Las guías de práctica clínica para la prevención, diagnóstico, evaluación y tratamiento de los trastornos minerales y óseos en la enfermedad renal crónica (TMO-ERC) en adultos, de la Sociedad Latinoamericana de Nefrología e Hipertensión (SLANH), comprenden un conjunto de recomendaciones elaboradas para dar apoyo al médico en el manejo de estas anormalidades en pacientes adultos con enfermedad renal estadios 3 a 5. No se incluyen las alteraciones asociadas al trasplante renal. Los temas abordados en las guías están distribuidos en cuatro capítulos: 1) Evaluación de las alteraciones bioquímicas; 2) Evaluación de las alteraciones óseas; 3) Evaluación de las calcificaciones vasculares, y 4) Tratamiento de los TMO-ERC. Las guías tienen como base las recomendaciones propuestas y publicadas por la Kidney Disease: Improving Global Outcomes (KDIGO) para la prevención, diagnóstico, evaluación y tratamiento de los TMO-ERC (KDIGO Clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder [CKD-MBD]), adaptadas a las condiciones de pacientes, instituciones y recursos disponibles en Latinoamérica, con el aval de KDIGO. En algunos casos, las guías corresponden a recomendaciones de manejo definidas directamente por el grupo de trabajo para su aplicación en nuestra región, basadas en la evidencia disponible en la literatura. Cada capítulo contiene las guías propiamente dichas y su fundamentación con base en numerosas referencias bibliográficas actualizadas. Desafortunadamente, existen pocos estudios controlados y con un poder estadístico suficiente en Latinoamérica para dar soporte a recomendaciones específicas para la región, por lo que la gran mayoría de las referencias utilizadas corresponden a estudios realizados en otras regiones. Esto pone en evidencia la necesidad de planificar estudios de investigación dirigidos a establecer la situación actual de los trastornos del metabolismo mineral y óseo en Latinoamérica, así como definir las mejores opciones terapéuticas para nuestra población.
The clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of chronic kidney disease mineral and bone disorders (CKD-BMD) in adults, of the Latin American Society of Nephrology and Hypertension (SLANH) comprise a set of recommendations developed to support the doctor in the management of these abnormalities in adult patients with stages 3-5 kidney disease. This excludes changes associated with renal transplantation. The topics covered in the guidelines are divided into four chapters: 1) Evaluation of biochemical changes, 2) Evaluation of bone changes, 3) Evaluation of vascular calcifications, and 4) Treatment of CKD-MBD. The guidelines are based on the recommendations proposed and published by the Kidney Disease: Improving Global Outcomes (KDIGO) for the prevention, diagnosis, evaluation and treatment of CKD-MBD (KDIGO Clinical practice guidelines for the diagnosis, evaluation, prevention and treatment of Chronic Kidney Disease Mineral and Bone Disorder [CKD-MBD]), adapted to the conditions of patients, institutions and resources available in Latin America, with the support of KDIGO. In some cases, the guidelines correspond to management recommendations directly defined by the working group for their implementation in our region, based on the evidence available in the literature. Each chapter contains guidelines and their rationale, supported by numerous updated references. Unfortunately, there are few controlled studies with statistically sufficient weight in Latin America to support specific recommendations for the region, and as such, most of the references used correspond to studies carried out in other regions. This highlights the need to plan research studies designed to establish the current status of mineral and bone metabolism disorders in Latin America as well as defining the best treatment options for our population.
PREFACE
The preparation of the clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of chronic kidney disease-mineral and bone disorders (CKD-MBD) in adults is an initiative of the Latin American Society of Nephrology and Hypertension (SLANH). The SLANH’s Mineral and Bone Metabolism Committee, consisting of an international representation of leaders in the area of kidney disease mineral and bone metabolism, was in charge of its preparation (vide supra).
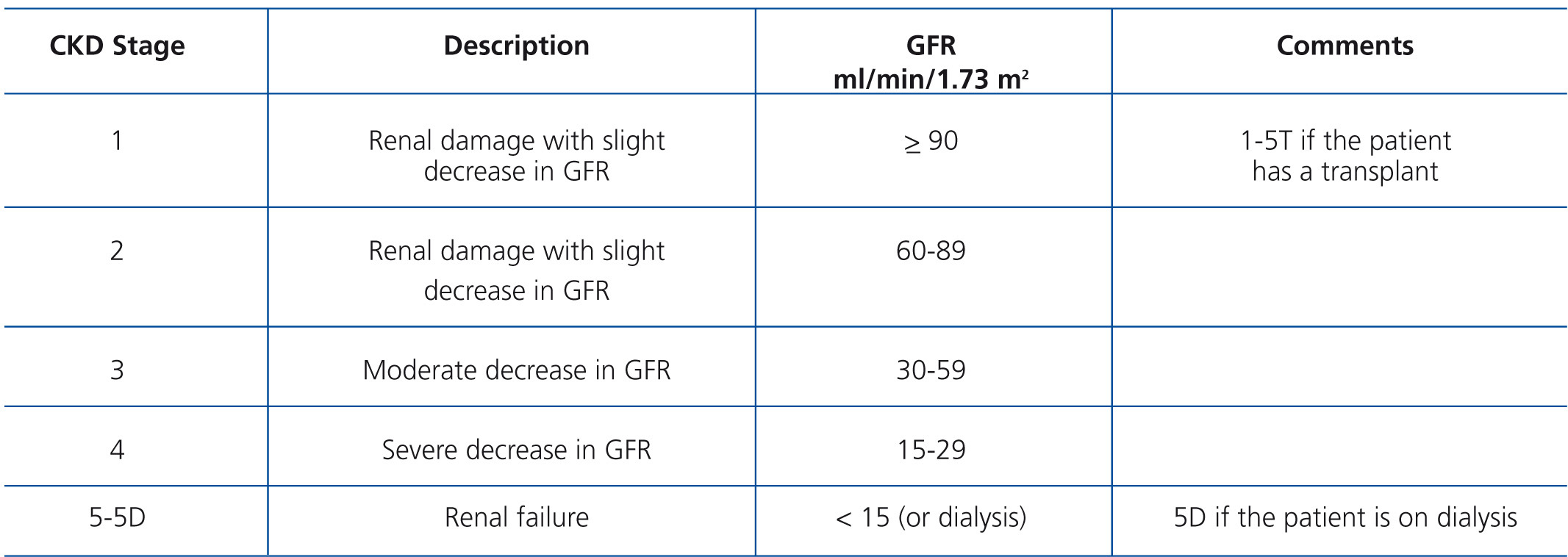
These are practical guidelines for the standardisation of diagnosis and treatment of chronic kidney disease (CKD) mineral and bone disorders disease in its different stages (Table A). In line with the trend of the global nephrology community, these guidelines are based on recommendations proposed and published by Kidney Disease: Improving Global Outcomes (KDIGO) for the prevention, diagnosis, evaluation and treatment of CKD-MBD (KDIGO Clinical practice guidelines for the diagnosis, evaluation, prevention and treatment of Chronic Kidney Disease Mineral and Bone Disorder [CKD-MBD]),1 and guidelines published by the Nephrology Societies of Argentina, Brazil, Mexico and Uruguay to meet the needs and conditions of patients, institutions and material resources available in Latin America. The elements used in the preparation of the KDIGO guidelines were summarised, updated and adapted without altering its essence. The use of statements and original concepts from the KDIGO guidelines has been approved by the aforementioned organisation. As such, the paragraphs that correspond to strict translations of the guidelines have been marked with the initials KDIGO at the end of the relevant paragraph, while those that correspond to modifications or adaptations of the guidelines drawn up by SLANH's Committee have been marked with the acronym SLANH.
