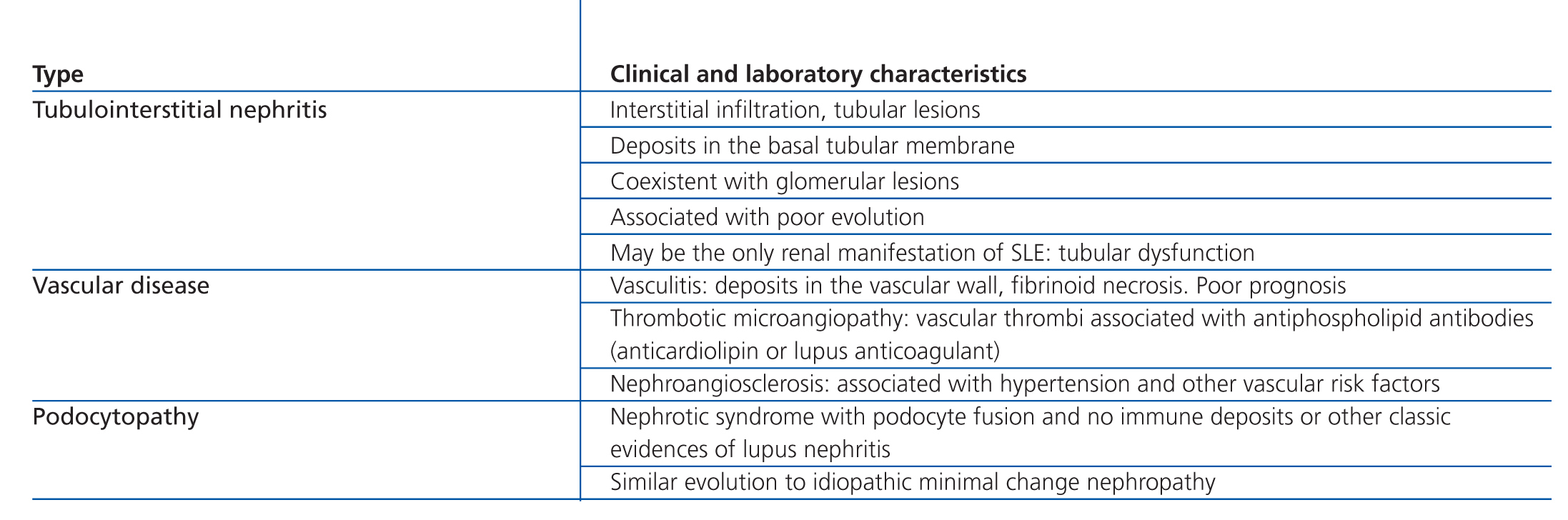
This classification system has demonstrated good inter-observer reproducibility6 and there is good correlation between clinical and histological data (Table 3). The primary issue is distinguishing between classes IV-S and IV-G, that is to say, the significance of global and segmental lesions in the disease’s clinical manifestations and especially in its prognosis.7,8 We must also point out that the renal lesions caused by lupus nephritis are not static, and transition between classes may occur, whether spontaneously or following treatment. There can also be a certain degree of overlap at any moment of the disease’s evolution.9 We should also point out the different levels of activity and chronicity, quantified according to the parameters detailed in Table 4. Finally, patients with SLE could have other renal lesions not contained in the ISN/RPS classification system, which are summarised in Table 5.
1.2.Indications for renal biopsy and second biopsy
Proposals
• Patients with SLE and proteinuria, haematuria, active urinary sediments, or renal failure should undergo a biopsy (NG).
• A second or successive biopsies would only be indicated if the findings could lead to a change to the treatment or prognosis of the disease (NG).
Justification
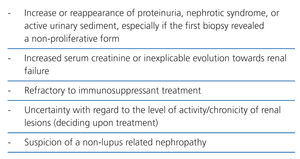
A renal biopsy is mandatory in patients with SLE and tests results indicative of renal involvement, such as increased creatinine, reduced glomerular filtration rate, proteinuria, haematuria, and active urinary sediments. The presence of isolated haematuria should be interpreted with caution due to the possibility of vaginal contamination, urinary infection, tumours, a history of treatment with cyclophosphamide, or familial haematuria. A renal biopsy provides essential information for a) identifying the ISN/RPS class; b) establishing a prognosis, and c) planning treatment.10 A biopsy should be included as a preferential or even emergency procedure based on the severity of the patient’s condition in all treatment protocols, following normal precautions.11 The indications for a first renal biopsy in patients with SLE are shown in Table 6. If patients have proteinuria <0.5g/24 hours and inactive urinary sediments, a renal biopsy is not indicated, but clinical and laboratory parameters should be monitored every six months, or every three months in the case of sustained elevated anti-nDNA antibodies and/or hypocomplementaemia. These intervals can be shortened based on clinical and laboratory criteria.
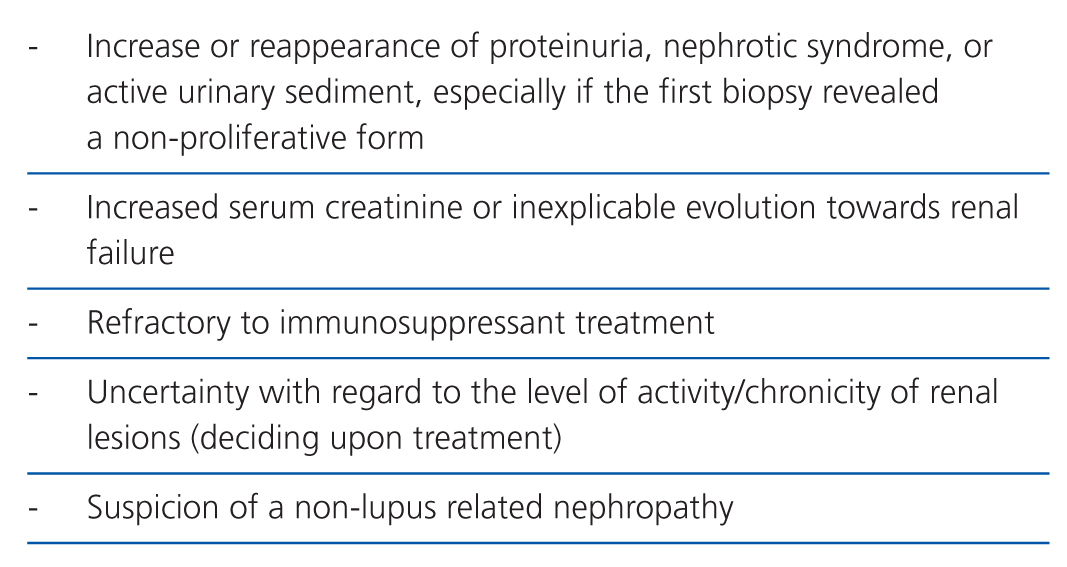
The indications for a second biopsy are more debatable for two reasons: the possible complications, and the doubts concerning their influence in treating patients.12 A repeated biopsy is not recommended if the patient has good evolution, or has reached an adequate response.13 However, there are other situations in which a second renal biopsy would be indicated (Table 7).14
1.3. Clinical and laboratory parameters
Proposals
• Patients with lupus nephritis should be tested for normal clinical and laboratory parameters in chronic patients, specifying the variables involved in the development of cardiovascular complications (NG).
• Renal involvement and immunological activity should be evaluated every 3 months, by determining creatinine, proteinuria, anti-nDNA, C3, and C4 (NG).
• Proteinuria should be measured in 24-hour urine samples, although the follow-upprotocol may only include the protein/creatinine ratio in first morning urine (NG).
Justification
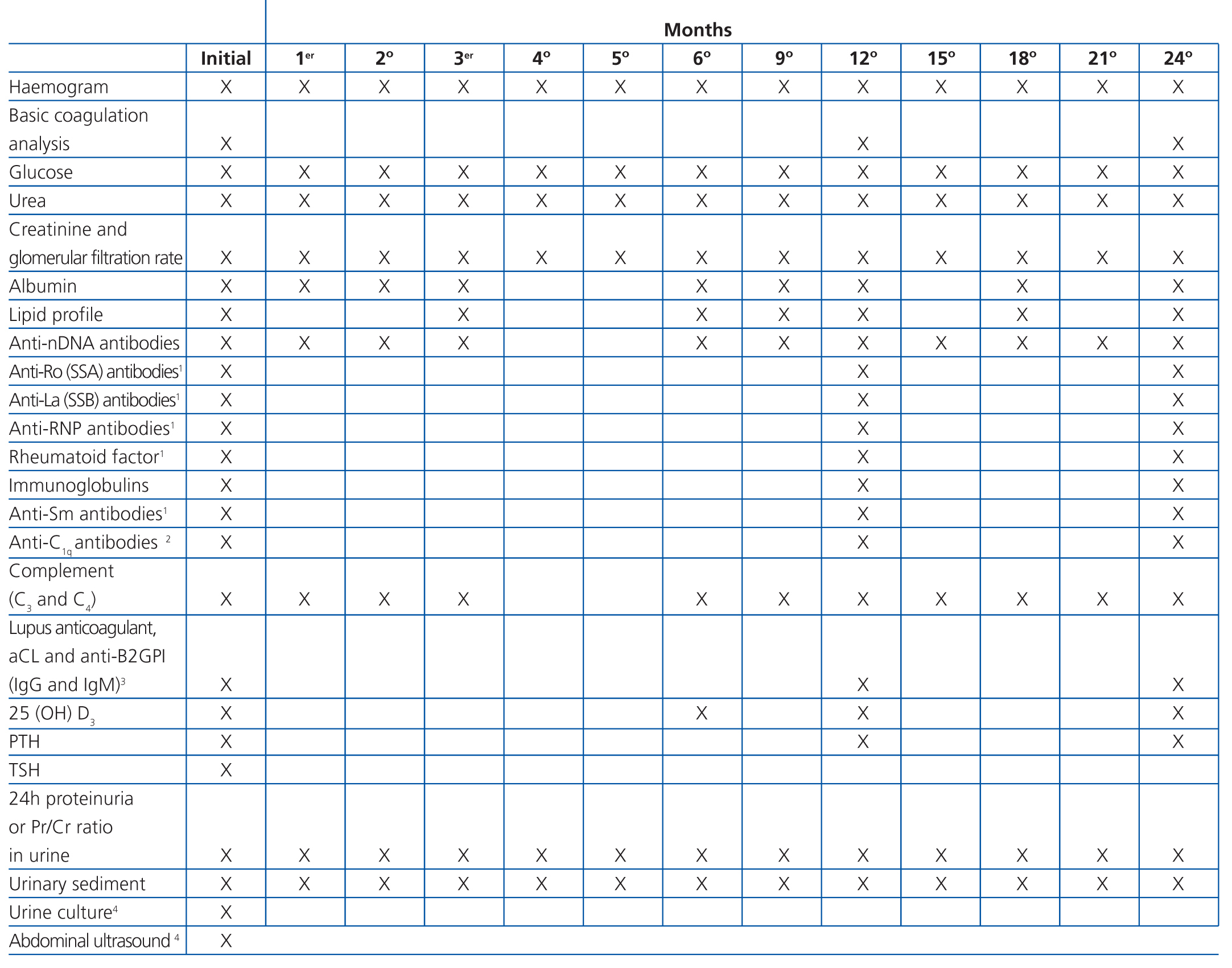
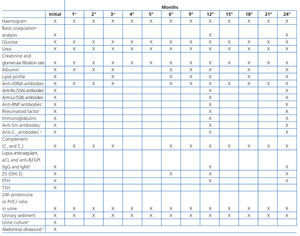
Although histological results are essential for evaluating lupus nephritis, it is also necessary to evaluate a series of basic clinical and laboratory parameters, both in the initial phase of patient treatment and in follow-up visits. Table 8 is a reference chart for the different parameters and the frequency with which each should be analysed following the diagnosis of lupus nephritis and in the first two years of follow-up. The parameters and their frequency of collection should be individualised based on the characteristics of each patient and their current stage of disease, taking into account that the patient will require life-long follow-up.
There is some debate regarding how to measure proteinuria. Although the gold standard is 24-hour urine samples, the protein/creatinine ratio, whether in 24-hour urine or first morning samples, can be equally useful in the initial evaluation and during follow-up.15 In a recent study, the use of the protein/creatinine ratio in 12-hour urine samples taken at night was defended as the best method for detecting renal recurrence and to monitor response to treatment,16 although we should point out the problem inherent in collecting the urine sample. In our opinion, the protein/creatinine ratio in first morning urine samples is a good method for evaluating the evolution of proteinuria, although 24-hour urine is more convenient to use in initial phases. There are not data regarding the use of microalbuminuria as a marker for renal involvement in lupus nephritis, although it may be of value as a marker for cardiovascular risk.
There are three serological markers of activity that are very useful in lupus nephritis: anti-nDNA, C3, and C4. Additionally, anti-C1q are very specific for renal activity, although they are not available for use in clinical practice in the majority of health institutions. Immunological activity is very unlikely to occur if all of these markers are at normal levels.17 Although the diagnostic and prognostic value of other biological markers have been examined, none have shown sufficient sensitivity and specificity to be used in clinical practice.18 Some patients may have circulating anti-neutrophil cytoplasmic antibodies (ANCA), but with no specific clinical significance.19
1.4. Criteria for complete and partial responses
Proposals
• The responses must be evaluated according to the criteria for complete and partial responses (NG).
