The development of new direct-acting antivirals (DAA) for the treatment of hepatitis C virus (HCV) has been a revolution in the control of this infection, achieving sustained virological response (SVR) rates close to 99%.1–4 Some extrahepatic (EH) manifestations associated with this infection may persist or recur after achieving SVR with DAA.4 Few cases have been reported of the development of new EH manifestations after achieving SVR. We present a case of mixed cryoglobulinaemic vasculitis (MCV) and membranoproliferative glomerulonephritis, accompanied by sicca syndrome (SS), which manifested 28 months after achieving SVR with DAA.
This patient was a 70-year-old woman with HCV infection without liver or EH involvement, treated with elbasvir/grazoprevir in December 2017, achieving SVR. Six months after achieving undetectable viraemia, she began to experience xerophthalmia, xerostomia and parotitis, for which she began follow-up by rheumatology. Blood tests showed: hypergammaglobulinaemia (polyclonal IgG), positive ANA (titre 1/640), negative anti-RO antibodies (anti-Ro) and anti-La antibodies (anti-LA), rheumatoid factor (RF) 55mg/dl (0–40); serum creatinine (sCr) 0.87mg/dl; basic urine negative for proteins and red blood cells. With these abnormalities, she was diagnosed with SS, as she did not meet the criteria for primary Sjögren's syndrome due to negative anti-Ro and anti-La, and previous HCV infection.
In January 2020, the patient began to experience purpuric skin lesions on her lower limbs associated with type III or mixed positive cryoglobulins (polyclonal IgG and polyclonal IgM), without prior testing having been performed; her RF had risen (130 IU/mL) and renal function remained normal (sCr 0.87mg/dl), along with a basic urine sample with no abnormalities. Skin biopsy was inconclusive. However, based on the rest of results, it was treated as type III or mixed cryoglobulinaemic vasculitis with skin involvement and she was started on oral prednisone (0.5mg/kg/day).
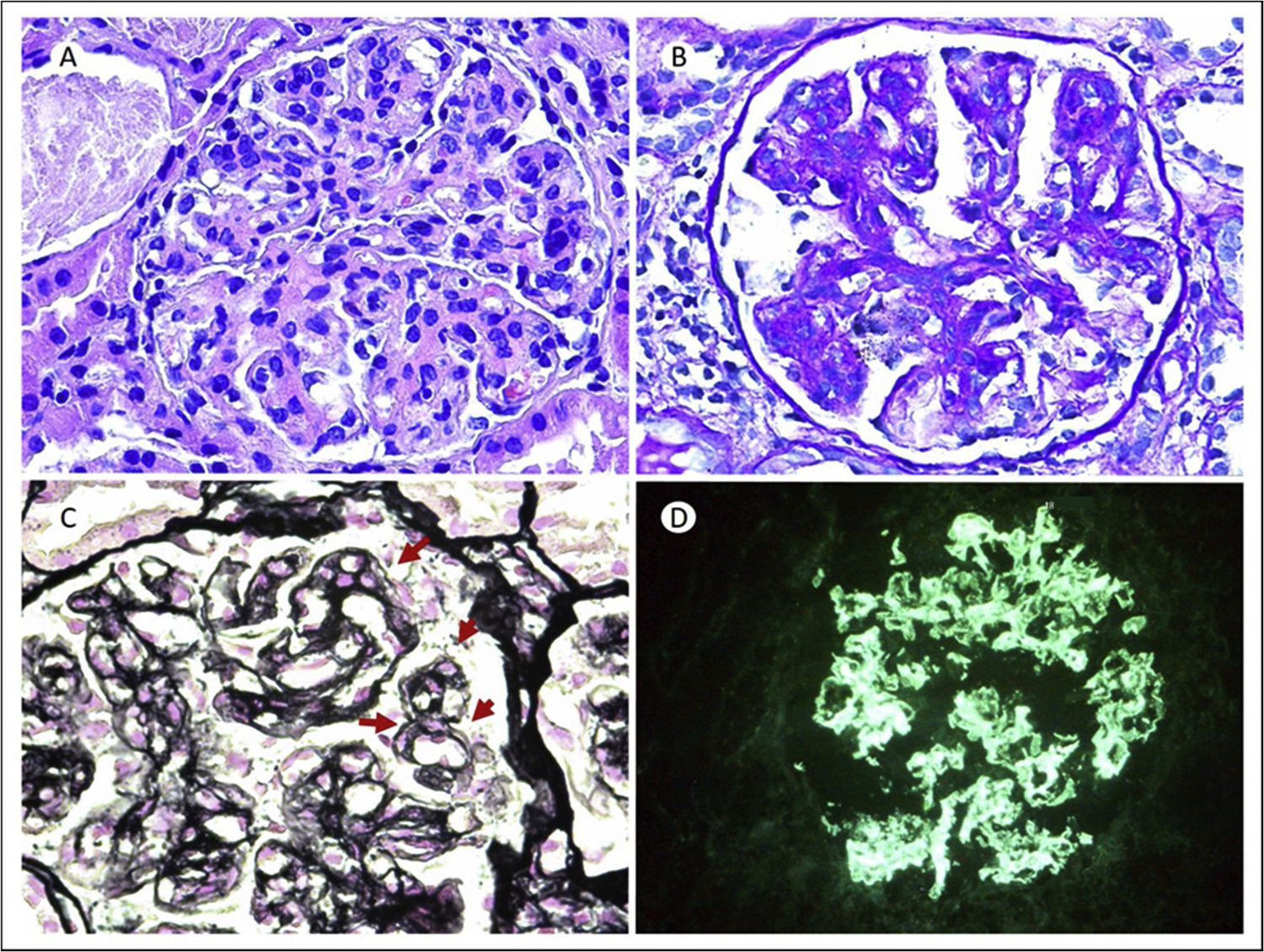
In May of that same year, she was admitted for a respiratory infection, documenting simultaneously a clinical and biochemical nephrotic syndrome (sCr 1.35mg/dl; urea 55mg/dl; albumin 3.2g/dl; cholesterol 275mg/dl; 24-h urinary protein 5,900mg/24h; urinary sediment with 5–10 dysmorphic red blood cells/field, and hypocomplementaemia with C3 of 47.80mg/dl and C4 of 2.20mg/dl). The rest of the autoimmunity study was negative (negative antiPLA2R, ANCA and proteins). Kidney biopsy showed 18 glomeruli, all of them hypertrophic with lobulated morphology and occlusion of vascular lumens by inflammatory cells, mesangial expansion with mesangial hypercellularity (Fig. 1A and B). Using the silver technique, double contour images in the capillary loops (Fig. 1C). Immunofluorescence showed capillary positivity for IgG (+++) in a coarse granular pattern and with a subendothelial and mesangial location (Fig. 1D). All of this is consistent with a pattern of membranoproliferative glomerulonephritis (MPGN) mediated by immune complexes.