The arguments that support the recommendations in these guidelines have been updated by the inclusion of new references. In many cases they were the subject of extensive discussion in which the aim was to adapt them to regional conditions and facilitate their implementation.
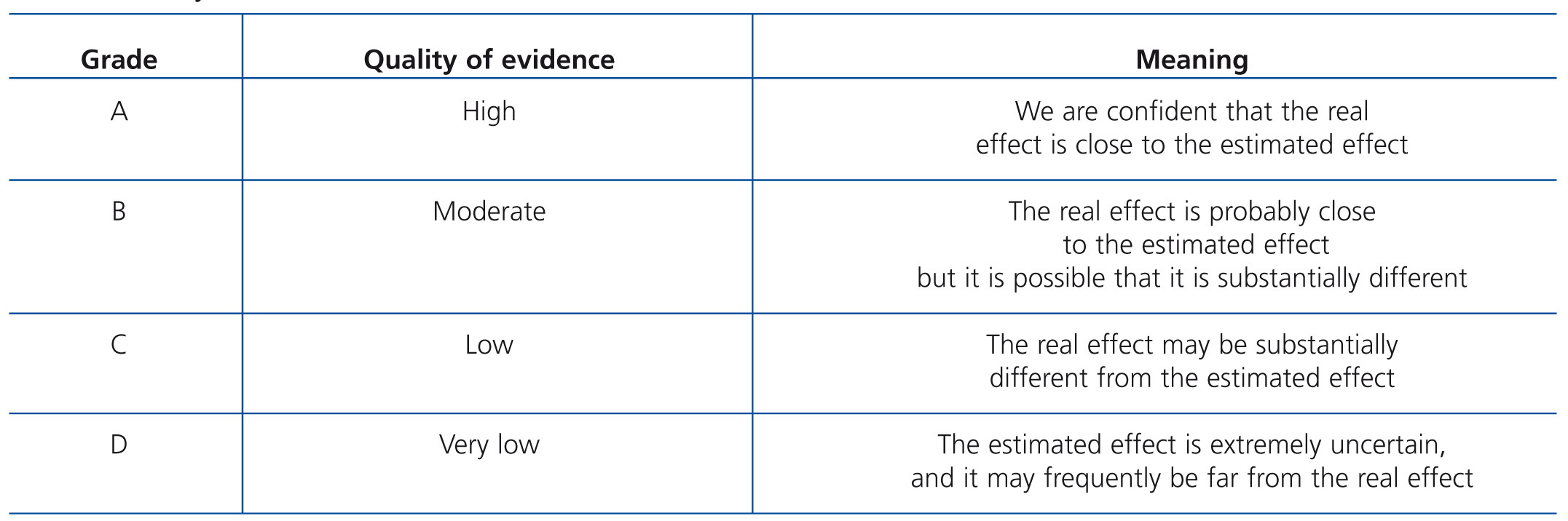
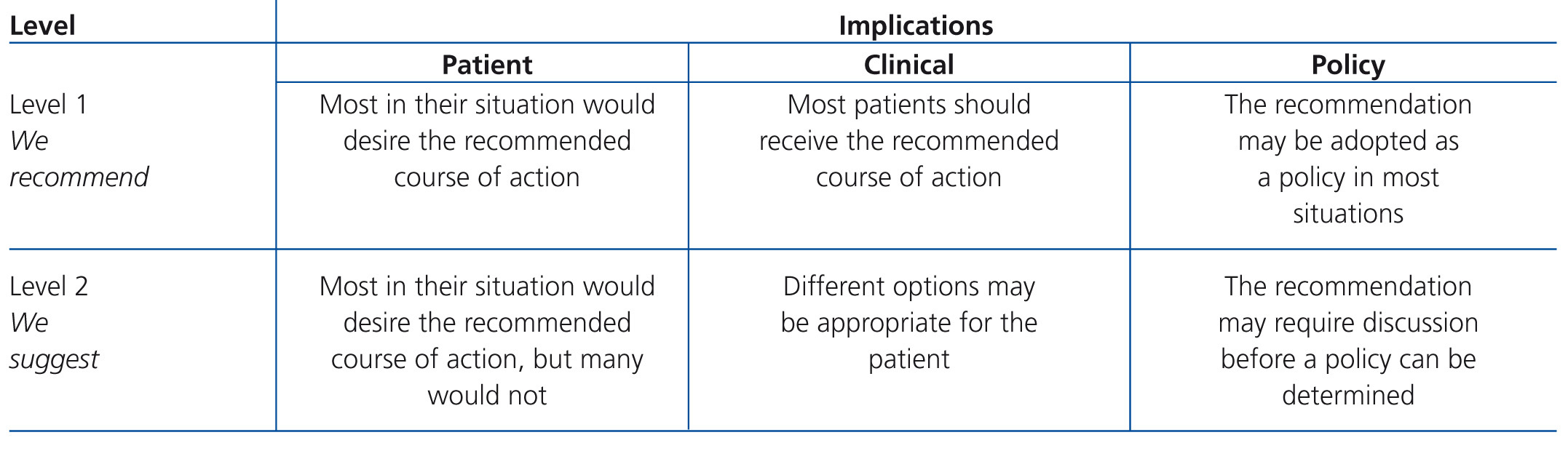
The issues in the guidelines are addressed in four chapters. Each chapter contains guidelines, followed by a rationale based on a number of references. The rationale is a summary of the literature on each set of guidelines in question, which is designed to justify the recommendation therein. The term evidence was used when the guidelines were based on evidence published in the literature, regardless of its grading. However, the term opinion was used, in accordance with the views contained within the guidelines consulted, often adapted to the personal experience of forum members. Table B and Table C display the criteria used to define the strength and quality of the evidence.1
It is important to highlight that, as with any clinical practice guidelines, sound judgement should be used in their consultation, and it should always be borne in mind that medical behaviour is individual and sovereign in each patient. This forum will be open for future revisions, and criticism and suggestions will always be welcome.