• Responses are based on the evolution of creatinine, proteinuria, and urinary sediment values as compared to baseline values (NG).
Justification
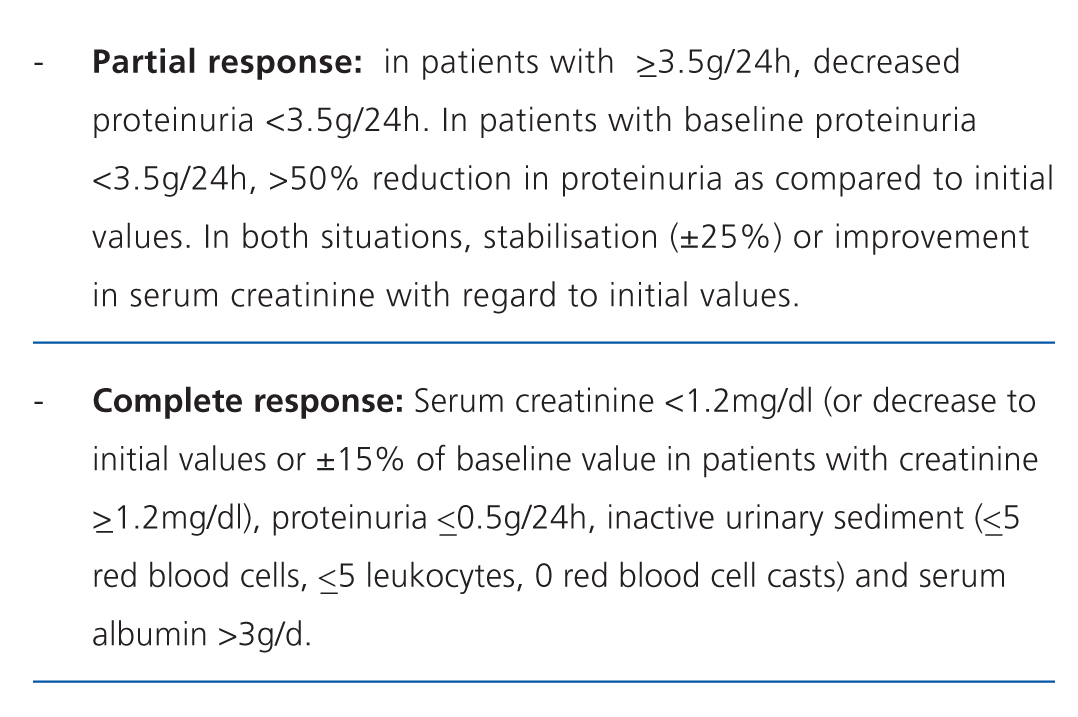
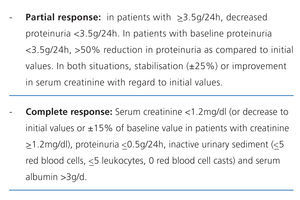
There is no standard definition for the patterns of response to treatment, although all are based upon the values observed in basic laboratory variables.20 The most commonly used criteria, which are divided into partial and complete response, are summarised in Table 9.21 The inclusion of haematuria as a deciding factor is debatable, since it can be confounded by the procedure of taking the urine sample, and in patients that have received cyclophosphamide, it does not necessarily indicate inflammatory activity. However, dysmorphic red blood cells and cell casts are very specific to glomerular damage.
1.5. Criteria for renal recurrence
Proposal
• Recurrences in patients that have reached a good response to treatment should be evaluated for the appearance of proteinuria, increased creatinine levels, changes in urinary sediments, and, in general, the presence of immunological activity (NG).
Justification
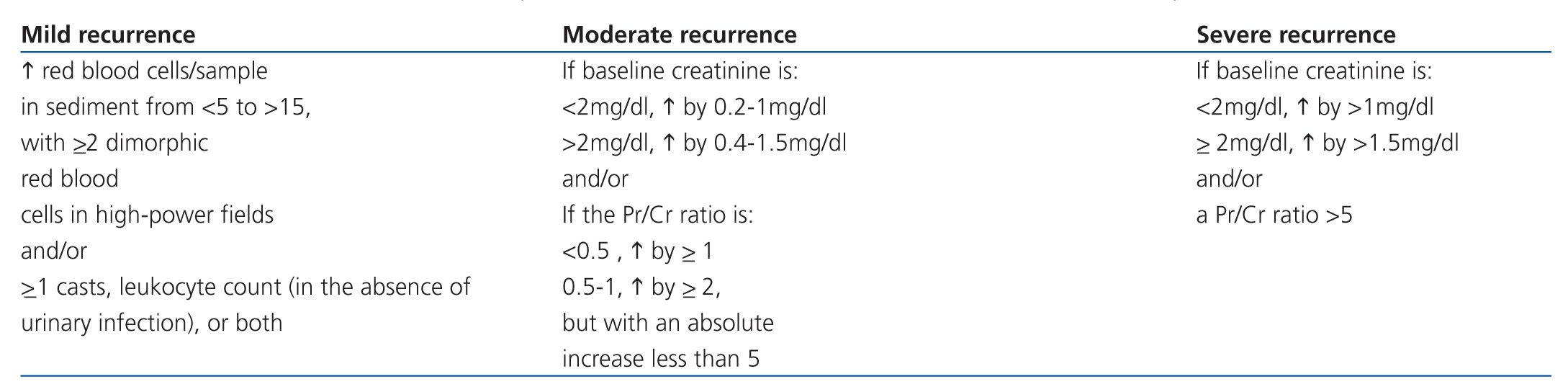
There is no unanimous decision regarding the criteria used for renal recurrence, which is also based on the changes observed in creatinine, proteinuria, and urinary sediment levels as compared to baseline values. Recurrence tends to be accompanied by reduced complement and increased anti-nDNA titres. In cases of severe recurrence, renal function deterioration may be present. The most commonly accepted criteria for renal recurrence are summarised in Table 10.
When treatment fails (failure to respond or recurrence), it is important to rule out non-compliance with treatment, especially in young patients that may have problems adapting to a chronic disease.22
2. THERAPEUTIC GENERALISATIONS
2.1. Corticosteroids
Proposals
• Given the level of associated morbidity, the use of oral corticosteroids has been recommended only at the lowest doses and shortest time spans possible (1B).
• In severe cases, intravenous pulses of methylprednisolone at 250mg-1000mg is recommended at the start of treatment and as adjuvant therapy during the induction phase (1B).
• Depending on the starting dosage, the amount of prednisone prescribed should be rapidly reduced, until reaching a maintenance dose no greater than 5mg/day, or even halting this treatment based on the activity of the disease (1C).
Justification
Oral prednisone is the normal baseline treatment for lupus nephritis. It has been clear for many years that combination therapy with immunosuppressive drugs is more effective than monotherapy with corticosteroids.23 However, there are no comparative studies that have tested prednisone at different doses. In fact, the formula of 1mg/kg/day is established only as the customary dosage.
From the pharmacological point of view, the relationship between beneficial and adverse effects of prednisone doses is not completely linear. Different doses activate different pathways, with important differences in the potency and speed of the anti-inflammatory activity of the drug, as well as the undesirable effects, the majority of which have to do with genomic mechanisms.24 Genomic effects are maximum at >30mg/day, with increased potency of non-genomic effects (greater speed and potency) after doses >100mg/day.25
Observational studies and clinical trials, most of which included patients with rheumatoid arthritis, show that adverse effects such as osteoporosis, osteonecrosis, diabetes, severe infections, cataracts, and cardiovascular disease do not or rarely appear at doses ≤7.5mg/day, and they exponentially multiply after doses >10mg/day.26-28
In patients with SLE, observational studies have shown that prednisone is an important cause of irreversible organ damage,29-31 with increased associated risk of osteoporotic fractures, avascular necrosis, cataracts, coronary disease, and stroke.30 On the contrary, methylprednisolone pulses have not been shown to be related to any of these complications. Other cohort studies have established the association between maximum doses of prednisone and thrombosis,32 and still others between the duration of steroid treatment and the presence of carotid plaques33 and ischaemic heart disease34 in patients with lupus.
With regard to the efficacy of these drugs in patients with lupus nephritis, and in the absence of direct comparisons, the different clinical trials that have been published all show similar results using induction doses of 1mg/kg/day of prednisone21,35,36 or 0.5mg/kg/day,37,38 or even less.39 The addition of methylprednisolone pulses, both at the start of treatment37,38 and during the entire induction phase,38 can improve the long-term prognosis of these patients, without increasing the incidence of adverse effects. Recent studies examining what dose of methylprednisolone should be used have shown that regimens <1000mg/day are equally effective in severe flares of lupus, and with a lower frequency of associated infections.40
Without a doubt, there is no evidence that can indisputably support induction therapy for lupus nephritis with elevated doses of prednisone, and indirect results suggest that lower doses can be equally effective. Additionally, the association of methylprednisolone pulses increases the potency of the treatment without increasing the rate of adverse effects. On the other hand, we do have solid evidence indicating that corticosteroids produce severe adverse effects, with a notable correlation between these drugs and the development of irreversible damage and cardiovascular disease in patients with lupus. The adverse effects of prednisone are dosage-dependent, and on a long-term basis, are clearly correlated with doses >5mg/day.
2.2. Hydroxychloroquine
Proposals
• We recommend that patients with SLE receive hydroxychloroquine on a long-term basis if no contraindications exist. Lupus nephritis, sustained remission, and pregnancy should not indicate halting hydroxychloroquine treatment (1B).
• We recommend including a yearly ophthalmological examination in the monitoring regimen for adverse effects, especially when dealing with cumulative doses of hydroxychloroquine >1000g (1C).
Justification
A recent systematic review has revealed that the use of anti-malarial medications increases the survival of lupus patients, with a >50% decrease in long-term mortality rates.41 A cohort study published afterwards confirmed these results, and also demonstrated an effect dependent on the duration of treatment.42 Other effects that stand out from anti-malarial treatments are protection against organ damage and thrombosis and the prevention of flares of lupus activity.41
In the specific field of lupus nephritis, three retrospective studies have pointed to the usefulness of anti-malarial medications as adjuvant treatment. In a cohort of 29 patients with membranous nephritis treated with mycophenolate mofetil, the rate of remission was greater in patients that received hydroxychloroquine (64% vs 22%; P=.036).43 A second case-control study analysed the factors associated with extended remission,44 finding that 94% of patients in extended remission had received hydroxychloroquine, as compared to 53% of controls (P=.01). Finally, a retrospective study with 206 patients with lupus nephritis showed that previous treatment with hydroxychloroquine was associated with decreased progression to stage 5 renal failure, thrombosis, cardiovascular disease, infection, and death.45 More recently, a study involving the LUMINA prospective cohort demonstrated that patients with lupus nephritis that receive hydroxychloroquine suffered less severe development of kidney damage (hazard ratio: 0.29; 95% confidence interval [CI]: 0.13-0.68).46
Given its better safety profile, hydroxychloroquine is recommended over chloroquine.41 Although the frequency of maculopathy is low, it does increase after cumulative doses of 1000g, and so an ophthalmological examination is recommended before using the drug and active monitoring should follow on a yearly basis.41,47
2.3. Anti-proteinuria drugs
Proposals
• We recommend that patients with lupus nephritis, proteinuria, and/or arterial hypertension receive renin-angiotensin-aldosterone system (RAAS) blockers (1B).
• We recommend weight loss if the patient is obese due to the beneficial effects on proteinuria and the progression of the renal disease (1C).
Justification
A sustained increase in protein elimination through the urine is considered to be an additional risk factor for the progression of renal disease.48 Since the studies involving lupus nephritis are limited, the beneficial effects of RAAS blockers in chronic glomerular diseases and diabetic glomerulonephritis may be extrapolated.49,50 These drugs include angiotensin II-converting enzyme (ACE-II) inhibitors, as well as angiotensin receptor blockers (ARB). The anti-proteinuria effect of these drugs is independent on the decrease in blood pressure.51 In certain patients with no deterioration in glomerular filtration rates, proteinuria may be further reduced using combined ACE/ARB inhibitors, closely monitoring the development of adverse effects on glomerular filtration rate and the possibility of hyperkalemia. There is no information available regarding the possible additional benefit of aliskiren on proteinuria, whether alone or in combination with ARB.
Recently, the LUMINA multi-ethnic prospective study concluded that the treatment of lupus patients with ACE inhibitors delays the development of nephritis as measured using renal biopsy.52 The probability of avoiding renal involvement after 10 years was 88.1% in the group of patients receiving ACE-inhibitors, as compared to 75.4% in those that did not (P=.0099). The group treated with ACE inhibitors also developed a lower percentage of proteinuria and/or lupus nephritis, as measured using renal biopsy, than the untreated group (7.1% vs 22.9%; P=.016). Long-term, prospective studies are needed to evaluate the administration of ACEi/ARB and clear up doubts regarding the efficacy and safety of these drugs in patients with different types and stages of lupus nephritis.
Obesity is considered to be a risk factor for the progression of chronic nephropathies. Five controlled studies have shown that weight losses achieved through various methods were associated with decreased mean proteinuria of up to 1.7g. The meta-regression analyses show that each kg of weight lost in obese patients with proteinuria allowed for a mean decrease in proteinuria of 110mg, regardless of the changes in blood pressure.53
2.4. Cardiovascular risk and arterial hypertension
Proposals
• We recommend evaluating cardiovascular risk and implementing both pharmacological and non-pharmacological measures to decrease the probability of developing accelerated arteriosclerosis (1B).
