Chronic kidney disease (CKD) is an emerging global burden with an increasing number of patient's requiring renal replacement therapy (RRT), with hemodialysis being the most prevalent dialysis modality. A functioning vascular access remains the main constrain for an adequate treatment. Clinical and, in some patients, ultrasound evaluation are fundamental for better access planning. Access planning is dependent not only on patient clinical characteristics and preference but also in vascular patrimony. As such, ultrasound evaluation aids in characterizing patient arterial and venous upper arm anatomy and provides information for which access would better suit each patient. Doctors dealing with CKD patients should be familiar with the role of ultrasound and Doppler use in access planning.
La enfermedad renal crónica (ERC) es una carga global emergente con un número creciente de pacientes que precisan tratamiento renal sustitutivo (TRS) y la hemodiálisis es la modalidad de diálisis más prevalente. Un acceso vascular funcional sigue siendo la principal limitación para un tratamiento adecuado. La evaluación clínica y, en algunos pacientes la ecográfica, son fundamentales para una mejor planificación del acceso. La planificación del acceso depende no solo de las características clínicas y las preferencias del paciente, sino también de la anatomía vascular. Por tanto, la evaluación ecográfica ayuda a caracterizar la anatomía arterial y venosa de la parte superior del brazo del paciente y ofrece información sobre qué acceso sería más adecuado para cada paciente. Los médicos que tratan a pacientes con ERC deben estar familiarizados con el papel de la ecografía y el uso del Doppler en la planificación del acceso.
Chronic kidney disease (CKD) is an emerging global burden with an increasing number of patient's requiring renal replacement therapy (RRT).1 Although RRT comprises many options (hemodialysis (HD), peritoneal dialysis, renal transplantation and conservative management), HD remains the predominant form of dialytic therapy.1 Many developments in HD treatment were accomplished (better machines, water treatment and so on) but a functioning vascular access remains the main constrain for an adequate treatment.2 Nowadays there are many options for vascular access, including innovative solutions for more complex patients (for instance HeRO graft for patients with central venous stenosis)3 or even less invasive techniques (percutaneous endovascular arteriovenous fistula (AVF) creation).4 AVF's are the preferred vascular access (because of lower risk of complications as well as lower mortality/morbidity risk)5 but, in certain patients, a fistula first approach may not be the most adequate.6 Other options include arteriovenous grafts (AVG) or central venous catheters (CVC). The latter are usually used in non-planned dialysis induction, but may have a role in certain situations like short term HD as a bridge for living kidney donor transplant as well as patients with presumed short life expectancy which are not candidates to autologous or prosthetic AVF. Although AVF creation is a simple procedure, it is associated with high failure-to-mature rate.7 This can be mitigated with an adequate clinical history and physical examination prior to AVF construction which will help to plan the most suitable AVF location. Nonetheless physical examination is not sufficient in many patients (obese, peripheral arterial disease, multiple comorbidities and risk factors for central stenosis). Also, many patients present anatomical variants which can impact on access planning and can only be identified with ultrasound mapping, some examples include presence of collaterals, early drainage of the basilic vein into the brachial vein and narrowing of the cephalic vein along its trajectory. Vascular mapping provides anatomic and hemodynamic information aiding in the preoperative evaluation and is a noninvasive, cheap and accessible exam. Its routine use may contribute for better AVF outcome, especially in the patients with higher risk of access failure.8 A meta-analysis performed by Cochrane in 2015 that included 4 randomized controlled trials (RCT) concluded that preoperative vessel imaging did not improve fistula outcomes compared with standard care.9 In the same year another meta-analysis (including data of one additional RCT) concluded that preoperative mapping could significantly reduce the immediate AVF failure rate thus recommending routine use of Doppler ultrasound previous to AVF creation.10 Regarding existing guidelines there are also some differences. KDOQI 2006 guidelines recommended preoperative ultrasound in all patients11 but the recent 2019 update recommends selective preoperative ultrasound (only patients with high risk of access failure).8 On the other hand, Spanish 2017 Access Guidelines recommend routine preoperative ultrasound vascular mapping in all patients.12 The 2019 ERBP guidelines for peri and postoperative access care do not mention any recommendation regarding preoperative ultrasound vascular mapping.13 Although there is some disparity regarding this topic, vascular mapping definitively plays a role in preoperative evaluation for access creation (at least in certain high-risk patients). Ideal vascular access must take into account a series of factors including clinical history, physical examination, ultrasound vascular evaluation and patient preference.12
Clinical evaluationA comprehensive clinical history and physical examination is fundamental when planning a vascular access.12 This includes patient age, comorbid conditions, plans (for instance, prospect kidney transplantation in the near future), CKD stage prevision for dialysis start.12 Patient's professional activity as well as dominant limb should also be assessed and, when possible, the contralateral limb should be used for access creation.