INTRODUCTION
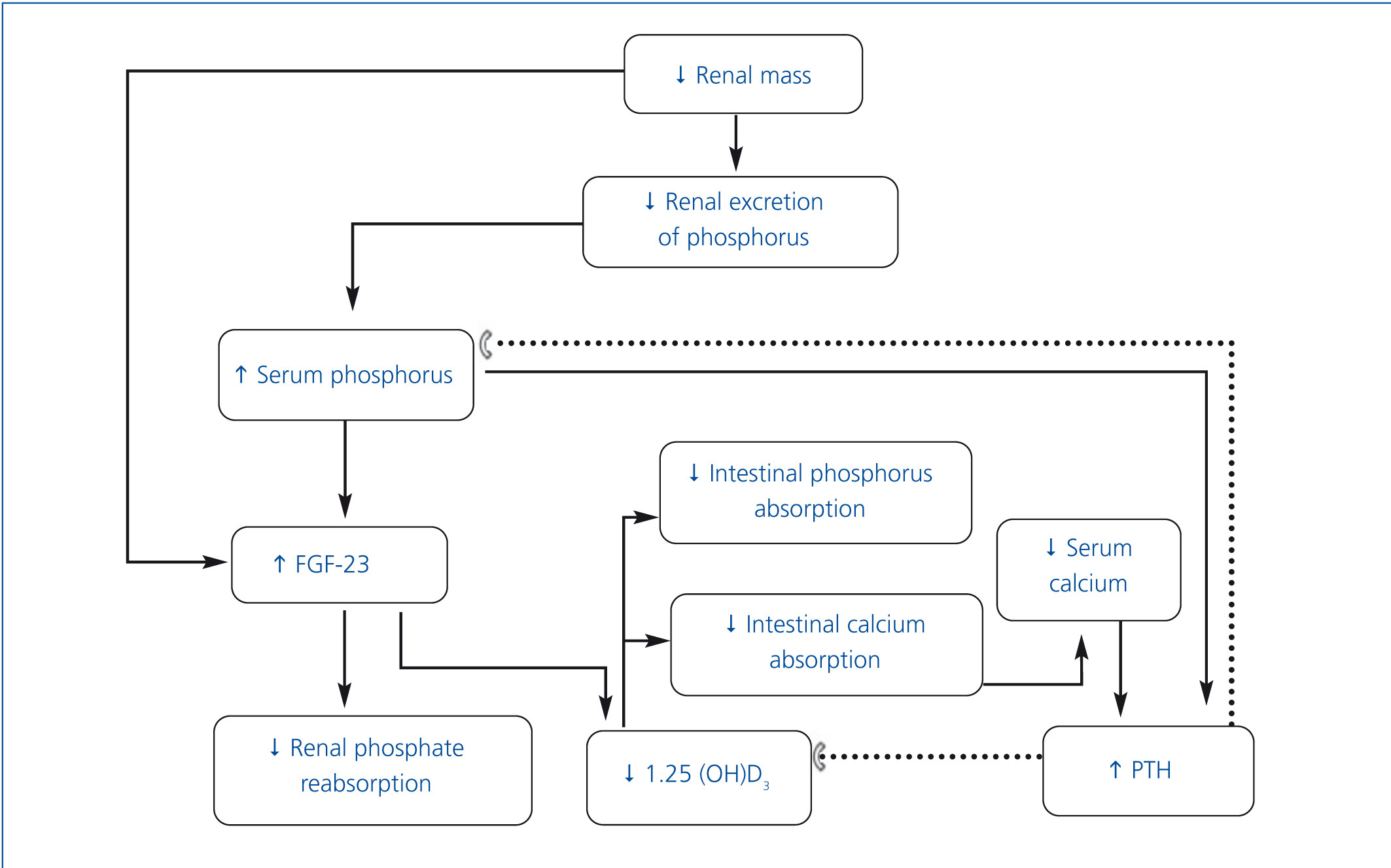
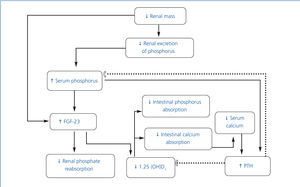
• Chronic kidney disease mineral and bone metabolism disorders (CKD-MBD) include: a) biochemical alterations (calcium, phosphorus, parathyroid hormone [PTH] and vitamin D); b) alterations in bone turnover, mineralization, volume, linear growth and strength and c) the presence of vascular and soft tissue calcifications. The term renal osteodystrophy should only be used to define the changes in bone histology that occur in patients with CKD.1 The pathophysiology of this complication is complex and is outlined in Figure 1.