• We recommend precise control of blood pressure, since this decreases the incidence of cardiovascular events and improve renal survival (1B).
Justification
It is well-established that patients with SLE have a higher incidence of arteriosclerosis than the general population,54,55 as well as a higher risk of acute myocardial infarction.34 This is probably due to the combination of several causes, some of which are common in the general population (age, hypertension, hypercholesterolemia, tobacco use) and others related to genetic factors, the stage of the chronic inflammatory disease, and the treatments received. In patients with lupus nephritis, over 40% of all deaths, both in the short and long term, are due to cardiovascular problems.56
In these circumstances, the risk of developing arteriosclerosis and ischaemic heart disease will influence both the quality and quantity of life of the patient, and so it is essential to evaluate the cardiovascular risk of lupus patients so as to modify diet, avoid harmful habits (tobacco use, sedentary lifestyle, and high salt intake) and start pharmacological treatment. An altered lipid profile, which is frequently observed in lupus patients with atheromatous plaques in the carotid artery, is closely associated with the cumulative dose of corticosteroids.57 The LASER study showed that patients with SLE and a background of ischaemic heart disease were older, with a higher proportion of males, and had received corticosteroids or azathioprine at higher doses than those with no background of coronary disease.58 Hypercholesterolemia should be treated intensely, regardless of treating the possible underlying causes.
Optimal control of blood pressure slows down the progression of chronic kidney disease and also reduces the risk of cardiovascular events.59 Chronic tubulointerstitial damage occurs in patients with chronic kidney disease and proteinuria. Controlling proteinuria with losartan, diuretics, and a no-salt diet is associated with reduced excretion of tubular damage markers and improved glomerular filtration rates and proteinuria.60 Thus, RAAS-blocking drugs would be the first option because of their anti-proteinuria, anti-hypertensive, and protective effects for the kidney. However, some patients may require combined therapies that may involve diuretics and calcium antagonists.
The blood pressure levels that should be reached in patients with lupus nephritis can be similar to the currently recommended values for other groups with high cardiovascular risk, such as patients with diabetic nephropathy, and should not exceed 130/80mm Hg. In order to ensure these objectives, outpatient blood pressure monitoring may be useful.
Tobacco use has been established as an additional risk factor that predicts the appearance of the first cardiovascular event in patients with SLE,61 mainly in patients of African descent.62
2.5. Gastric protection
Proposal
• We recommend the use of drugs for gastric protection in patients with a history of gastrointestinal haemorrhage or ulcerative disease and those under concomitant treatment with corticosteroids and non-steroidal anti-inflammatories (1B).
Justification
Monotherapy with glucocorticosteroids causes a slight increase in the risk of gastrointestinal complications (bleeding or perforation).63 Another case is the concomitant use of prednisone and non-steroidal anti-inflammatories, which is associated with a risk of gastrointestinal toxicity 12 times higher.63 On the other hand, chronic suppression of gastric acidity may produce adverse effects, since it can interfere with the absorption of iron, calcium, and vitamin B12, as well as increasing the rate of colonisation of the upper digestive tract by enterobacteria and Clostridium.64,65 As such, it must be used with caution.
2.6. Bone protection
Proposals
• We recommend that patients treated with corticosteroids receive oral supplements of calcium and vitamin D, as long as no contraindications exist (1A).
• We recommend administering bisphosphonates as a preventative measure against osteoporosis and fractures in patients older than 50 years, or at younger ages if the patient has a history of fracture (1A).
• We recommend regular monitoring of circulating 25(OH) Vitamin D and treatment if levels are abnormal (1C).
• We recommend preventing secondary hyperparathyroidism in lupus patients with renal failure (1C).
Justification
The presence of osteopaenia and osteoporosis presents a serious problem for patients with SLE of both sexes. The analysis of risk factors is complex, since the available studies have been performed with patients from very different periods and varied treatment regimens. Without a doubt, it is difficult to determine the exact role of corticosteroids in the loss of bone mass in adults with lupus, since age, weight, and menopause are additional risk factors.66,67
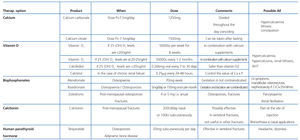
Furthermore, after a systematic review, the American College of Rheumatology has recently recommended, with the highest possible level of evidence, oral calcium supplements (1000mg/day-1500mg/day) and vitamin D preparations in patients that are to receive treatment with corticosteroids for over three months.68 Additionally, 25(OH) Vitamin D and levels must be measured regularly in patients with lupus, since deficiencies of this vitamin are very common due to several factors, especially photoprotection.69 Although the physiological and clinical consequences of 25(OH) Vitamin D and deficit in patients with SLE is under debate, some authors have suggested an inverse relationship between 25(OH) vitamin D concentrations and the activity of the lupus disease and increased cardiovascular risk.70
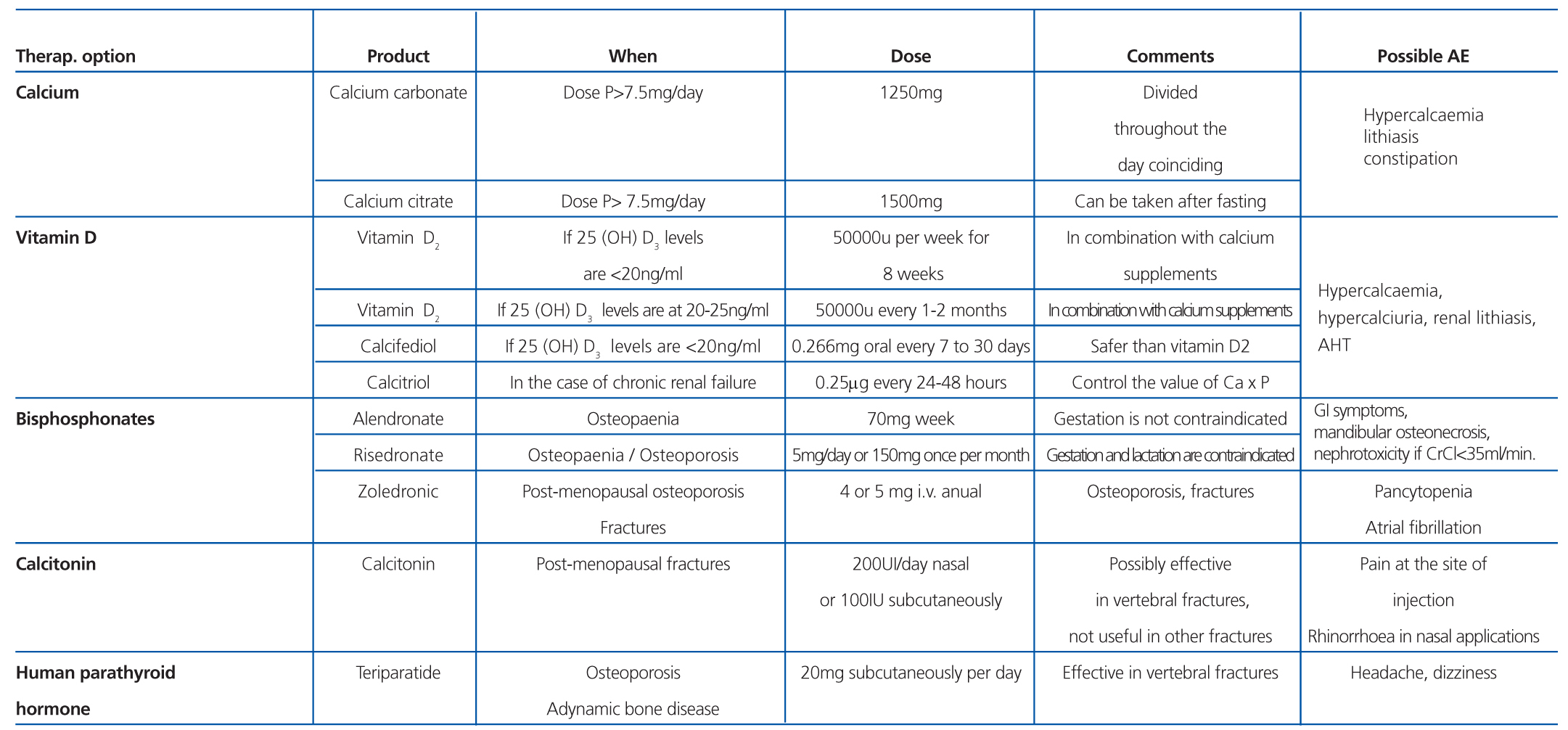
In post-menopausal patients and males older than 50 years that are to receive corticosteroids for more than three months, we recommend administering bisphosphonates (Table 11). In pre-menopausal women and men younger than 50 years, the recommendations are less clear, since the risk of fracture is not well defined. Additionally, the risks of taking bisphosphonates for extended periods and the adverse effects on gestating mothers recommend caution in using these drugs. Compounds with a shorter half-life, such as alendronate, should be used in these patients when also taking prednisone doses equal to or greater than 7.5mg/day.68
We also recommend taking other actions, such as quitting smoking, limiting the daily intake of alcohol, and regularly monitoring bone density. Patients with glomerular filtration rates <50ml/min should have parathyroid hormone (PTH) levels regularly checked, since secondary hyperparathyroidism may be present. In addition, serum phosphorous levels should be controlled through proper diet, and oral phosphorous binding agents, and PTH secretion-suppressing drugs, such as calcitriol and paricalcitol, can be used along with regular measurements for preventing the appearance of hyperkalemia and excessive parathyroid suppression.71
2.7. Ovary protection and hormonal contraception
Proposals
• We recommend not exceeding a cumulative dose of 10g cyclophosphamide in order to minimise the risk of ovarian toxicity (1C).
• We recommend using GnRH analogues in order to preserve ovary function in women older than 35 years if the dose of cyclophosphamide is >10g (1C).
• We advise against the use of oestrogen-based contraceptive methods in women with active lupus nephritis or antiphospholipid antibodies (1C).
Justification
A recent review72 established a 50% risk of permanent amenorrhea in women older than 32 years that received a cumulative dosage >8g/m2. However, other authors have published values lower than 15%.37,73 In any case, ovarian toxicity produced by cyclophosphamide is clearly determined by the cumulative dose and age of the patient. Women that receive cumulative doses >10g of cyclophosphamide, particularly if they are older than 30-35 years, should be considered at a high risk of suffering early menopause.72
GnRH analogues (leuprolide), administered on a monthly basis intramuscularly, are effective at reducing the incidence of amenorrhea. A recent meta-analysis that included studies (mostly observational) of women with lupus and different types of cancer treated with cyclophosphamide concluded that the probability of maintaining fertility was 68% greater (relative risk: 1.68; 95% CI: 1.34-2.1) when using leuprolide.74 However, this difference was not significant in any of the three studies analysed that included women with SLE. The total dose of cyclophosphamide administered was not specified in the majority of studies. Although it has been suggested that leuprolide may increase the risk of thrombosis or worsen lupus activity, the adverse effects observed so far have been mild and uncommon.75
The safety of oral contraceptive medications containing oestrogens in women with SLE has been analysed in two recent clinical trials.76,77 Neither study observed an increase in lupus activity in patients taking contraceptive medications, although women with severe lupus activity were excluded from the study. As such, we cannot ensure that oestrogen-based contraceptive medications do not worsen cases of active lupus nephritis.
On the other hand, the very small number of thrombotic episodes registered were produced in patients with antiphospholipid antibodies, even at low levels.76,77 One population-based study carried out recently demonstrated that the risk of stroke was much higher in women younger than 50 years on lupus anticoagulants that received oestrogen-based treatments.78 As a consequence, the presence of antiphospholipid antibodies should be considered as a contraindication for administering oestrogen-based contraceptives.
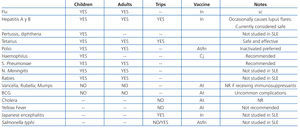
2.8. Vaccinations and infectious prophylaxis
Proposals
• We recommend carrying out all vaccination protocols based on age and to avoid vaccines containing live or attenuated viruses during immunosuppression (1B).
• We recommend vaccinating patients before they are to receive B lymphocyte-depleting agents (1B).
Justification
Extreme caution should be taken to avoid whenever possible the appearance of infection by opportunistic micro-organisms, which can occur with greater frequency in the case of active disease and intense immunosuppression, especially during the induction phase. Recording the history of all vaccines received in the initial clinical history is recommended in order to plan a tentative vaccination schedule.79 Infections can be favoured by a state of pharmacological immunosuppression or immune deficit associated with lupus. This includes congenital or acquired immunoglobulin and complement deficits and decreased splenic function, with a greater risk of infection by encapsulated bacteria and Salmonella.
