Dear Editor,
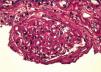
We read with interest the report of Dr. Guang-Yu Zhou, about a case of Membranous Glomerulonephritis with crescentic transformation.1 Acute crescentic transformation is a rare but well described event in patients with membranous glomerulonephritis.2 The concomitant occurrence of a vasculitic glomerulonephritis and membranous nephropathy in the same patient is unusual.3 We report herein our similar experience. A caucasic 66-year-old man presented for rapid declining of renal function. For nearly 10 years he was suffering from hypertension and for 4 years he is having paresthesias, muscle aches in the legs and significant reduction in muscle strength with an ataxic walking. This condition was interpreted as a mixed sensori-motor polyneuropathy. He performed therapy with prednisone and azatioprine, suspended for 1 year; six months before, laboratory tests showed a serum creatinine concentration 220μmol/L and a 24-h protein excretion 0,75g/d. On admission, the physical examination showed edema and severe hypertension; we detected a 24-h protein excretion 1.1g/d, Hb: 9.1g/dl, serum creatinine concentration 550.8μmol/L, the urinalysis 2 + urine protein and 1 + urine blood. ANCA determined in serum screening test by indirect immunofluorescence and other immunological tests, including anti-nuclear antibodies (ANAs), anti-Sm antibody, anti-dsDNA antibody, anti-cyclic citrullinated peptide (CCP) antibody, and anti-glomerular basement membrane (GBM) antibody were negative; serum complement and immunoglobulin levels were normal. Liver function tests and other routine parameters were within the normal range. There was no evidence of systemic lupus erythematosus (SLE), infection, malignancy. For the clinical suspicion of a vasculitis leading to renal and neurological involvement, we performed a kidney biopsy. In the light microscopic visualization of renal tissue, 11 out of 17 glomeruli were globally sclerotic; the remaining glomeruli showed: diffuse, global and marked thickening of capillary wall (Figure 1), focal and diffuse sub-epithelial vacuolation; global and segmental sub epithelial deposits with sub-epithelial spikes, formation of 11 crescents (n° 3 cellular crescents, n° 1 fibrocellular crescent and n° 7 fibrotic crescents (Figure 2). Immunofluorescence examination displayed granular deposition of IgG, kappa and lambda light chains and a strong, diffuse and global granular staining along the glomerular capillary (subepithelial) walls. Therefore renal histology and laboratory examinations supported diagnosis of membranous GN with crescentic overlap. We diagnosed a vasculitis ANCA-negative and the patient was treated initially with methylprednisolone pulse 125mg/d for 3 days followed by prednisone 50mg/d, and i.v. cyclophosphamide 0.25g once every 21 days. Because of no sign of improvement shown 2 months later, we stopped the cyclophosphamide therapy and the patient started chronic haemodialysis treatment. Several authors have reported acute crescentic transformation in patients with primary membranous glomerulonephritis. Vasculitic or crescentic glomerulonephritis is rarely seen in membranous nephropathy, except in those cases associated with systemic lupus. The immunopathogenesis of this unusual transformation is unclear. It is well recognized that patients with a crescentic glomerulonephritis have severe and often rapidly deteriorating failure. Unlike membranous nephropathy, which often has an insidious course progressing to renal failure over a period of years, patients with superimposed crescentic glomerulonephritis appear to have a more aggressive clinical course. The importance of recognizing this group of patients with membranous nephropathy and crescentic glomerulonephritis is that immunosuppressive therapy may ameliorate the progression of renal damage and in some cases early treatment was associated with useful recovery of renal function.4 In our case, the discontinuation of prednisone and azathioprine therapy may have facilitated the rapid progression of kidney disease.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1. PAS stain shows global and prominent thickening of the glomerular basement membranes, in the absence of evidence of cellular proliferation (PAS, x 400)
Figure 2. PAS stain shows a fibrocellular crescent in the urinary space that compresses the residual glomerulus and its tuft (PAS, x 400)