• As CKD progresses, renal excretion of phosphorus decreases, which leads to its retention in the organism.2 However, under clinical conditions, the phosphorus load stimulates the production of fibroblast growth factor 23 (FGF-23) by osteocytes,3 which in turn inhibits Na/P cotransport at the proximal convoluted tubule, resulting in increased renal phosphorus excretion. FGF-23 inhibits 1-α-hydroxylase, which reduces the production of calcitriol by the kidney, and consequently increases the production of PTH.4,5 The resulting hyperparathyroidism increases renal phosphorus excretion.6 Although FGF-23 acts on the parathyroids, thus suppressing PTH production, the rise in the latter, despite high levels of FGF-23 in patients with uraemia, suggests resistance of parathyroids to the suppressive effect of PTH by FGF-23.7,8
• While there is a direct relationship between the concentration of serum phosphorus and increased FGF-23 production, some recent evidence indicates that this factor increases in early CKD stages, when there is still no hyperphosphataemia. In fact, hyperphosphataemia is uncommon with a glomerular filtration rate over 20ml/min. The mechanisms that increase FGF-23 in these stages are unclear. A recent study in children demonstrated the presence of FGF-23 in osteocytes in patients with stages 2-4 CKD.9 FGF-23 expression in osteocytes did not show any difference between patients with early-stage CKD and patients on dialysis. However, serum levels were lower in patients with CKD not on dialysis, probably as a result of renal clearance of FGF-23.
• In more advanced stages of CKD, serum calcitriol levels decrease further still, which leads to a decrease in calcium absorption, thus constituting an additional stimulus for PTH synthesis.6,10,11 The decrease in calcitriol is also the result of a reduction in the number of functioning nephrons and the direct effect of phosphorus overload in the proximal tubule.12 Additional mechanisms contributing to the excessive production of PTH include a reduction in the number of vitamin D receptors and the calciumsensing receptor in parathyroids and greater resistance to the effect of PTH on bone due to the decrease of its receptors.10,13
• Secondary hyperparathyroidism tends to maintain calcaemia by stimulating subperiosteal resorption, renal production of 1,25-dihydroxycholecalciferol and tubular calcium reabsorption.14 Although this compensation mechanism can normalise calcaemia and phosphataemia temporarily, it does so at the expense of inducing alterations in bone turnover.14
• Another potential complication is inadequate calcification of bones (osteomalacia) resulting from calcitriol deficiency.
• Bone biopsy makes it possible to distinguish between different types of renal osteodystrophy.15
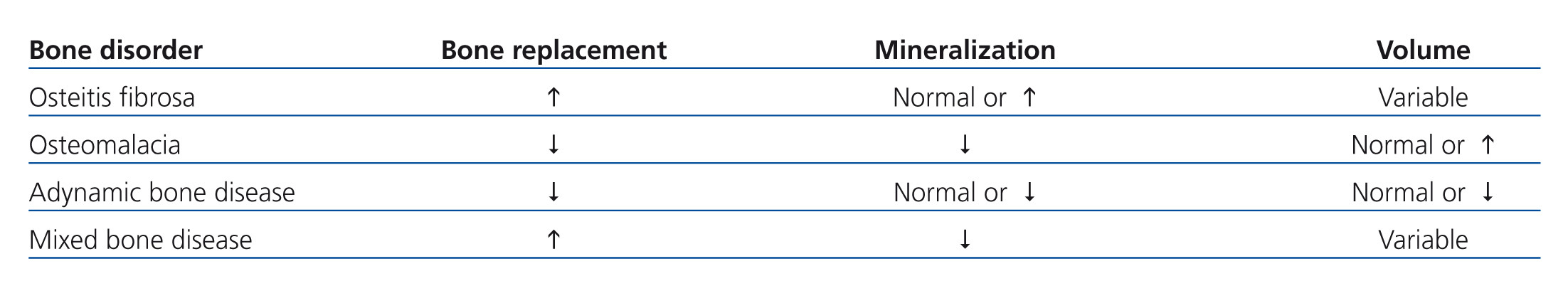
• The term renal osteodystrophy refers to the set of histological bone lesions resulting from mineral metabolism alterations in CKD which include: a) secondary hyperparathyroidism, b) osteomalacia, c) adynamic bone disease and d) mixed disease.16
• Although renal osteodystrophy may be present in most patients with advanced CKD, very few have symptoms before starting treatment with dialysis.1,17-19
• Renal osteodystrophy can be prevented or alleviated by proper control of calcium, phosphorus and PTH from early stages of CKD.20
• Secondary hyperparathyroidism is characterised by rapid bone turnover, a higher number and activity of osteoclasts and osteoblasts and increased bone resorption, resulting in the typical osteitis fibrosa cystica.15
• It may present clinically as bone pain, and x-rays may display subperiosteal resorption, which is most evident in the radial edge of the middle phalanges of the index and middle fingers, at the distal end of the clavicle and pubic symphysis. In severe cases cysts can be observed in long or flat bones, as well as sclerotic lesions in the upper and lower vertebrae. “Salt and pepper” lesions can also be observed in the skull due to a combination of areas of osteopenia and osteosclerosis, although they are not restricted to hyperparathyroidism.21,22
• Osteomalacia in CKD stages 3-5D is rare nowadays; it is characterised by low bone turnover, a decrease in the number of osteoblasts and osteoclasts, and increased osteoid volume due to a defect in the mineralisation.15,23 It may present clinically as bone pain and fractures. Although the origin of osteomalacia in CKD stages 3-5 is related to vitamin D deficiency, aluminum accumulation in bones may be involved when phosphorus binders containing this element are used.20,24
• Adynamic bone disease is another type of renal osteodystrophy of low bone turnover, but unlike osteomalacia, osteoid accumulation is not present.15 This bone alteration is one of the most common lesions in the earliest stages of CKD;25 it is also more prevalent in diabetics and the elderly. It may appear clinically as hypercalcaemia due to decreased calcium uptake in bone, low serum PTH and alkaline phosphatase concentrations, as well as an increased risk of fractures and vascular calcification.26
• Another component of CKD-MBD is vascular, non-vascular and extraosseous calcifications.27,28 Arterial calcification is usually detected radiologically and can affect any artery of the organism.29,30
• Cutaneous calcifications may present as small macules or firm papules. Calciphylaxis is a rare but particularly severe form, characterised by medial calcification of the arteries, distal ischaemia, progressive development of necrosis and ulcers on the skin of the toes and fingers, thighs, legs and ankles.31,32 This complication, rarely observed in early stages of CKD may appear in advanced CKD during replacement therapy; its pathogenesis is not fully understood.