The efficacy of vaccination programmes in patients with chronic inflammatory diseases (rheumatoid arthritis, vasculitis, and lupus) has been shown to have a similar or slightly lower response rates than those obtained in healthy control subjects. However, treatment with tumour necrosis factor alpha (TNFα) antagonists and, to a much greater extent, rituximab, has been associated with inadequate immunological responses to vaccines against the cold virus and pneumococcus. In contrast, the response to the tetanus vaccine under the same circumstances is not altered. As such, we recommend programming vaccinations at least four weeks before treatment with rituximab, or to wait six months for vaccinations after administering this drug.79
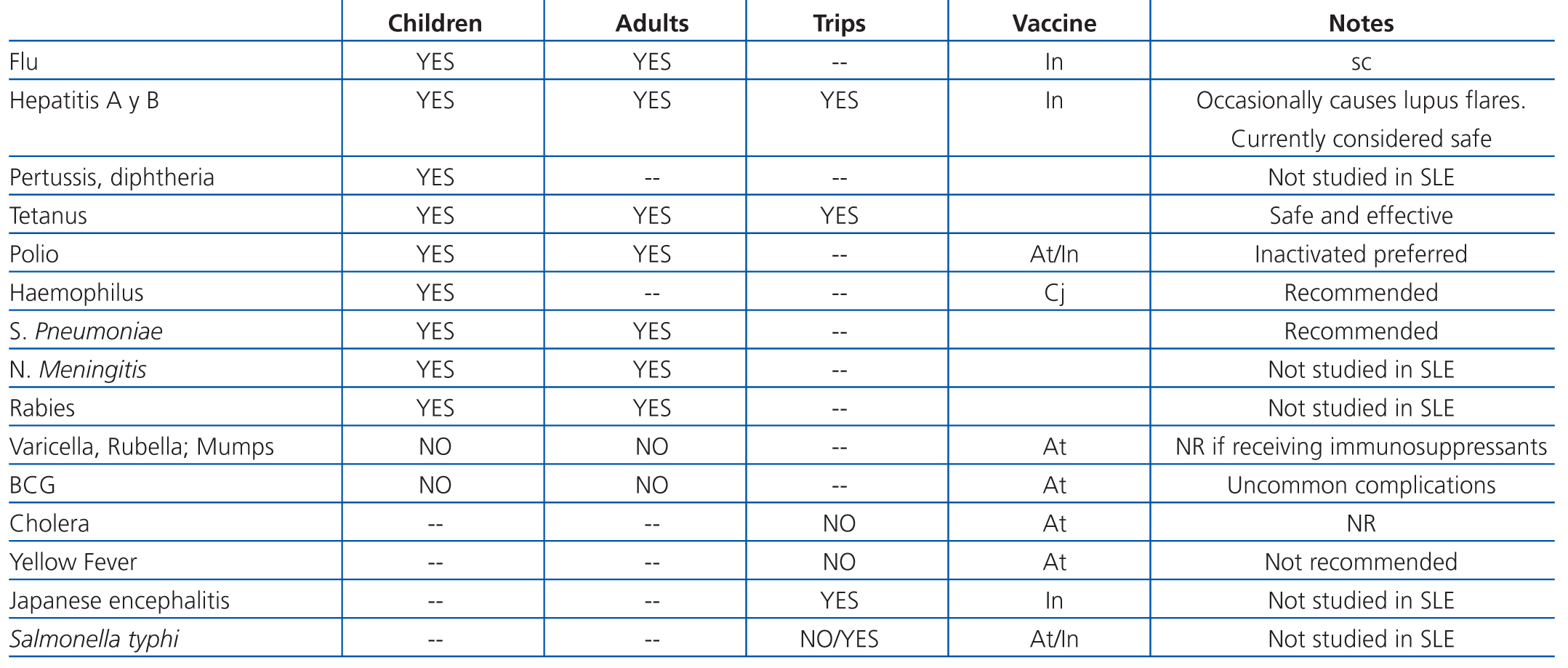
Studies that have specifically addressed vaccinating lupus patients are limited.80 It has been suggested that certain vaccines act as triggering factors for flares of lupus activity, although this suspicion is based on case series with a low level of evidence. Table 12 summarises the recommendations for vaccinating patients with lupus nephritis.79
Before starting immunosuppressive therapy, the presence of active or latent infection by Mycobacterium tuberculosis should first be evaluated. So far, there is no evidence for recommending prophylaxis against cytomegalovirus or herpes virus with drugs such as ganciclovir or acyclovir, although the risk of infection by these viruses should be taken into account. Some authors recommend prophylaxis against Pneumocystis jiroveci with trimethoprim-sulfamethoxazole during treatment with cyclophosphamide in patients with auto-immune disease, despite the lack of precise evidence. They justify this recommendation by extrapolating the benefits obtained in patients with human immunodeficiency virus (HIV) and those that have undergone a kidney transplant (KT), taking into account that the prevalence of colonisation exceeds 10% and can be exacerbated in situations of pharmacological immunosuppression.81 In any case, risk should be evaluated on an individual basis for each patient (immunosuppressant load, lymphocytopenia, etc.)
3. INDUCTION AND MAINTENANCE THERAPY WITH IMMUNOSUPPRESSIVE DRUGS.
TREATMENT DISCONTINUATION
3.1. What are induction and maintenance therapy?
The treatment of the most severe histological classes of lupus nephritis (classes III and IV) and class V is divided into two phases.82 The first phase, or induction of response, has the objective of producing early remission of the renal flare and to avoid progression towards chronicity. This is achieved using intensive immunosuppressive treatment. The duration of this phase can vary, but in general it lasts from three to six months, or even longer if the patient still has signs of active disease in the kidney. The second phase, or maintenance, has the objective of avoiding the development of renal flares during the evolution of the disease, and to maintain the improvements achieved during the induction phase. In general, this phase involves a less intense immunosuppression regimen. The duration of this phase, and thus the duration of treatment for lupus nephritis, is indefinite, but it generally lasts at least two years after remission is established. It is also important that all of this treatment be undergone with the least possible secondary side effects.
The number and type of immunosuppressive drugs used, their dosage, and duration of treatment must all be decided upon based on the clinical manifestations of the patient, his/her renal function, the histological characteristics observed in the renal biopsy, and the evolution towards a complete or partial response.
3.2. Treatment categorised according to the histological class of the renal biopsy
3.2.1. Class I
Proposals
• Given that the diagnosis of class I lupus nephritis is only histological and is not accompanied by altered clinical or laboratory parameters, immunosuppressive treatment should not be administered. The treatment of these patients should be determined by their extra-renal manifestations (NG).
• The appearance of significant proteinuria, nephrotic syndrome, or macroscopic haematuria in patients diagnosed with class I lupus nephritis would require ruling out associated glomerular processes or histological evolution towards other classes of lupus nephritis with a new renal biopsy (NG).
Justification
Class I lupus nephritis is an exclusively histopathological finding, since patients remain asymptomatic from a clinical and laboratory analysis point of view, and as such do not present any indications for performing a renal biopsy.
There are very few studies that have evaluated the prevalence of histological classes I and II in patients with SLE.83 These studies are based on renal biopsies in patients with no indication of renal involvement. So far, and despite some authors considering class I to be the initial stage of lupus nephritis, there is no evidence to support a renal biopsy at the moment of diagnosing SLE in patients with no signs of renal involvement, nor the need to treat these patients with class I lupus nephritis.84
3.2.2. Class II
Proposals
• Immunosuppressive treatment would not be initially indicated in patients with class II lupus nephritis. The treatment of these patients should be determined by their extra-renal manifestations (NG).
• In the presence of significant proteinuria (>1-2g/day despite renal protective treatment) and/or deteriorated renal function that is not attributable to functional factors, we suggest that patients receive steroid treatment (up to 0.5mg/kg/day) whether accompanied or not by immunosuppressive drugs (azathioprine, mycophenolate), such as corticosteroid-sparing drugs for 6-12 months (2D).
Justification
There is no data with a high level of scientific evidence regarding the optimal treatment of class II lupus nephritis. In a systematic review of cases published on mesangial nephritis, some of which had histological characteristics of proliferative mesangial disease, the resulting recommendations were to treat patients with significant proteinuria (the majority being in nephrotic range), active sediment, arterial hypertension, or deteriorated renal function using glucocorticosteroids.85 The dose of glucocorticosteroids is not specified, although it is high in all cases published. In patients with recurrence, azathioprine is proposed as the first choice, although some patients were treated with other immunosuppressive drugs.
3.2.3. Classes III (A and A/C) and IV (A and A/C)
3.2.3.1. Induction therapy
Proposals
• In patients with class III or IV lupus nephritis, we recommend treating with glucocorticosteroids (1A) accompanied by one of the following therapeutic options:
o Cyclophosphamide (1B).
o Mycophenolate mofetil (1B) or enteric-coated mycophenolate sodium (2C).
• We recommend that glucocorticosteroids be started with a dose of prednisone up to 1mg/kg/day (maximum dose of 60mg/day), although smaller doses as low as 0.5mg/kg/day can be used with concomitant pulses of methylprednisolone (1B).
• We suggest using intravenous (IV) pulses of methylprednisolone (250mg/day-1000mg/day for three consecutive days) in the presence of extracapillary proliferation in the renal biopsy or in patients with acute deterioration in renal function (2C).
• In order to reduce the cumulative dose and avoid the well-known toxicity that can be produced when using this drug, we recommend administering cyclophosphamide in IV pulses under one of the following administration regimens:
o Monthly IV pulses of 750mg/m2 body surface area for six consecutive months. (1B)
o IV pulses every two weeks with a fixed dose of 500mg for three months (a total of 6 pulses) (1B). If this regimen is used, the patient must first receive pulses of methylprednisolone (750mg/day for 3 days) followed by prednisone at doses of 0.5mg/kg/day.
• If mycophenolate is chosen for induction therapy, it should be commenced at oral doses of 1g/day (divided into two doses) in the form of mycophenolate mofetil or 720mg (divided into two doses) in the form of enteric-coated mycophenolate sodium. These doses should be progressively increased over two weeks until reaching a dose of 2g/day-2.5g/day (mycophenolate mofetil) (1B) or 1440mg-1880mg (mycophenolate sodium) (2C), divided into 2-3 daily doses.
• We suggest that the treatment regimen include IV cyclophosphamide in cases of severe deterioration in renal function (serum creatinine >3mg/dl) and those with cellular crescents or fibrinoid necrosis lesions in the biopsy (2C).
• The patient’s race, socioeconomic conditions, and the probability of proper compliance with the prescribed treatment are all factors shat should be considered before deciding between a treatment regimen that includes IV cyclophosphamide or mycophenolate (NG).
Justification
Histological classes III and IV are more severe and so require intensive treatment based on combined therapy with corticosteroids and immunosuppressive drugs.86 The majority of studies performed until now have involved glucocorticosteroids at high levels initially, up to 1mg/kg/day, or IV pulses for more severe cases.87 As previously mentioned, this dose is based on nothing more than customary practice, since the only available studies simply compared different doses of prednisone.
In this sense, there is indirect evidence that lower initial doses of corticosteroids could be sufficient to push a renal flare of disease into remission. In studies by the Euro-Lupus Nephritis Trial (ELNT), comparing two different treatment regimens with cyclophosphamide, the initial dose of glucocorticosteroids was three pulses of 750mg methylprednisolone followed by 0.5mg/kg/day prednisone for four weeks, and then a progressively descending dosage.37,88 In another study sponsored by the National Institutes of Health (NIH) in the USA, monthly pulses of methylprednisolone were compared with monthly pulses of cyclophosphamide, and with a combined treatment scheme.38 All patients also received treatment with prednisone at initial doses of 0.5mg/kg/day for four weeks, followed by progressively decreasing amounts. As was described in the previous section, a small randomised clinical trial with 29 patients with proliferative lupus nephritis compared one continuous dose of oral cyclophosphamide with pulses of cyclophosphamide combined with methylprednisolone,39 and this second group was also treated with prednisone at 0.3mg/kg/day, as opposed to 0.85mg/kg/day in the first group. No significant differences were observed between the two groups in terms of a clinical response. Finally, a study of 81 patients with class III and IV lupus nephritis analysed the efficacy of mycophenolate sodium in combination with two different dosages of glucocorticosteroids.89 All patients also received a daily pulse of methylprednisolone (500mg) during three days and were then randomised to receive initial doses of prednisone at 1mg/kg/day or 0.5mg/kg/day, both in similar progressively descending amounts. The lowest dose of glucocorticosteroids achieved the same results regarding complete remission (19% vs 18%), although not in partial remission (48% vs 33%), with better percentages in terms of the appearance of adverse effects (10.3% vs 16.7%).
According to these studies, it is possible that initial doses of prednisone of 1mg/kg/day may not be needed, and that smaller doses along with pulses of methylprednisolone could be sufficient for achieving remission in patients with proliferative lupus nephritis, with a lower associated toxicity level. However, this fact must be confirmed through future randomised studies.