Comorbid conditions that may influence access maturation must be recognized.11,12 These include risk factors for arterial disease (such as diabetes mellitus, smoking habits, intermittent claudication, previous isquemic stroke and obesity) as well as factors that may be associated with altered anatomy such as trauma/surgery (including breast cancer surgery and possible upper limb lymphedema, presence of a pacemaker). In addition, it is important to identify factors that can be associated with scarce venous territory: previous AVF/AVG, chemotherapy, radiotherapy, presence of pacemaker, previous central catheters and multiple peripheral venous catheterizations. After creation, AVF/AVG will bypass some arterial circulation leading to potential risk for cardiac recirculation. This is important in patients with congestive heart failure, whose therapy must be optimized previous to access creation and, when feasible, a distal vascular access should be attempted (lower risk of high output). Patients with significant reduction in systolic function (ejection fraction lower than 30%) or classified within the NYHA Class IV should probably be candidates for tunneled catheter placement.14 Thrombophilia, antiplatelet drug therapy and anticoagulation should also be taken into account.
Global evaluation of the patient's performance status is fundamental, especially in the very elderly group (> 80 years-old).6 Although in this subgroup there is a higher prevalence of multiple comorbidities, chronological age alone is not a marker of frailty/disability. Access creation (especially AVF) in this subgroup of patients is frequently a challenge: many will need an intervention after AVF creation (increasing the time span between AVF creation and use)15 or even a CVC because of failure of maturation or other complications.16 Therefore, a fistula first approach may not be suitable for all elderly patients. Some guidelines recommend that a more conservative approach (CVC placement) may be used according to comorbid conditions and life expectancy.12 Also, many studies support an AVG first strategy in the very elderly population especially those with uncertain prognosis of survival, poor vasculature, or variable rate of progression to ESRD.15,17,18
Regarding physical examination, both the arterial and venous circulation must be assessed.11,12 Examination should document arterial patency and pulse amplitude as well as performance of the Allan's test. The latter allows assessment of the circulation in the hands and may identify a small subgroup of patients in which blood supply to the hand is not assured by both the radial and cubital artery. In these patients an access creation may pose them in risk for hand ischemia after the procedure.19 Blood pressure in both arms must also be evaluated.
For venous evaluation, the clinician must search for a suitable vein for AVF construction: it must be superficial, have a linear trajectory and dilate sufficiently after compression with a tourniquet. It is also important to document the presence/absence of scars from previous CVC's (as these may be associated with central stenosis) and to assess for signs of central venous stenosis (collateral circulation in the thorax and upper arm or limb edema). In case of suspicion of a central venous stenosis, an imaging exam should be performed (contrasted computerized tomography (CT) or venography).
Ultrasound evaluationFor vascular mapping and assessment, the operator must be familiarized with the vascular anatomy (and possible anatomic variations) of the upper extremity, have basic knowledge of the types of transducers and their correct placement as well as B-mode and Doppler mode for imaging evaluation.20 Particularly, a good understanding of Doppler physics and hemodynamic physiology is fundamental for good exam accuracy.