CHAPTER 1. EVALUATION OF BIOCHEMICAL ALTERATIONS
Guidelines
1.1. It is recommended to measure serum levels of calcium, phosphorus, intact parathyroid hormone (iPTH) and alkaline phosphatase when the glomerular filtration rate (GFR) is <60ml/min (1C).(SLANH)
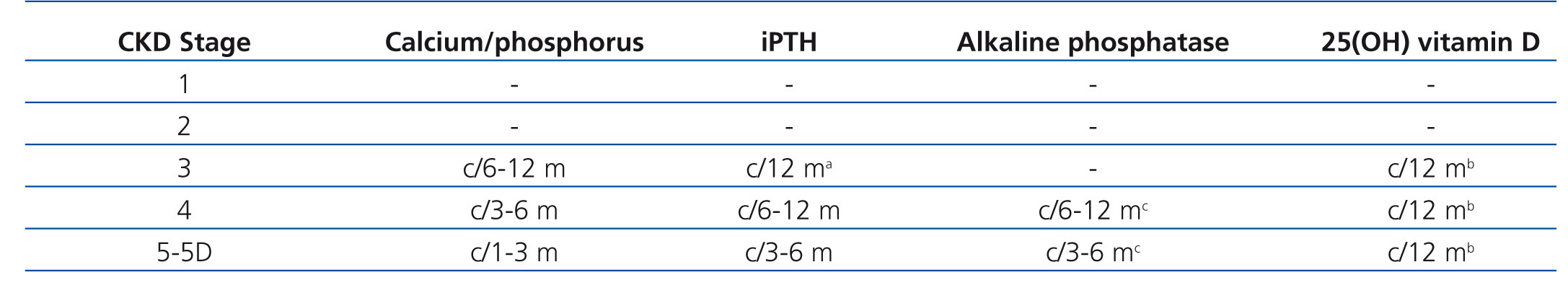
1.2. The frequency of calcium, phosphorus and iPTH measurement must be based on the presence of abnormalities in the rate of CKD progression (Table 1). In patients who are being treated for CKD-MBD and in those in whom biochemical alterations have been detected, it is reasonable to take measurements more frequently, in order to monitor trends and evaluate the effectiveness of treatment, as well as side effects (no grade).(SLANH)
1.3 In patients with CKD stages 3-5D, it is suggested to measure serum levels of 25-hydroxyvitamin D [25(OH)D] (calcidiol) and repeat in accordance with the baseline value and therapeutic intervention (2C). It is also suggested to treat vitamin D insufficiency and deficiency in accordance with the strategies recommended for the general population (2C).(KDIGO)
1.4 It is recommended that doctors be informed of the methodology and changes in techniques, sample type (plasma or serum) and processing used by laboratories in the biochemical determinations in stages 3-5D CKD-MBD, in order to obtain an adequate interpretation of the results (1B). (KDIGO)
Guideline 1
The detection of biochemical alterations in mineral metabolism is essential in the diagnosis of CKD-MBD. These alterations usually occur from CKD stage 3. For this reason, it is recommended to start the determination of serum calcium, phosphorus and iPTH levels from the aforementioned stage. However, the severity and rate of progression of mineral metabolism biochemical alterations is extremely variable, and as such, measurement frequency must be determined by the presence, duration and extent of the alterations encountered as well as the degree and progression of CKD and the use of medication to correct the anomalies. Thus, in a study of incident dialysis patients, serum levels of calcium and phosphorus increased during the first six months of renal replacement therapy.33
• Although there are numerous reports of cross-sectional studies on serum calcium, phosphorus and iPTH levels in the CKD stage 5D population, the DOPPS study (Dialysis Outcomes and Practice Patterns Study) provides the best overview of the prevalence of calcium (corrected by albumin), phosphorus and PTH disorders.34 Unfortunately, there is no global standardisation of PTH trials.
• Recently, high levels of total serum alkaline phosphatase have been recognised as a potential independent variable associated with an increased relative risk (RR) of mortality in patients with CKD stage 5D.35,36 Regidor et al.35 described a link between total serum alkaline phosphatase levels and mortality among haemodialysis (HD) prevalent populations, in addition to U or J curves for calcium, phosphorus and PTH, further emphasising the complexity of the relationship between these laboratory abnormalities and the results. However, there is no evidence that lowering these levels will lead to better results.
• Although the recommendations on the frequency of calcium, phosphorus and iPTH measurement are based on opinion, they constitute a general framework that is useful in clinical practice. There are no data that show that routine determination improves clinical results in patients. However, the reasonable frequency for laboratory measurements of these parameters in CKD-MBD may be suggested. Physicians should adjust the frequency of the former in accordance with the extent of the anomalies and the rate of CKD progression. The frequency of measurements must be individualised for patients being treated for CKD-MBD, with the aim of monitoring both the beneficial effects of treatment and the side effects.
• In patients with CKD stages 3-5D, it is recommended that treatment decisions be based on the trend and not on a single laboratory value and all available evaluations of CKD-MBD should be taken into consideration (1C).
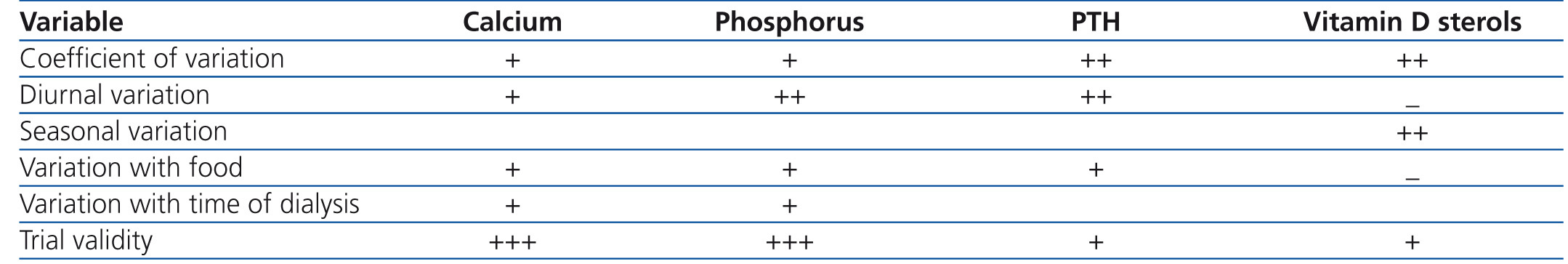
• The interpretation of biochemical and hormonal values in the diagnosis of CKD-MBD requires knowledge of the type of trial and its precision, inter-trial variability, blood sample management and the normal postprandial, diurnal and seasonal variations. It is because of these variations in the trial that we consider that the trends found in laboratory values and not individual values should be used preferentially, in order to determine when to start and/or adjust treatment. Table 2 describes the origin and extent of variation in the measurements of serum calcium, phosphorus, PTH and vitamin D sterols This table serves as a guide for doctors and it is the basis for the recommendation that laboratory tests should be measured using the same trials and similar times of the day or week for a given patient. Health care providers should become familiar with the problems and limitations of the trial. This variability highlights the importance of using trends instead of absolute single values when making decisions about diagnosis or treatment.