As regards immunosuppressive drugs, both cyclophosphamide and mycophenolate mofetil are supported by sufficient scientific evidence to be considered as first lines of induction therapy for proliferative lupus nephritis. Until now, at least four systematic reviews and six meta-analyses have analysed the available randomised and observational studies that have compared cyclophosphamide (oral or in pulses) with mycophenolate mofetil.90-95 In general, mycophenolate mofetil presents a better profile for secondary side effects, as well as a lower incidence rate of leukopenia and amenorrhea. In the first studies on the subject, mycophenolate mofetil also demonstrated itself to be more effective for inducing remission,90-92 as well as in some composite variables such as death and terminal chronic renal failure.92 However, after the publication of the ALMS study, the largest study published with 370 patients,21 the conclusion of the most recent meta-analyses is that mycophenolate mofetil is similar to cyclophosphamide in terms of efficacy.93-95 Few studies have evaluated the effectiveness of enteric-coated mycophenolate sodium in patients with proliferative lupus nephritis, although the results are positive and similar to those achieved using mycophenolate mofetil.89,96,97
As for the two different methods for administering cyclophosphamide pulses, the ELNT studies demonstrated that the low dose (500mg pulses every 14 days for 3 months) is similar in terms of efficacy to the high dose (750mg/m2 every month for 6 months), both at 5 and 10 years.37,88 One possible limitation to these studies is that all patients included in the studies were Caucasians, and most did not have severe renal involvement, making debatable the extrapolation of the results to other types of patients, such as those of African descent or Latin Americans, or those with severe initial forms of lupus nephritis.
Finally, the choice of one immunosuppressant or another could be made based on other variables such as race, socioeconomic conditions, or the probability of compliance with the prescribed treatment. Regarding race and the ethnic origin of the patient, we consider that a sub-analysis of the ALMS study21 showed that patients of African descent and mixed race responded worse to cyclophosphamide than to mycophenolate mofetil. In a similar manner, Latin American patients had a better response to mycophenolate mofetil.98 On the other hand, the cost of treatment with mycophenolate mofetil can be greater than with cyclophosphamide, although several studies show conflicting results in this regard.99,100 In patients suspected of a low level of compliance with prescribed oral treatment plans, IV pulses of cyclophosphamide allow for ensuring that the patient will receive proper immunosuppression.
3.2.3.2. Maintenance therapy
Proposals
• We suggest that, once they have completed the induction therapy and have reached at least a partial response, patients with class III or IV lupus nephritis receive maintenance treatment with low doses of steroids and mycophenolate mofetil or enteric-coated mycophenolate sodium (2D) as the first option over azathioprine (2A).
• We recommend that the maintenance dose for mycophenolate mofetil range between 1.5g/day and 2g/day (mycophenolate mofetil) (1B) or 1080mg/day-1440mg/day (mycophenolate sodium) (2C), divided into two doses.
• The duration of treatment with mycophenolate should be at least two years once remission has been reached (2C). The dose of mycophenolate should be progressively reduced before completely halted (2C).
• The initial dose for maintenance therapy using azathioprine should range between 1.5mg/kg/day and 2mg/kg/day (1B). The duration of treatment and gradual reduction in dosage should follow the same trend as in the case of mycophenolate (2C).
• If a response has been achieved at the start of maintenance therapy, the dose of prednisone should be set at a maximum of 10mg/day. After this moment, the prescription should be progressively reduced, always attempting to reach the lowest possible amount (≤5mg/day) (2B).
• Steroids should be administered at the lowest dose possible, but for as long as the patient is being treated with mycophenolate or azathioprine (2C).
• After discontinuing treatment with mycophenolate or azathioprine, prednisone should be maintained during a period that will vary based on the characteristics of the patients. If there is no clinical or laboratory evidence of activity in patients that have not experienced previous recurrences, we suggest a slow and gradual reduction of the dose until suspending it entirely (2D).
Justification
In 2004, Contreras, et al101 showed in a randomised clinical trial that both mycophenolate mofetil and azathioprine were more effective and safer than pulses of cyclophosphamide during the maintenance therapy phase in proliferative lupus nephritis, with no significant differences between the two drugs. There were no differences in efficacy and safety between these two treatments in other studies, although the number of patients included in these publications was small.36,102 In fact, two meta-analyses published between 2007 and 2010 concluded that there were no differences in efficacy or the appearance of adverse effects between mycophenolate mofetil and azathioprine in maintenance therapy regimens for proliferative lupus nephritis.91,95
Following these meta-analyses, the results for two different randomised clinical trials were published that analysed the same issue but included a larger number of patients. The MAINTAIN study103 involved a total of 105 patients that were randomised to receive either mycophenolate mofetil at maximum mean doses of 2g/day or azathioprine at maximum mean doses of 124mg/day. After a mean follow-up period of 48 months, and after performing an intention to treat analysis, the primary variable, defined as time to renal flare, was similar between the two study groups. In fact, there were no differences in any of the final variables studied: number of renal flares, time to severe flares, time to mild flares, and time to remission of the renal flare. Additionally, the activity scores for the disease and the laboratory variables analysed were similar between the two groups. In terms of adverse effects, the two drugs were also similar, except for a greater frequency of leukopenia in patients treated with azathioprine.
A second recently published phase III clinical trial constitutes the maintenance phase of the ALMS study.104 This trial included 227 patients that had reached remission according to the criteria of the responsible physician, and were randomised to azathioprine (n=111) at a mean dose of 119.7mg/day or mycophenolate mofetil (n=116) at a mean dose of 1.87g/day, with a 36-month follow-up period. The primary variable analysed was the time to treatment failure, which was a composite variable of death, terminal chronic renal failure, doubled creatinine value, renal flare (proteinuric or nephritic), or the need for salvage therapy due to deterioration or exacerbation of the nephritis condition. At the end of the study, mycophenolate was shown to be superior to azathioprine in terms of the primary variable (hazard ratio: 0.44; 95% CI: 0.25-0.77; P=.003) and treatment failure (P=.03). There were no differences in adverse effects. Furthermore, the frequency with which patients reached complete remission was similar in both groups. One interesting finding from this study was that the patients that had received induction therapy using cyclophosphamide had better results in both groups, and that patients of African descent showed the greatest difference in favour of mycophenolate.
These contradictory results could be due to several reasons. The number of patients included in the different studies varies, as does their ethnic origins. Furthermore, in the MAINTAIN study, patients were included in the maintenance phase even if they had not achieved renal remission using the induction therapy. In this study, all patients received cyclophosphamide during the induction phase, and this subgroup had the best evolution during the maintenance phase in the second study, both with azathioprine and mycophenolate. Finally, the primary variable was not the same in both studies.
Moreover, the dose of corticosteroids used in these studies at the start of the maintenance therapy phase was different. In the ALMS study, the maximum dose was 10mg/day prednisone or its equivalent, and the progressively decreasing dose was decided upon by the treating physician. In the MAINTAIN study, the initial corticosteroid dose at the start of the maintenance therapy phase was 7.5mg/day prednisone in week 24 and 5mg/day in week 52. Additionally, after week 76, the amount administered continued to decrease, and was completely suspended whenever possible (21 and 20 patients in the azathioprine and mycophenolate mofetil groups, respectively). This information is important, because there is a notable heterogeneity in the amounts of corticosteroids used in maintenance therapies for lupus nephritis, as has been recently revealed.105
There is no reliable data regarding what the optimal duration for the maintenance therapy is in proliferative lupus nephritis. In the majority of randomised studies, immunosuppressive therapy lasts approximately 3.5 years. One retrospective study identified a mean duration of maintenance phase immunosuppression of less than three years as an independent prognostic factor for negative renal evolution, defined as the development of terminal chronic renal failure, doubled creatinine levels, or death.106 Some authors recommend progressively suspending immunosuppressive treatment after a minimum of five years.107
3.2.4. Class V
3.2.4.1. Induction therapy
Proposals
• In patients with class V lupus nephritis, we recommend initial treatment with prednisone up to 1mg/kg/day (with maximum dose of 60mg/day and later reduction of the dosage in a similar manner to classes III and IV), accompanied by one of the following treatment options:
o Cyclophosphamide (1B).
o Calcineurin inhibitors: cyclosporine (1B) or tacrolimus (2C).
o Mycophenolate mofetil (1B) or enteric-coated mycophenolate sodium (2C).
o Azathioprine (1C).
• If cyclophosphamide or mycophenolate are used, they should be used in the same doses as for classes III and IV. If azathioprine is used, the initial dose should be between 1.5mg/kg/day and 2mg/kg/day, and in the case of calcineurin inhibitors, between 2mg/kg/day and 5mg/kg/day for cyclosporine and between 0.15mg/kg/day and 0.2mg/kg/day for tacrolimus (1B).
• Patients with class V lupus nephritis and renal biopsies with criteria for coexisting class III or IV lupus nephritis should be treated as is indicated for these last two types (2C).
Justification
Few studies have analysed which the most adequate treatment for membranous lupus nephritis is, and as such, the level of supporting evidence is generally low. Recently, a prospective study of 40 patients demonstrated that a combined treatment regimen with prednisone and cyclosporine or cyclophosphamide administered intravenously is more effective than treatment with prednisone alone.108 Specifically, patients were treated with a regimen of prednisone on alternate days with an initial dose of 40mg/m2 (approximately 1mg/kg) for eight weeks followed by a gradual decrease (5mg/week) until reaching a dose of 10mg/m2 that was maintained for the one-year study period. One group of patients also received six bi-monthly pulses of cyclophosphamide (0.5-1g/m2) and the third group of patients received cyclosporine at initial doses of 200mg/m2 (approximately 5mg/kg). The median level of proteinuria at the start of the study was 5.4g/day (range: 2.7g/day-15.4g/day) and the median glomerular filtration rate was 83ml/min/1.73m2 (range: 32ml/min-189ml/min). The primary study variable was remission of proteinuria, which was achieved in 27%, 60%, and 83% of patients treated only with corticosteroids, cyclophosphamide, and cyclosporine, respectively. The use of cyclosporine in this type of lupus nephritis is also supported by other studies performed with a smaller number of patients.109
With regard to the use of mycophenolate mofetil, Radhakrishnan et al110 published a study that compiled specific information from patients with class V lupus nephritis included in two previous randomised studies in which mycophenolate mofetil was compared to pulses of cyclophosphamide as an induction therapy.21,101 They analysed 84 patients, 65 of which followed the treatment schedule for 24 weeks. The mean dose of mycophenolate mofetil ranged between 2.6g/day and 2.8g/day, and the mean dose of cyclophosphamide ranged between 760mg/m2 and 829mg/m2. The initial dose of prednisone used ranged between 35mg/day and 54mg/day. There were no differences observed between the two drugs in the percentage change in proteinuria or creatinine after 24 weeks. They did not found any difference when analysing patients with nephrotic-range proteinuria either. There is very little evidence regarding the use of enteric-coated mycophenolate sodium in patients with class V lupus nephritis. In the previously mentioned studies, only 6 cases were described.
One open study examined 28 patients treated with azathioprine (mean dose of 1.7mg/kg/day) together with prednisone at a mean initial dose of 0.85mg/kg/day. After 12 months, the percentage of complete and partial remissions was 67% and 22%, respectively, and the drug was very well tolerated.111
The use of tacrolimus to treat membranous lupus nephritis is anecdotal.112,113 The study involving the largest sample size held only 18 patients, with mean proteinuria at 4.5g/day and a glomerular filtration rate of 102.8ml/min/1.73m2 The dose of tacrolimus was between 0.1mg/kg/day and 0.2mg/kg/day in order to achieve a plasma level of 3ng/ml-8ng/ml, along with prednisone at an initial dose of 30mg/day. After 12 weeks, the partial and complete remission rates were 50% and 27.8%, respectively. These results were similar to those achieved in a historical cohort of patients treated with oral cyclophosphamide or azathioprine.113
Finally, there are retrospective studies that show that the prognosis for renal function after 10 years in patients with membranous lupus nephritis and evidence of proliferation in the renal biopsy is worse than in patients with “pure” forms of the disease,114 which would support treating these patients as if they suffered from proliferative forms (classes III and IV).
3.2.4.2. Maintenance therapy
Proposals
• After they have completed the induction therapy regimen and reached at least a partial response, we suggest that patients with class V lupus nephritis undergo maintenance treatment with low doses of steroids and one of the following treatment options (2B):
o Mycophenolate
o Calcineurin inhibitors
o Azathioprine
• We suggest maintenance therapy duration and dosage similar to those described for classes III and IV, with regard to steroids, mycophenolate, and azathioprine. The duration of treatment would also be the same in the case of calcineurin inhibitors (2D).
Justification
No studies have been specifically designed to investigate which is the proper treatment for maintenance therapy in patients with class V lupus nephritis. In general, these patients have been included in randomised prospective studies involving proliferative lupus nephritis, but due to the scarcity of this disease, it has not been analysed independently.
The data available comes from the studies cited in the previous section and suggest that azathioprine,101,111,113 mycophenolate mofetil,21,101,103 and cyclosporine108 are valid options for the maintenance therapy phase. Additionally, the treatment regimen involves the same duration as in patients with proliferative lupus nephritis.
4. SPECIAL SITUATIONS
4.1. Chronic renal failure class VI lupus/dialysis/transplant
4.1.1. Concept of class VI lupus nephritis. Histopathology and diagnosis
Class VI lupus nephritis is the final phase of renal damage produced by this immunological disease. The current histological classification of lupus nephritis includes class VI for cases that are in the final stage of the disease and that, from the point of view of renal function, correspond to stage 5 on the KDOQI scale for the classification of advanced chronic kidney disease (ACKD) based on glomerular filtration rate, which in these cases is less than 15ml/min.5,115 In stage 5 ACKD, the kidney cannot reach adequately with its functional objectives, despite the activation of effective compensation and adaptation mechanisms, which causes a clinical syndrome known as uraemia. At this point, almost all organs and systems are affected and the body quickly deteriorates into a life-threatening state unless renal replacement therapy (RRT) is commenced in the form of dialysis or KT. This stage of the disease becomes very important in the life of patients with SLE and lupus nephritis, and is a determining factor in their prognosis.116
The histopathology of class VI lupus nephritis reveals a form of renal sclerosis that is the result of the evolution of the different types of lupus nephritis, which is indistinguishable from the other aetiologies. At least 90% of glomeruli are sclerotic and there is interstitial fibrosis with distortion of tubular and vascular structures. Immunofluorescence techniques can show residual complement and immunoglobulin deposits, but there are little or no histological signs of immunological activity.5,117 Under these circumstances, the progression of the nephropathy is independent of the immunological aggression, relentlessly advancing to a terminal stage.