(A) H&E: 20×. Glomerulus with lobulated morphology, mesangial expansion due to matrix and mesangial and endocapillary proliferation. (B) PAS 20×: PAS positive mesangial expansion, with increased cellularity. (C) Methenamine silver 64×. Images of double contours in the glomerular capillary loops (red arrows). (D) Direct immunofluorescence (DIF) IgG 20×. Intense deposit (3+) in a coarse granular pattern and with a subendothelial and mesangial location for IgG.
Although there was no sample for electron microscopy and no thrombi or fibrinoid necrosis were found, the rest corresponded to type III MCV with skin and kidney involvement in the form of MPGN, probably related to past HCV infection. A lymphoproliferative syndrome was ruled out by computed tomography, and the HCV viral load was always negative. The patient was given rituximab (500mg x 2 doses 15 days apart) and steroid therapy in a tapering regimen. After three months she was in complete remission: sCr 0.8mg/dl; albumin 4.2g/dl; C3 66.90mg/dl (normal); C4 5.40mg/dl (normal); negative cryoglobulins. Urinalysis with 24-h urinary protein of 294mg/g and normal sediment. She has remained stable over the subsequent 18 months of follow-up, receiving maintenance doses of rituximab (500mg) every six months since then.
The development of new DAA against HCV has represented a paradigm shift in the treatment of this infection.1 They are clearly effective in eradicating the virus, with SVR rates close to 99%. Despite this, the effectiveness in controlling some EH manifestations is not so evident.1
MCV is the most common EH manifestation.2 Typically, those associated with hepatitis C virus infection are type II and/or type III cryoglobulinaemia, also known as mixed cryoglobulinaemias.3 Some 70–90% are associated with HCV infection.2 In our case, it was a type III mixed cryoglobulinaemia because it was formed by polyclonal IgG and IgM, with rheumatoid activity.
The renal involvement usually associated with MCV due to hepatitis C virus is MPGN.3 DAA have highly variable clinical and immunological response rates on MCV (64%–96% and 48%–89%, respectively).4 Furthermore, some authors have suggested that the use of DAA alone could be insufficient in controlling renal manifestations and cryoglobulinaemic vasculitis.4 Recent publications have reflected persistence of MCV with or without renal involvement despite SVR with DAA.5–8 In these patients, the use of immunosuppressive treatment such as rituximab can be effective in controlling the disease.9 In our case, what makes it unusual is that the manifestations appeared after SVR. Prior to treatment with DAA, no EH involvement had been documented. To the best of our knowledge, there are few published cases similar to ours.
The mechanism by which patients with eradicated HCV infection can develop EH manifestations after treatment with DAA is not fully understood. HCV infection causes different immune system regulation abnormalities. One well-known theory is that HCV "settles" inside the cell it infects, thereby avoiding attack by the immune system.10 HCV acts on the usual cellular DNA repair mechanisms, producing an increase in unrepaired DNA alterations that are associated with greater genomic instability, increasing the risk of development of neoplasia and dysfunction of the immune system.10,11 Among other factors, this would cause the abnormal activation of B lymphocytes and their monoclonal expansion, which would give rise to the formation of cryoglobulins responsible for MCV.11 Some studies have found that patients who achieved clinical remission of MCV after the use of DAA have shown a decrease in activated B lymphocytes and an increase in regulatory T lymphocytes.12 Other authors, such as Hegazy et al., have found that patients with HCV infection treated with DAA have a higher concentration of B lymphocyte activation factors (BAFF and APRIL) during the first 12 months after receiving treatment.13 This could lead to hyperactivity of B lymphocytes after the use of DAA, which could explain the appearance of autoimmune manifestations after treatment.
Lastly, contrary to what might have happened with the immunomodulatory effect of interferon, free regimens of this drug could lose this anti-inflammatory effect, and not be sufficient in all cases to control EH manifestations.13
In conclusion, DAA improve hepatic morbidity and mortality rates in almost all cases. The exact mechanism behind the development of autoimmune manifestations that appear once SVR is achieved with these drugs is unknown. Therefore, in the first few months after treatment with DAA, it is important to closely monitor for autoimmune manifestations as, if they do appear, they may require additional immunosuppressive therapy.
FundingThe authors declare that they have not received any funding for publishing this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.