• Calcium: Serum calcium levels are routinely measured in clinical laboratories with automated equipment using colorimetric methods and quality control standards. As such, the trial is generally accurate and reproducible. In healthy individuals, serum calcium is rigidly controlled within a narrow range, generally 8.5-10.0 or 10.5mg/dl (2.1-2.5 or 2.6mmol/l), with some, although minimal, diurnal variations.37 However, the normal range may vary slightly between laboratories depending on the type of measurement used. In CKD patients, serum calcium levels fluctuate more due to change of homeostasis and concomitant therapies. In patients with CKD stage 5D, there are additional fluctuations associated with dialysis-induced changes, haemoconcentration and the subsequent haemodilution. There are also variations in accordance with the day on which the sample is taken; therefore, Mondays or Tuesdays that are the first day of dialysis of the week show higher calcium values (0.01mg/dl) than measures in Wednesday or Thursday sessions.34 Therefore, it is important to highlight that biochemical determinations in these patients should be carried out at midweek before dialysis (opinion).
• Serum calcium concentration is a poor reflection of total body calcium. The extracellular compartment contains only 1% of total body calcium, and the remainder is stored in bones. The serum ionised calcium, generally 4050% of the total serum calcium is physiologically active, whereas non-ionised calcium is bound to albumin or anions such as citrate, bicarbonate and phosphate, and is therefore physiologically inactive. In the presence of hypoalbuminaemia, there is an increase in ionised calcium in relation to total calcium; thus, total serum calcium may underestimate physiologically active serum (ionised) calcium. A formula for estimating ionised calcium in total serum calcium is commonly used, which consists of adding 0.8mg/dl (0.2mmol/l) for each gram of serum albumin decrease below 4g/dl (40g/l). This formula for the “corrected calcium” is routinely used in many centres and in many clinical trials. Unfortunately, some recent data show that this method is not better than total calcium and is less specific than ionised calcium measurements.38 Additionally, the method used for albumin could affect the measurement of corrected calcium. Unfortunately, ionised calcium measurements are not routinely available and, in some cases, may require additional cost for measurements.
• Phosphorus: Inorganic phosphorus is critical for many normal physiological functions including skeletal development, mineral metabolism, content and function of phospholipids in the cell membrane, cell communication, platelet aggregation and transfer of energy through the mitochondrial metabolism. Because of its importance, the body maintains serum concentrations between 2.5 and 4.5mg/dl (0.81-1.45mmol/l). The terms phosphorus and phosphate are often used interchangeably, but strictly speaking, the term phosphate means the sum of two inorganic ions that physiologically exist in serum and other body fluids: hydrogen phosphate and dihydrogen phosphate. However, most laboratories report this measurable inorganic component as phosphorus. Unlike with calcium, a significant component of phosphorus is intracellular, and factors such as pH and glucose may cause displacement of phosphate ions into and out of cells, and as a result they may alter serum concentrations without changing total body phosphorus.
• Phosphorus is routinely measured in clinical laboratories with automated equipment using colorimetric methods and quality control standards. As such, the trial is generally accurate and reproducible. Haemolysis during sample collection produces erroneously high levels of phosphorus. In healthy individuals, there is diurnal variation, both in serum levels and in the urinary excretion of phosphorus. Serum phosphorus reaches a low level in the early hours of the morning, increasing to a plateau at 4:00 p.m. and further increasing to a peak value between 1:00 a.m. and 3:00 a.m.39,40 However, a study in patients with CKD stage 5D found no diurnal phosphorus variation when studied on a day on which they did not receive dialysis.41 Generally, levels are higher after a long dialysis period. In the DOPPS study, HD patient samples that were collected immediately before the Monday or Tuesday sessions were higher than those of the Wednesday or Thursday sessions by 0.08mg/dl (0.025mmol/l). As such, the determination of phosphorus is generally valid and reproducible, but it could be affected by normal and postprandial diurnal variations. Again, the progressive increase or decrease trends may be more accurate than small variations in individual values.
• Alkaline phosphatase: Alkaline phosphatases are enzymes that digest phosphate of proteins and nucleotides and have optimum performance in alkaline pH. Measurement of the total alkaline phosphatase level is carried out by a colorimetric test that is routinely performed in clinical laboratories with automated equipment, using quality control standards. The enzyme is found in the body in the form of isoenzymes that are characteristic of the tissue of origin. The highest concentrations are found in the liver and bones, but it is also found in the intestines, placenta, kidneys and leukocytes. The specific alkaline phosphatase isoenzyme for identifying the tissue from which it originates can be determined by fractionation and inactivation by heat, but the availability of these procedures in clinical laboratories is scarce. Bone specific alkaline phosphatase is measured with an immunoradiometric assay; its high levels are generally due to abnormal liver function (in which case, other tests are abnormal), high bone activity or bone metastases. The levels are normally higher in children with growing bones than in adults.
• It is advisable that laboratories report the method used and any change to this, the type of sample analysed (plasma or serum) or the sample management requirements, since the correct interpretation of the results requires knowledge of the aforementioned variables as well as the normal fluctuations of the parameters evaluated. For example, determination of iPTH by various assays detect differently fragment 7-84 of the PTH, and as such, the measurement of this hormone by two different tests may even yield different results even in the same sample.42
• Vitamin D: The term vitamin D represents both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Ergocalciferol is synthesised in plants from ergosterol and together with fish oil cholecalciferol it is a dietary source of vitamin D for humans. However, 90% of vitamin D requirements come from the conversion of 7dehydrocholesterol to cholecalciferol through a reaction catalysed by sunlight in skin. Both vitamin D2 and vitamin D3 undergo hydroxylation in the liver to become 25(OH)D, and subsequently in the kidney to become 1,25(OH)2D3 or calcitriol, the most active form of vitamin D. Vitamin D calcitriol (hormone) plays an important role in mineral homeostasis and skeletal muscle function. Furthermore, modulatory effects of endothelial and immunological function and cell cycle regulation have been described, including pleiotropic effects.