Patient positionThe patient can be either seated or lying down in a comfortable position with the area of examination exposed and his arm abducted and the palmar face of the hand exposed (if seated the limb should be comfortably placed on top of a pillow). Room temperature should be warm (cold or excessive heat could promote vascular compression/dilation and change the exam's results).20
TransducerA high-frequency Doppler ultrasound linear transducer is the ideal probe for vascular evaluation. A convex probe might be necessary in case of obese patients and/or to evaluate central vessels. An extensive review of transducer characteristics is beyond the scope of this article but operators performing ultrasound evaluation should be familiar with them.20
Optimal scanning techniques/Doppler settings and limitationsDoppler mode is extremely useful when evaluating vascular structures because it allows the detection of flow direction, measurement of blood velocities, volume flow, and hemodynamic evaluation of an arterial stenosis.20 As stated above, ultrasound evaluation is an operator-dependent technique and professionals using it must be familiar with optimal settings for correct evaluation. Correct position of the transducer to achieve an angle of insonation between 45° and 60°, as well as an angle correction cursor parallel to the direction of blood flow is essential.20 This is important because incorrect assignment of these parameters is a common source of operator error and leads to incorrect velocity measurements. Also, for correct flow direction assessment the operator must be aware of which colors represent which direction of flow: normally red is used to show flow directed towards the transducer and blue flow directed away. But since most scanners allow scale color inversion the operator must confirm the color scale used in each examination. Correct color box steering and size are also important for color image optimization and must be adjusted. Sample volume length adjustment is needed to correctly calculate volume flow (sample size should measure between 50-70% of the vessel caliber).20
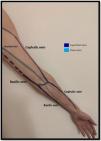
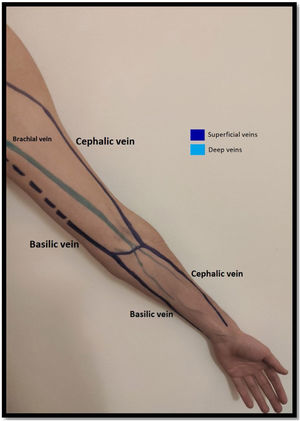
Venous evaluationVenous circulation in the upper extremity can be divided into superficial and deep veins, the first being more commonly used for AVF creation (Fig. 1). The superficial venous system is comprised by the cephalic (lateral) and basilic (medial) veins.20 The cephalic vein drains the dorsal surface of the hand and runs from the lateral aspect of the forearm until it drains into the axillary vein. The basilic vein drains the palmar surface of the hand and runs from the distal medial aspect of the forearm until it drains into the brachial vein (although its anatomy can be quite variable, sometimes draining directly to the axillary vein).20 Despite being superficial, the basilic vein penetrates the fascia in the more proximal aspect of the forearm (as represented with doted lines in Fig. 1). When used for access creation the basilic vein must be superficialized/transposed (which may sometimes imply a second surgical time and a longer maturation process). Because of this, the cephalic vein is the preferred vein for AVF creation. Many anatomical variations may occur and the clinician performing the exam must be aware of this when performing Doppler ultrasound.21
The deep venous circulation is composed by the veins that accompany the radial and cubital arteries and normally join at the elbow to form the brachial veins (usually paired and accompanying the brachial artery). This in turn drains to the axillary, subclavian and brachiocephalic veins. The deep venous veins normally are used only in complex access creation in patients with paucity of autologous material in whom a graft is normally needed (for example: axillary artery to axillary vein graft; HeRO grafts, brachio-brachial fistula).22–25
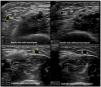
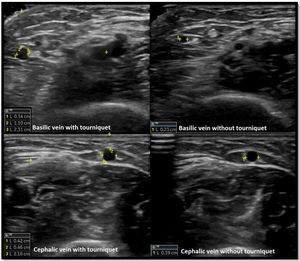
Venous evaluation includes demonstration of patency. This is achieved by applying pressure to the transducer and totally compressing the vein. Besides patency, vein diameter is also measured (Fig. 2) as many studies demonstrated that this parameter has implications for prognosis of the access. The existing guidelines do not define a minimum acceptable vein diameter for AVF/AVG creation but it is generally accepted that diameter superior to 2mm is associated with better outcomes. Vein diameter changes with the use of a tourniquet can be measured, as this will provide information about venous distension capacity (distention capacity superior to 40% is a good prognostic factor).26–28 Also, any anatomical variation must be characterized as this may have implication when planning access construction (different drainage site, presence of accessory veins, different basilic vein course).21