Proposals
• The diagnosis of class VI lupus nephritis should essentially be clinical, and a renal biopsy should not be routinely indicated (NG).
• Taking a renal biopsy should only be justified in the presence of rapid deterioration in glomerular filtration rate with no evident cause in patients with a history of lupus nephritis renal flares (NG).
Justification
The diagnosis of the nephropathy in this phase of the disease is based on clinical evidence. There is a progressive decrease in glomerular filtration rate with increased nitrogenous products and the appearance of biochemical signs and clinical manifestations of uraemia (acidosis, anaemia, hyperkalemia, altered metabolism of phosphorous and calcium, etc.), with no clinical or immunological signs of lupus activity. Proteinuria and altered urinary sediment can persist.5,116 A renal biopsy is not indicated at this stage of the disease, except for in certain clinical contexts (see farther on). Occasionally, there may be a rapid deterioration in renal function in patients that already had CKD secondary to lupus nephritis. These changes can be secondary to a spontaneous evolution of the disease or due to the occurrence of exterior, interacting factors (excessive blood pressure control, dehydration, medication, etc.) or can also occur in the event of an acute flare of lupus nephritis within a kidney chronically damaged from previous renal flares. As such, a decrease in glomerular filtration rates in patients with lupus nephritis that already had experienced deteriorated renal function may present a differential diagnosis problem between irreversible class VI lupus nephritis (which would not require immunosuppressive treatment), a reversible or improvable functional disorder that can be returned to similar renal function levels from before (if proper specific treatment is applied), and an acute recurrence of the lupus disease (requiring immunosuppressive treatment), inflicted on a renal parenchyma already damaged by chronic lesions from the evolution of the previous lupus nephritis. The diagnosis is fundamental for the future of the patient, and in many cases may a renal biopsy may be necessary to establish one of these therapeutic alternatives.5,116,118
4.1.2. Risk factors for developing renal failure in lupus nephritis. Evolution towards advanced renal failure in lupus nephritis
There is a very large variability in the rate of patients with lupus nephritis that evolve towards ACKD requiring RRT (7%-70%; median: 20% at 10 years). Despite the advances made in the treatment of these patients, the rates for CKD secondary to lupus nephritis does not appear to have improved in the last decade. An analysis of 9199 cases in the United States revealed rates of 4.4 per million population (pmp) in 1996 and 4.9pmp in 2009.119 However, there are considerable differences between the different studies published.
There is a marked variability based on race that can affect the evolution of lupus nephritis. The progression towards ACKD is significantly more common in patients of African descent, followed by Latin American patients, and the best results are obtained in Caucasian and Chinese patients.120-122 Korbet et al,122 in a multi-centre North American study, observed a 38% renal survival rate 10 years after diagnosis in African American patients, as opposed to 61% in Latin Americans and 68% in Caucasians.
Patients with proliferative forms of lupus nephritis (class III, IV) evolve towards ACKD with the need for RRT at a greater rate than those with class V patients. The incidence of ACKD in class V lupus nephritis in classic studies is lower (8%-12% after 10-12 years), and its evolution slower.123-125 However, another study showed an incidence of ACKD or death in class V lupus nephritis of 28% at 10 years, a similar rate to those found in proliferative forms.126 Class I and II lupus nephritis do not evolve towards ACKD.115,117
Before the introduction of the various methods used for intensive immunosuppression in 1970, renal survival at 10-15 years in the proliferative forms of lupus nephritis did not exceed 20%-25%, improving to 60%-80% afterwards, especially following the introduction of cyclophosphamide.37,73,101,121,127-129 The results also seem to be improving with the most recent therapeutic options. For example, in the ELNT study by Houssiau et al,88 renal survival at 10 years was 93%.
Certain histological risk factors have been described for the evolution towards ACKD, including the index of chronicity, the index of activity, and the presence of focal vs diffuse glomerular lesions, but only the presence of interstitial fibrosis and index of chronicity are common in the majority of published studies. They have been established as independent risk factors for the development of ACKD.130-132
Proposal
• We recommend early treatment at the proper intensity at the start and upon recurrence, since the severity of the clinical presentation of lupus nephritis and an early clinical management of the patient are determining factors in the evolution of the disease to advanced phases of CKD (1B).
Justification
A joint analysis of the studies available on the prognosis of lupus nephritis reveals that serum creatinine levels at the start of treatment is the risk factor most clearly related to the development of ACKD (hazard ratio: 1.3-5). This has been reported by most of the authors.122,133-136 In a less consistent manner, the level of baseline proteinuria has also been established as a risk factor for developing ACKD.122,135 As such, the clinical severity of lupus nephritis is the clearest determining factor in the evolution towards ACKD.
Secondly, prospective studies have shown that delaying the start of induction therapy more than three months after diagnosis is associated with a progression towards ACKD.122,135 On the other hand, the initial response to treatment also influences the long-term evolution of the disease: complete and partial remission are accompanied by greater renal survival than in those cases with no response to treatment.137-139
Additionally, recurrence is important in determining the evolution of the disease. Each episode of lupus nephritis recurrence involves added damage to the renal parenchyma that will favour evolution towards ACKD, even in the absence of immunological activity.129,140 In a population of paediatric and young adult patients with lupus nephritis, no response to initial treatment and the presence of recurrence were significant predictive variables for the development of ACKD (hazard ratio: 5.5 and 11.8, respectively).139
4.1.3. Pre-dialysis management of patients with lupus nephritis
Proposals
• We recommend that patients undergo assessment in special ACKD centres when estimated GFR drops below 30ml/min (stage 5 ACKD) in order to begin procedures in preparation for RRT and to monitor uraemic changes closely (1C).
• We suggest the following for class VI lupus nephritis in stage 5 CKD: maintaining RAAS blockers and monitoring closely for any complications that may arise, mainly hyperkalemia and decreased renal function (2C).
• For class VI lupus nephritis, we recommend slowly decreasing immunosuppressive drugs until they can be discontinued, unless they are necessary due to extrarenal lupus activity (1B).
• In cases of lupus nephritis that reach ACKD (stage 5) during an acute flare with rapidly progressing kidney failure, induction treatment should be prolonged for at least 4–6 months after beginning dialysis, until lack of recovery is confirmed (NG).
Justification
It has been stated that early initiation of preparations for RRT and proper monitoring of treatment for uraemic complications by a nephrology specialist influence the overall prognosis of ACKD.141,142 The main steps at this moment in the course of the disease are as follows: a) provide correct information to the patient and family members regarding the characteristics and expected outcomes of different types of RRT; b) select the best procedure for each case (haemodialysis, peritoneal dialysis or KT);c) prepare for that procedure (create the arteriovenous fistula for haemodialysis, prepare catheter for peritoneal dialysis, etc.); d) monitor arterial blood pressure; e) provide dietary information and meticulously adjust medication for treating biochemical changes in uraemia (anaemia, hyperkalemia, acidosis, calcium/phosphate metabolism disorders, etc.) It is also very important to pay attention to nutritional factors. Studies by Siu et al143 and Huang et al144 showed that lupus nephritis patients with predialysis ACKD presented serum albumin levels that were lower than those of the patient control group with ACKD but without SLE.
RAAS blockers may be used with caution in lupus nephritis; ACEi and ARB may be used either alone or in combination. The purpose of these anti-proteinuria and anti-hypertensive drugs is to preserve residual renal function that could delay the onset of RRT. However, handling these drugs in a case of ACKD may be difficult due to induction of hyperkalemia or their harmful affect on GFR, which is already in terminal values.145 Measures such as controlling blood pressure and administering lipid lowering treatment are important, since these patients present two cardiovascular risk factors: ACKD as an independent risk factor, and SLE itself, which has a higher prevalence of vascular complications (see section 2.4).
Lupus activity decreases in the final phase of ACKD and remains at a low level throughout RRT (haemodialysis and peritoneal dialysis).146,147 The study by Nossent et al147 is a good example of this situation: in more than half of a cohort of 55 SLE patients on haemodialysis that were monitored prospectively, lupus activity decreased or disappeared. Only 15% of the patients needed to continue treatment with low doses of prednisone, and only 7% needed immunosuppressive drugs. Decreased renal function in this stage of lupus nephritis is not usually secondary to immunological activity, but rather to chronic kidney failure’s self-perpetuating and progressing mechanisms. We should therefore try to decrease immunosuppressive treatment in order to minimise its side effects, which are the main cause of morbidity and mortality at this stage of the disease. They should only be maintained if extrarenal lupus activity persists. Medication should be adjusted with great care as some authors have warned us about the presence of significant levels of lupus activity during the first year of dialysis treatment, especially in black patients.148-150
In cases requiring dialysis due to progression to acute renal failure after a flare, significant immunological activity with active renal lesions tends to persist during the first months of RRT. These patients may therefore improve under immunosuppressive treatment. In fact, an improvement in renal function has been described in 10%–20% of these cases, and RRT can be discontinued, at least for a few months.116,150 We must remember that treatment directed at extrarenal manifestations of lupus must be maintained.
4.1.4. Alternatives to renal replacement therapy: Haemodialysis vs. peritoneal dialysis in lupus nephritis.
Proposals
• A type of dialysis – haemodialysis or peritoneal dialysis – should be offered to patients with ACKD and lupus nephritis only after assessing their clinical condition and individual preferences (NG).
• We suggest offering peritoneal dialysis to patients with class VI lupus nephritis without significant lupus activity and with no need for intensive immunosuppressive treatment (2C).
• We suggest haemodialysis in cases in which the lupus is extremely active and immunosuppressive treatment is required (2C).
• For SLE patients on RRT, prophylactic measures protecting against infection should be greatly increased, both in peritoneal dialysis and haemodialysis. Furthermore, immunosuppressive drugs should be decreased to the lowest level needed in order to prevent extrarenal lupus activity (1B).
Justification
In most published studies, survival rates in SLE patients undergoing dialysis are similar to that of patients on dialysis due to other causes.116,146-149 However, other authors point to a worse prognosis, particularly during the first 3 months of dialysis. This is fundamentally due to an increased incidence rate of infections as a complication of chronic treatment with high doses of steroids.150-152 Few studies compare the two basic procedures — haemodialysis and peritoneal dialysis — in patients with lupus. In a prospective study of 55 lupus patients on dialysis, Nossent et al147 found no significant differences in survival rates between the two techniques. However, Weng et al153 found that mortality and infection rates were higher in the peritoneal dialysis group, even when excluding infections having to do with the peritoneal dialysis catheter or the arteriovenous fistula (more information provided below). These data were confirmed by other authors.146,154 Consequently, haemodialysis is preferred to peritoneal dialysis in patients with active SLE who need intensive immunosuppressive treatment. Peritoneal dialysis, however, is a procedure that may be carried out on an outpatient basis. It allows the patient to be more independent, thereby providing a greater degree of recovery. These characteristics may be of particular interest for younger patients, in fact, most lupus nephritis patients are below retirement age and many are still raising their children. After weighing the risks and benefits of both techniques, we believe that peritoneal dialysis should be offered to patients with class VI lupus nephritis with no major lupus activity and no need for intensive immunosuppressive treatment.
Infections are the main cause of mortality and morbidity in SLE patients on RRT. They are more common in these patients than in the general population, and cause 80% of all deaths during the first 3 months of RRT.143,151,152 Some authors have found infections to be more common in SLE patients treated with peritoneal dialysis than in the rest of the population except for diabetics. They also report that SLE patients have higher incidence rates of peritonitis and infections, both at the catheter exit site and at unrelated locations, therefore leading to higher rates of treatment discontinuation.143,144,152 Infections are also one of the main causes of death among SLE patients on haemodialysis, and the mortality rate is higher than in patients undergoing HD due to other causes.154
The use of immunosuppressive drugs has a considerable effect on the appearance of infections. In a study of patients on peritoneal dialysis who were treated with immunosuppressive drugs for whatever reason, the peritonitis rate in the treatment group was 1.8 episodes per patient/year, compared to 0.6 in those not receiving immunosuppressive drugs.155 These data emphasise the need for being extremely cautious and increasing prophylactic measures against infection in peritoneal dialysis and haemodialysis patients, as well as decreasing immunosuppressive treatment, particularly corticosteroids (see sections 2.1 and 2.8).
4.1.5. Kidney transplantation in patients with systemic lupus erythematosus
Proposals
• We recommend cadaveric or living kidney transplant as the RRT procedure of choice in patients with SLE (1B).
• We recommend that KT as treatment for rapidly-progressing kidney failure in ACKD patients with lupus be postponed as long as lupus activity persists during dialysis.
• The pre-KT examination of SLE patients must include lupus activity variables and phospholipid antibodies in addition to routine parameters measured in all patients (NG).
• Standard immunosuppressant doses should be used for KT (NG).