• Patients with CKD have a decrease in the activity of renal 1-α-hydroxylase with the consequent reduction in serum calcitriol levels, which contributes to the hypocalcaemia that may accompany this disease. Calcitriol deficiency promotes the development of secondary hyperparathyroidism, since this hormone regulates the production and release of PTH through specific receptors.
• Most studies consider a serum concentration of 25(OH)D lower than 15ng/ml to be deficient and level between 15 and 30ng/ml to be insufficient. However, there is no consensus on the definition of “adequate” and “toxic” levels of vitamin D. Deficiency of 25(OH)D has been associated with increased risk of mortality in patients with CKD43,44 and is one of the factors involved in the pathogenesis of secondary hyperparathyroidism. However, it has not been proven that vitamin D, until achieving a certain serum concentration, decreases mortality, nor have its optimum figures been established. While the benefit of the correction of serum 25(OH)D concentrations has not been demonstrated, we consider that the measurement of vitamin D in patients with CKD stages 3-5 can be useful45,46 (Table 3).
CHAPTER 2. EVALUATION OF BONE ALTERATIONS
Guidelines
2.1. The most accurate diagnostic method for determining the type of bone disease associated with CKD (renal osteodystrophy) is bone biopsy with histomorphometric analysis (1A).(SLANH)
2.2. It is reasonable to perform a bone biopsy on patients with CKD stages 3-5D in certain situations, including, amongst others (Not graded)(SLANH)
- Fractures or bone pain with no apparent cause.
- Suspected bone disease associated with aluminium.
- Suspected osteomalacia.
- Unexplained hypercalcaemia or hypophosphataemia.
- Before beginning treatment with bisphosphonates.
- Before parathyroidectomy.
2.3. In patients with CKD stages 3-5D with evidence of CKD-MBD, we suggest that bone mineral density (BMD) testing not be performed routinely, because BMD does not predict fracture risk as it does in the general population, and BMD does not predict the type of renal osteodystrophy (2B).(KDIGO)
2.4. In patients with CKD stages 3–5D, we suggest that measurements of serum PTH or b-alkaline phosphatase can be used to evaluate bone disease because markedly high or low values predict underlying bone turnover (2B).(KDIGO)