Finally, assessment of central venous circulation by ultrasound is very difficult, and exclusion of central vein stenosis by this mean is challenging. The subclavian vein is visualized from either the infraclavicular fossa (distal end) or the supraclavicular fossa (mid-subclavian vein). While compression of the subclavian vein is difficult to assess because of the underlying anatomy, its patency may be evaluated using spectral Doppler.20 This will show spontaneous phasic flow with respiration regarding there is no outflow obstruction. Diminished respiratory phasicity and diminished transmitted cardiac pulsatility in the subclavian and jugular veins may suggest an occlusion proximally (proximal subclavian vein, brachiocephalic trunk and superior vena cava), but a normal flow does not exclude occlusion.29 If occlusion is suspected, internal jugular vein patency can be evaluated as a thrombus there may be visualized. In case of central stenosis suspicion a venography/contrasted CT scan must be performed.20
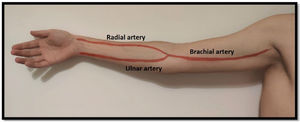
Arterial evaluationArterial circulation in the upper extremity is ensured proximally by the subclavian artery which turns into the axillary artery between the clavicle and the first rib. Distally it converts to the brachial artery (in the upper arm) and usually divides into the radial and ulnar arteries 1–2cm below the elbow (but this bifurcation can be more proximal/distal)20 (Fig. 3). The radial artery runs lateral and the ulnar artery runs medial until they communicate in the palmar arch. As stated above, some people do not have this communication between the radial and ulnar artery (and the Allan test helps identifying them).
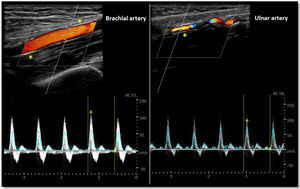
As for the venous circulation, arterial patency and diameter must be evaluated. Markers of arterial disease such as vascular calcification (hyperechogenic contour of the vessel sometimes severe enough that an acoustic shadow is seen),20 stenotic disease (post-stenotic doubling of peak systolic velocities indicates a >50% diameter reduction)20 and altered flow pattern must be assessed (Fig. 4). The upper arm arterial circulation is comprised of high resistance flow arteries and this will translate into triphasic flow pattern in the Doppler evaluation20 (Fig. 4). Alteration of the flow pattern (biphasic/monophasic flow) indicates diseased arteries (although this does not preclude access creation). Radial artery systolic peak velocity (SPV) measurement as well as artery flow rate are also useful, as a value below 50cm/s and below 50mL/min respectively, have been associated with higher rates of primary failure.30,31
Arterial diameter has been shown to be associated with better access outcomes. Severe calcification may hamper arterial diameter measurement because of acoustic shadow. Although current guidelines do not establish a minimum arterial diameter for access creation, it is settled that when <15mm chances of success are lower.12,32,33 Nonetheless, a small diameter should not preclude access creation, since other variables should also be taken into account (for instance functional quality of the artery).12,34
A useful test when performing ultrasound is assessing for reactive hyperemia.34 This consists of clenching the fist for 1–2min and evaluating the arterial flow pattern afterwards. A reduction in the resistance index below 0.7 after this maneuver is related to increased rate of success after fistula creation.35
ConclusionClinical management of the patient with CKD is challenging, and preparation for RRT is an important step in the course of CKD. A functioning vascular access is a key factor for better quality of treatment and patient wellbeing. CKD is becoming more common, especially in elderly patients because of the increasing patient longevity seen nowadays. Unfortunately, AVF's still have a high rate of primary failure, and this is even more prevalent in the elderly patients as well as those with comorbid conditions that damage their vascular system. Thorough clinical evaluation helps in better planning of the access placement, but may not be sufficient, especially in certain subgroups of patients mentioned above. Ultrasound and Doppler evaluation are therefore of invaluable aid in access planning. Although some RCT's have not shown better fistula outcomes in patients submitted to pre-surgical sonographic evaluation, it remains a major tool because of its advantages (low cost, accessibility and non-invasive technique), especially in patients in whom physical examination fails to identify a suitable vessel for access creation. As such, some guidelines already recommend that ultrasound assessment should be routinely performed in all patients previous to access creation.12 Therefore, doctors dealing with CKD patients should be familiar with the role of ultrasound and Doppler use in access planning.
Financial disclosureNone declared.
Conflicts of interestNone declared.