Justification
Patient and graft survival rates among SLE patients are similar to those in patients undergoing KT for other reasons.116,146,147,156-158 Analysis of European transplant registry data showed that 3 year survival rates for SLE patients were similar to those for the rest of the cohort.159 On the other hand, the analysis by Ward et al119 of data from the United States Renal Data System found no differences in graft survival or mortality rates among 772 transplant recipients with lupus and 32 644 control subjects. However, this opinion is not unanimous and some studies report lower patient and graft survival rates for SLE patients.157,160 In lupus, as in other diseases, living-donor kidney transplant offers better results than cadaveric-donor kidney transplant.156, 157,160 Graft survival rates in 390 living-donor recipients and 772 cadaveric-donor recipients were 83.3% and 58.1% respectively. Patient survival rates were 94.4% and 77%.
As we stated above, lupus activity decreases in the end stage of ACKD, including the dialysis period, and is particularly low during the KT period.116,146,147 A study by Nossent et al147 shows that the lupus activity index drops significantly in transplant patients with SLE. There can be no question that this occurs because the immunosuppressive drugs administered during KT are also effective for treating lupus, which would explain the low rates of lupus nephritis relapse in the graft. Different studies show this rate between 4.3% and 8.6%.146,154,161 Furthermore, a lupus nephritis relapse in a kidney recipient usually responds well to treatment, and causes graft loss in only a small percentage of cases (3.8% in the study by Stone et al).160 In some cases, significant lupus activity may persist during the first months of dialysis treatment, particularly when ACKD has developed in only a few months as a result of rapidly-progressing renal failure.146,158 KT in such a situation may lead to loss of the graft. In these cases, we therefore recommend waiting 6-12 months until the activity has been brought under control and the patient’s general condition has improved.
The pre-transplant study in a lupus patient must include all common parameters for the ACKD population on the waiting list, in addition to the following specific checks:
1. Immunological parameters for lupus activity, for the reasons stated above.
2. Phospholipid antibodies: 30%–40% of SLE patients test positive, and phospholipid presence may hold clinical consequences for the KT.160,162 In addition to the normal complications arising from antiphospholipid syndrome (APS), authors have also described an increased risk of early graft thrombosis.160,163 In the study by Marcén et al,163 early thrombosis of the graft occurred in 37.5% of the cases in which the patient tested positive for antiphospholipid antibodies, in 2.8% of the cases in which patients were not tested, and in none of the 13 cases that were negative (P<0.0001).
On the other hand, lupus patients have a high prevalence (30%–90% of anti-lymphocyte antibodies that may cause false positives in the pre-KT crossmatch. The test should therefore be carried out with autologous lymphocytes from the donor.164
There are no controlled studies that show whether any immunosuppressive agent proposed for KT management offers demonstrable benefits to SLE patients. In addition to their anti-rejection properties, the immunosuppressive agent must control lupus activity, minimise the corticosteroid dose, not cause nephrotoxicity and minimise atherogenic effects. In general, calcineurin inhibitors, tacrolimus or cyclosporin A165 are recommended during the induction period (the first 6-12 months after KT), and mycophenolate mofetil or mycophenolate sodium is recommended during the maintenance phase. They are useful in SLE treatment, both have antiatherogenic effects and are proven to be effective in preventing rejection.156,164 All of them are associated with low doses of corticosteroids. However, since suspending prednisone in SLE patients is recommended whenever possible, some authors have proposed routine use of steroid-free treatment programmes in KT patients with lupus.158
4.2. Pregnancy/postpartum
Proposals
Although prognoses have noticeably improved in recent years, pregnant women with SLE have a higher incidence of miscarriage and premature births, a worse perinatal and neonatal prognosis, a greater frequency of preeclampsia, and greater maternal mortality than healthy women. A recent analysis of 13 555 pregnant lupus patients from a database of 16.6 million pregnancies showed a maternal mortality rate 20 times greater in lupus patients, with a 1.7 odds ratio of C-section, 2.4 for premature birth, and 3.0 for preeclampsia.166 The presence of organ damage, especially in the kidney, results in a greater frequency of complications.
Proposals
• Pregnancy should be contraindicated in patients with lupus nephritis in remission and preserved renal function (1B).
• We recommend avoiding pregnancy in women with advanced renal failure (1B).
• The pregnancy should be monitored by multi-disciplinary teams in high-risk pregnancy units under consensus protocols (NG).
• A pregnancy should be properly planned in the case of lupus nephritis patients, i.e. after at least 6 months of remission, at least partial remission (1B).
• When planning a pregnancy in lupus nephritis patients, immunosuppressive and immuno-modulating treatments should first be adapted for maintaining control of renal and systemic activity, while avoiding the use of teratogenic drugs or medications with negative effects on gestation (1B).
• The patient should be closely monitored for flares of lupus nephritis with frequent urine analyses and measurements of other parameters for lupus activity, such that recurrences are early detected and treated using drugs that do not have contraindications during pregnancy (NG).
• We recommend adequate control of blood pressure in pregnant women with lupus nephritis, suspending angiotensin-renin system blockers due to their teratogenic effects, and in their stead using alpha methyldopa, labetalol, or nifedipine (1B).
• We recommend aspirin at low doses (100mg/day) before week 12 in pregnant women with lupus nephritis that is active or in remission in order to reduce the risk of preeclampsia and foetal loss (1A).
Justification
The evolution of pregnancies in women with SLE has improved notably in recent years. An epidemiological study carried out by Clark et al reported a percentage decrease of embryo-foetal losses from 40% in 1960-1965 to 17% in 2000-2003.167 Despite this, pregnancies in women with lupus should automatically be considered high-risk.
The presence of lupus nephritis produces a series of additional problems. In different cohorts of patients with lupus nephritis, a consistent increase has been described in foetal deaths, miscarriages, and perinatal deaths, which varies according to study.168-172 This disease also causes a high rate of premature births and underweight newborns.170,173 C-section births are also more frequent in some studies.170,173
As regards maternal complications, a recently published meta-analysis reported that women with an episode of lupus nephritis during pregnancy or with a previous history of lupus renal involvement may have a greater probability of developing arterial hypertension, preeclampsia, and premature/pre-term birth.172
It has been described that lupus activity can increase during pregnancy and during postpartum.174,175 During pregnancy, there may be a recurrence of lupus nephritis, which is more frequent in women with recent renal activity.170,171,176-178 A glomerular filtration rate <60ml/min, proteinuria greater than 1g/24 hours, and the absence of complete remission at the moment of conception have all been identified as predictive variables for a renal flare during gestation.170 The presence of renal activity during pregnancy is related with a greater probability of embryo-foetal loss, which ranges between 25% and 57% in women with active nephritis at the moment of conception vs 8%-12% in patients in renal remission.173,179 There are also severe complications that may affect the mother, including acute renal failure and death.170,177-179 In order to minimise the risks involved, SLE should be adequately controlled at least six months before conceiving. According to the majority of authors, patients with nephritis should be in a prolonged remission period, at least partial remission.168,173,179,180
Pregnancy can also cause rapid deterioration in renal function in patients with chronic renal failure.181 Renal failure is a risk factor for preeclampsia, premature birth, and delayed intra-uterine growth.181,182
All of these reasons establish pregnancy while suffering SLE as a high-risk situation that should be attended by a multi-disciplinary team, ensuring coordinated medical and obstetric action, proper pre-pregnancy planning and information, and a strict monitoring programme for the mother and foetus that should be continued through the first weeks post-partum.
There are only a small number of drugs that can be used to induce or maintain the remission of lupus nephritis during pregnancy (Table 13). Cyclophosphamide and mycophenolate, in addition to methotrexate, are absolutely contraindicated during gestation.183 Azathioprine is a safe medication since, although it does cross the placental barrier, the foetal liver does not have the enzyme inosinate-pyrophosphorylase, which converts azathioprine into 6-mercaptopurine, its active metabolite. Some experience has been garnered in providing calcineurin inhibitors to patients undergoing a renal transplant, which can also be used during pregnancy at the minimum possible effective dose.183 Hydroxychloroquine should be maintained, since it does not cause malformations, nor does it affect foetal growth. On the contrary, suspending this medication is associated with a greater frequency of flares during pregnancy.184 Finally, glucocorticosteroids do not pose a risk of foetal toxicity, since they are inactivated in the placenta in over 90% by 11β-hydroxysteroid dehydrogenase, except for its fluoride forms (betamethasone and dexamethasone), which cross the placental barrier at high concentrations.183 However, the list of potential maternal complications when using this drug is long, including the normal complications associated with corticosteroids and increased risk of gestational diabetes, preeclampsia, and premature rupture of membranes.185 Methylprednisolone pulses can be used in severe situations, as well as immunoglobulins,183 making them a good option in severe renal flares during pregnancy.
Arterial hypertension is an independent risk factor for the evolution of the disease towards terminal renal failure and foetal death in women with lupus nephritis.168,179,186 As such, proper control of blood pressure is essential in these patients. Target values less than 140/90mm Hg have been proposed, although the exact target values are unknown. In fact, an excessive reduction in blood pressure has been shown to lead to delayed foetal growth.187 Frequently, patients with lupus nephritis are treated with ACE inhibitors and ARB, which have the capacity to combat proteinuria and protect the kidneys in addition to their efficacy as anti-hypertensive agents. However, both therapeutic options are associated with congenital defects and altered foetal renal function, and as such, they must be suspended several weeks before pregnancy or as soon as possible after discovering it.188 During gestation, labetalol, nifedipine, or alphamethyldopa can be used as anti-hypertensive drugs.
Lupus nephritis increases the risk of preeclampsia.172 Aspirin reduces the risk of preeclampsia and perinatal death in high-risk pregnancies, including patients with renal disease. As such, it should be prescribed at low doses (100mg/day) in women with active or previous lupus nephritis, as long as contraindications are not present.189 Aspirin treatment should be commenced before week 12 of pregnancy, and it does not need to be suspended before birth.
It is difficult to make the differential diagnosis between a flare of lupus nephritis and preeclampsia in pregnant women with lupus, and it is important to specify that the treatment for these two different conditions is very different. Lupus nephritis flares can be produced at any moment during pregnancy. Preeclampsia is not produced before week 20, and is most common after week 32. In both cases, there is an increase in proteinuria (especially if ACE inhibitors have just been suspended), arterial hypertension, and a possible deterioration in renal function. Thrombocytopenia may exist in both cases, although it is more common in preeclampsia. The presence of active urinary sediment with microhaematuria and cell casts and immunological activity are products of the lupus nephritis flare and aid in making the differential diagnosis, although complement levels tend to increase with pregnancy and therefore normal values may be present in the case of lupus activity.170,171,179 Uric acid tends to be elevated in preeclampsia.
4.3. Antiphospholipid syndrome
Antiphospholipid syndrome (APS) is defined as thrombosis (arterial, venous, and/or small vessel) and/or obstetric complications (miscarriage, foetal death, and/or premature birth due to placental insufficiency), along with sustained positive antiphospholipid antibodies: lupus anticoagulant, anticardiolipin antibodies, and anti-ß2-glycoprotein I.
Approximately 30%-40% of patients with lupus have circulating antiphospholipid antibodies. Many of them remain asymptomatic. However, these antibodies are considered to be a risk factor for developing thrombosis and long-term irreversible damage. APS can affect the kidneys in several ways: thrombosis of renal arteries/veins or stenosis of the renal arteries with renovascular hypertension, along with a specific type of nephropathy associated with the antiphospholipid syndrome (APS), i.e. thrombotic lesions in the glomeruli (thrombotic microangiopathy), arterioles, and interlobular spaces, not involving larger vessels.190
Proposals
• Given the thrombotic nature of APSN, we suggest maintaining anticoagulant treatment in these patients (2C).
• We recommend treating thrombosis of major renal vessels with prolonged anticoagulant therapy (1B).
• No specific therapeutic approaches are recommended for patients positive for antiphospholipid antibodies who are undergoing dialysis.
• Anticoagulant treatment in KT patients with antiphospholipid antibodies should be adjusted on an individual basis (NG).
Justification
APSN may be present in more than 30% of SLE patients with antiphospholipid antibodies,190 as well as in individuals with primary APS.191 It may have a wide histological spectrum, but its underlying condition is usually the presence of thrombosis. In its acute forms, thrombosis can be found as thrombotic microangiopathy-like lesions of the glomerular arterioles and capillaries. In its chronic forms, we may find lesions with intimal thickening and fibrosis and capsular sclerosis/atrophy.190 The typical clinical presentation of renal thrombotic microangiopathy associated with APS consists of arterial hypertension, which may be severe; proteinuria, which may be within the nephrotic range; and the development of kidney failure.191 APSN lesions may appear over those caused by lupus nephritis and are independent from them. Some authors have found a correlation between testing positive for antiphospholipid antibodies and increased risk of developing kidney failure among patients with lupus nephritis. In addition, vascular thrombosis has been reported to develop during late follow-up in APSN patients, especially in arteries.190
In addition to the small-vessel lesions that are typical of APSN, thrombosis of large renal arteries and veins may appear in the clinical presentation of a case of APS. Renal vascular hypertension associated with stenosis of renal arteries is also frequent.193
There are no specific treatment guidelines for APSN. In line with recommendations for other thrombotic manifestations of APS, and in consideration of the high risk of developing thrombosis at a later date, we recommend indefinite anticoagulant treatment,191,193 although its effect on the course of renal lesions of this type is unknown. Recommendations for treating thrombosis of major renal vessels are the same as those for treating vascular thrombosis in other locations.194 Immunosuppressive treatment has not been shown to be superior to anticoagulant treatment in APS patients.194
The prevalence of antiphospholipid antibodies is higher in patients with advanced chronic renal failure undergoing haemodialysis than in the general population.195-197 There are many reasons why these antibodies might develop and they are not clear, since its association with bioincompatibility with dialyser membranes or other factors has not been proven. These antiphospholipid antibodies are independent from cofactor beta 2-glycoprotein I, and are therefore not associated with thrombosis development. Some authors believe that these antibodies are related to an increase in thrombosis of the vascular access for haemodialysis, but this phenomenon was not confirmed by other authors.198-200 In cases of thrombosis of the arteriovenous fistula and where antiphospholipid antibodies are present, treatment decisions should be made on an individual basis.201-203
Several studies have described a high rate of early thrombosis of the kidney graft (25%–37.5%) in KT patients positive for antiphospholipid antibodies and lower functional survival rates in those grafts. They also found lesions characteristic of APS-related vascular disease in biopsies.204,205 Early anticoagulant treatment may be indicated in patients with a history of thrombosis prior to the transplant and/or high titres of antiphospholipid antibodies. However, the high risk of patients experiencing major haemorrhages immediately after KT has prevented the widespread use of this measure. Every case requires careful assessment and a personalised treatment strategy.206,207
5. TREATING RELAPSES AND RESISTANT CASES. NEW DRUGS
5.1. Recurrence of lupus nephritis
The term recurrence refers to the reappearance of signs of renal activity after having achieved partial or complete remission using treatment (see previously described definitions). Recurrence tends to be associated with extra-renal symptoms and biochemical indicators of activity, although it can rarely occur in their absence.
Proposals
• In the case of renal recurrence, other diseases and processes must be ruled out (APS with thrombotic microangiopathy, nephritis secondary to NSAIDs, atheromatous ischaemic nephropathy, etc.) and to ensure proper compliance with treatment (NG).
• In the case of recurrence, the patient should follow general measures (body weight control, avoid exposure to the sun, anti-proteinuria drugs, hydroxychloroquine, controlling vascular risk factors, etc. (NG).
• In the case of moderate or severe recurrence after achieving complete or partial response, we recommend treating the patient with the same induction and maintenance therapies as were initially effective. The treatment should be given early in order minimise the risk of residual fibrosis (1C).
• If cyclophosphamide was indicated in the previous cycle of induction therapy, and the risk of toxicity is unacceptable due to high cumulative doses or adverse side effects, mycophenolate should be used (NG).
• A new renal biopsy may be needed if a change in the clinical manifestations of the disease is detected, such as signs indicating thrombotic microangiopathy, if a change in the class of lupus nephritis is suspected, or if the renal deterioration could be secondary to terminal/scarring nephropathy rather than lupus nephritis, as determined by discordance between extra-renal signs and symptoms, biological markers of activity, and signs of renal involvement (NG).
Justification
Although SLE is a condition characterised by a series of flares, the epidemiology of lupus nephritis recurrence following partial or complete remission is not well understood. Such flares may emerge more than 20 years after the first episode, although they are quite uncommon more than 1 decade after diagnosis.208 A prospective, observational study of a single cohort showed that up to 40% of patients who responded completely to treatment experienced recurrence after a median of 41 months. Among the patients who responded partially, the percentage rose to 63% after a median of 11.5 months.209 We lack a precise knowledge of the risk factors for recurrence, although it seems clear that incomplete remission is a strong risk factor.210 In addition, such recurrences are associated with decreased renal function and increased risk of presenting ACKD with a need for dialysis treatment.211 Thus, patients need to be monitored periodically, every 3-6 months at the longest, even if they have experienced no clinical or lab activity since remission. This contributes to early detection and treatment of relapses.
There are no randomised studies to compare the efficacy/safety of different treatments for achieving remission in the case of a recurrence. Observational studies suggest that remission may be achieved through administering either cyclophosphamide212 or mycophenolate mofetil213. There is no data suggesting that rituximab is more effective than mycophenolate or cyclophosphamide, or that it would prevent further recurrences.214 Several observational studies describe responses to rituximab in patients with relapsing forms of the disease.215
Although figures vary, we cannot overlook the fact that changes in the class of lupus nephritis have been described in as much as 50% of re-biopsies.216 The most common transition is from class III to class IV,13 and up to 20%-30% of patients whose initial biopsies showed no proliferation may present it during a subsequent recurrence. Transition from a proliferative class to a non-proliferative class is much less frequent.37 With this in mind, re-biopsy should be considered if there are signs of increase or reappearance of proteinuria, nephrotic syndrome or active sediment (particularly if the first biopsy revealed a non-proliferative class); increase in serum creatinine or unexplained progress toward renal failure; clinical doubt with respect to the degree of activity/chronicity of the renal lesions; or a suspicion of nephropathy not related to lupus (Table 7).
5.2. Resistant and non-responsive patients
Resistance to treatment is defined as an absence of complete or partial response of the renal involvement after completing the induction therapy phase. Renal resistance may or may not be associated with persistent signs of extra-renal activity.
Despite the apparent simplicity of this definition, there is actually no current consensus on how to define the minimum time for the induction therapy phase or the minimum cumulative dose of immunosuppressive drugs needed to consider the disease resistant to treatment.
Proposals
• We recommend changing the treatment scheme if there are no signs of response before completing the six months period of induction therapy (1B).
• In patients with acute or rapidly progressing renal failure, with elevated activity observed in the biopsy and/or extracapillary proliferation, treatment may be intensified if there is no improvement at the end of the fourth week (NG).
• In non-responsive patients, we should rule out the presence of other diseases or processes (APS with thrombotic microangiopathy, nephritis secondary to NSAIDs, atheromatous ischaemic nephropathy, etc.) and ensure proper compliance with treatment (NG).
• We believe it to be necessary to ensure compliance with the general indications for these patients (control weight loss, avoid overexposure to the sun, anti-proteinuria drugs, hydroxychloroquine, controlling vascular risk factors, etc.) (NG).
• Patients with criteria of resistance to cyclophosphamide or mycophenolate should be treated with an alternative induction therapy regimen (mycophenolate or cyclophosphamide, respectively (1A).
• In the case of resistance to effective induction therapies (cyclophosphamide and mycophenolate), we suggest using alternative treatment methods such as rituximab (2B), calcineurin inhibitors (2B), immunoglobulins (2C), or to use strategies based on combined drugs (2B).
• If there is still no response to treatment, a new renal biopsy may be necessary if the persistence of renal activity is not associated with extra-renal activity and if complement and anti-DNA levels are normal, in order to rule out the possibility that the resistance may be due to changes in the histological pattern of disease or advanced glomerulosclerosis (NG).
Justification
The results from cohort studies and clinical trials agree that the probability of response following induction therapy is similar whether using cyclophosphamide or mycophenolate, around 50% after the first year and 75%-80% after two years. Approximately 20% of patients do not reach a response under the currently used induction protocols. Most of the patients do have some level of response during the first year of induction therapy, although between 5% and 25% can take as long as 24 months to reach remission,217,218 which is an obstacle to standardising a definition of non-responsive patients. However, there are data indicating that an absence of improvement in renal function and/or a decrease in proteinuria to less than 1g/day after 6 months of induction therapy predicts patient evolution towards chronic renal failure,138 and so at this point the patient can be classified as resistant and the treatment strategy should be changed. In patients with rapidly progressing renal failure with severe histological signs, the time before changing treatment in the case of an absence of improvement should be 4 weeks, given the high risk of irreversible lesions in this type of disease.
It is important to rule out other possible causes that could explain the lack of response to treatment, especially in the presence of thrombotic lesions and in patients with antiphospholipid antibodies or drug toxicity. Additionally, proper compliance with treatment must be verified, both with immunosuppressive therapy and general medications. If pulses of cyclophosphamide were administered for induction therapy, it is advisable to check the date and dose for each cycle and to perform control haemograms two weeks after administering each cycle in order to optimise the dose based on leukocyte count. If mycophenolate was used for induction therapy, the attending physician must ensure that the maximum dose has been reached and the patient complied with the treatment regimen (circulating levels of mycophenolate do not correlate with the response, but may be useful for monitoring compliance).
There are no randomised prospective studies that have compared the efficacy/safety of the various treatments available for resistant lupus nephritis. However, the similar efficacy of cyclophosphamide and mycophenolate mofetil observed in clinical trials,21,94,95 as well as the different response rates observed in specific patient sub-groups (such as those of African descent),98 make of these two drugs the first choice to treat proliferative lupus nephritis. If resistance develops to one of these two treatments, the other should be used as an alternative.
The results from prospective observational studies (in which the criteria used for defining resistance vary) suggest that it is possible to reach remission in patients that have not responded to mycophenolate and cyclophosphamide using immunoglobulins, rituximab, and calcineurin inhibitors. There is evidence to suggest that immunoglobulins at high doses can induce a response in resistant patients, and the efficacy of this treatment has even been shown to be comparable to that of cyclophosphamide in patients with proliferative lupus.219 These drugs can be administered at the routine doses prescribed for systemic auto-immune diseases (400mg/kg during 5 days or 1g/kg for 2 days). Preparations rich in sucrose should be avoided due to the risk of nephrotoxicity, assuming that there may be a relative increase in proteinuria due to the renal elimination of these immunoglobulins.
Despite the failure of the EXPLORER220 and LUNAR221 trials in demonstrating the primary objectives of rituximab in lupus patients with extra-renal and renal involvement, respectively, there is evidence from observational studies on the benefits of this drug in patients with multiple recurrence or resistance to standard protocols,222-228 which also is useful in combination with mycophenolate without excessive toxicity.228 The routine regimen is four weekly doses of 375mg/m2 or two weekly doses of 1000mg. There is less experience with other biological agents, including infliximab, which was successfully used in a small number of patients, although with more secondary side effects than rituximab.229,230
Recent observational studies have suggested that calcineurin inhibitors (cyclosporine A at 2.5mg/kg/day and tacrolimus at 0.075mg/kg/day) in combination with other drugs such as mycophenolate can induce remission in patients with persistent activity after receiving standard induction therapy. However, the potential effect of this treatment regimen on the long-term prognosis of renal function has yet to be demonstrated.232-234 In patients with a glomerular filtration rate <60ml/min, treatment with calcineurin inhibitors should be avoided.
There is no evidence suggesting that plasmapheresis or immunoadsorption can induce remission in patients that are resistant to other treatments.235
There are several drugs and treatments (leflunomide, bone marrow transplant, stem cell transplant, ablative doses of cyclophosphamide, fludarabine, cladribine, chlorambucil, mizoribine, thalidomide/lenalidomide, imatinib, etc.) that have occasionally been used in these patients, and several therapeutic targets are under development, whose benefit is yet to be explored.
The decision as to when to intensify induction therapy or how long it should be prolonged before considering the patient to be resistant should be considered on an individual basis based on the severity of the nephropathy, considering the equilibrium between the risk of infection brought on by excessive immunosuppression and the risk of developing irreversible renal fibrosis lesions associated with late or absent response to treatment. On the other hand, the lack of a response in the absence of other signs of renal or extra-renal treatment could suggest the need for a renal biopsy before intensifying this type of treatment, in order to rule out the presence of irreversible lesions with no potential for improvement using immunosuppressive drugs.
CONFLICTS OF INTEREST
Laboratorios Novartis has collaborated on the organisation of meetings of this group and the publication of the manuscript.
No organisation outside of the S.E.N. or GEAS-SEMI scientific societies has participated in the election of authors or the selection of references, nor had any access to the different drafts of the manuscript before publication.
Guillermo Ruiz Irastorza. He declares no conflicts of interest with respect to the contents of this article.
Gerard Espinosa. He declares no conflicts of interest with respect to the contents of this article.
Miguel Ángel Frutos. He declares no conflicts of interest with respect to the contents of this article.
Juan Jiménez Alonso. He declares no conflicts of interest with respect to the contents of this article.
Manuel Praga. He declares no conflicts of interest with respect to the contents of this article.
X. Lucio Pallarés. He declares no conflicts of interest with respect to the contents of this article.
Francisco Rivera. He declares no conflicts of interest with respect to the contents of this article.
Ángel Robles Marhuenda. He declares no conflicts of interest with respect to the contents of this article.
Alfons Segarra. He declares no conflicts of interest with respect to the contents of this article.
Carlos Quereda. He declares no conflicts of interest with respect to the contents of this article.
Table 1. Quality levels and grades of recommendation used in this consensus document*
Table 2. Classification system for lupus nephritis according to ISN/RPS (2003)
Table 3. Clinical-pathological correlations
Table 4. Active or chronic lesions
Table 5. Other renal lesions in systemic lupus erythematosus
Table 6. Indicaciones de biopsia renal
Table 7. Indications for repeating a renal biopsy
Table 8. Laboratory variables proposed for the follow-up of patients with lupus nephritis
Table 9. Response criteria
Table 10. Criteria for recurrence
Table 11. Possible therapeutic options for the prevention and treatment of osteopaenia/osteoporosis induced by glucocorticosteroids in adult patients
Table 12. Recommendations for vaccines in patients with systemic lupus erythematosus