2.5. In patients with CKD stages 3-5D, it is suggested to not routinely measure specific bone turnover markers based on the synthesis of collagen (such as the C-terminal propeptide of type I procollagen) and its catabolism (such as type I collagen telopeptide, pyridinoline and deoxypyridinoline) (2C).(KDIGO)
2.6. In patients with CKD stages 3-5D, the measurement of iPTH and alkaline phosphatase have not consistently demonstrated a correlation with the histological alterations of renal osteodystrophy (2B).(SLANH)
2.7. It is suggested to detect and eventually correct metabolic acidosis in order to prevent loss of bone and muscle mass in patients with CKD stages 3-5 (no grade)(SLANH).
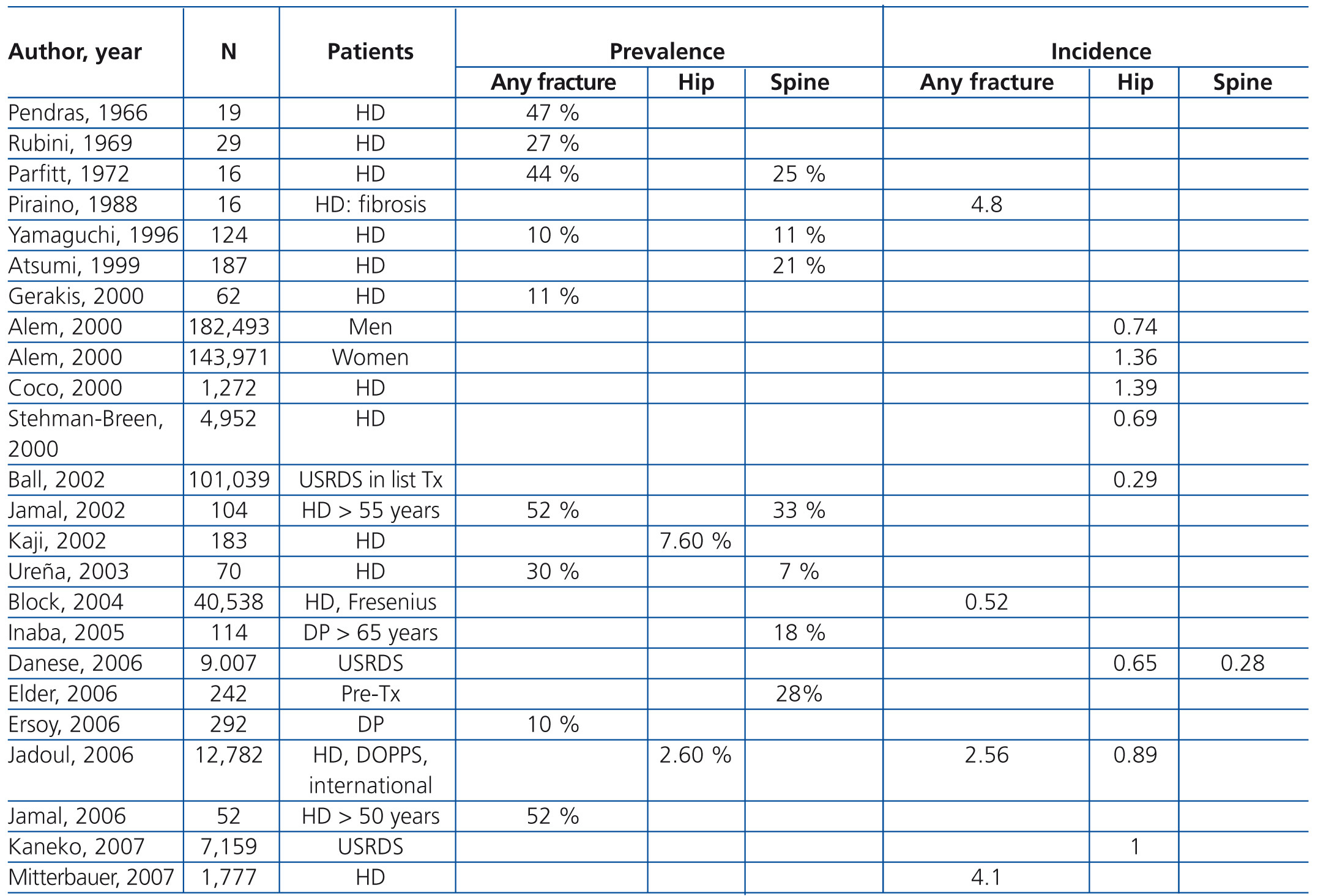
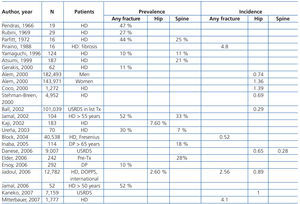
Guideline 2
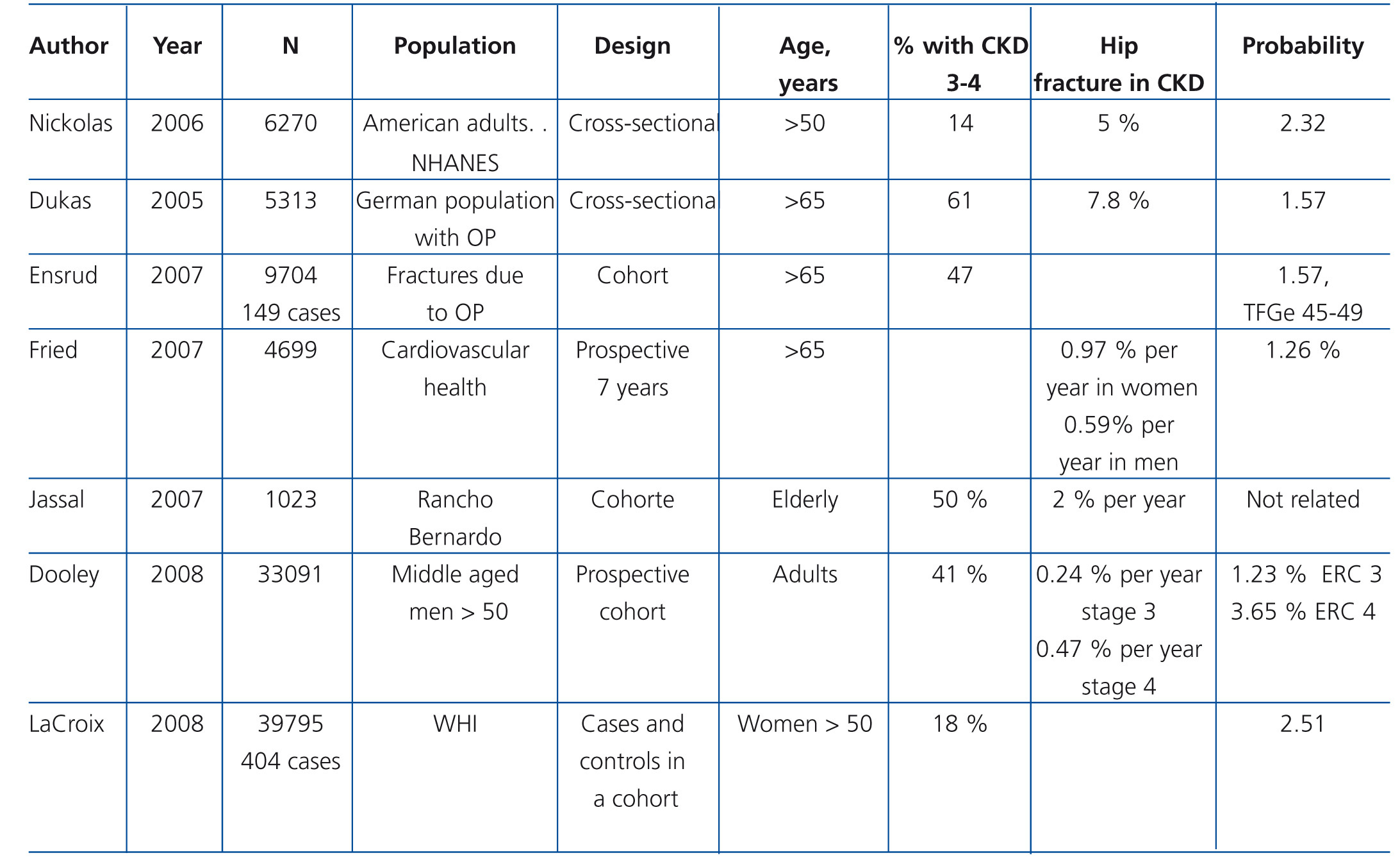
Patients with CKD stages 3-5D have a higher frequency and risk of fractures compared with the general population (Table 4 and Table 5) and are associated with increased morbidity and mortality.
• The main methods that have been used in the study of bone disease associated with CKD are bone biopsy and bone densitometry. Biochemical markers of bone formation and resorption are other potential indicators of the aforementioned alterations.
• Bone fragility may be secondary to alterations in mass and/or bone quality. The various types of renal osteodystrophy may be associated with any of these alterations.
• Histomorphometric analysis of bone biopsy is the gold standard for the diagnosis of renal osteodystrophy and as such it should be considered in patients with bone symptoms or biochemical abnormalities with no clear aetiology. It is also recommended for those with suspected aluminium-induced bone disease, osteomalacia and before initiating bisphosphonate therapy, since these drugs may aggravate the low turnover disease.
• Bone biopsy allows evaluation of bone quality and the type of predominant change, in accordance with the pathophysiology (osteitis fibrosa, osteomalacia, adynamic bone disease and mixed disease). The parameters that allow this classification are: bone turnover, mineralization and volume. However, the different types of renal osteodystrophy bear little relation to the clinical repercussions, with the risk of fractures and arterial calcification.47,48 Volume is another of the parameters evaluated in the bone biopsy, and although it does not form part of the traditional classification of renal osteodystrophy, it has been determined that it is a variable that independently influences bone fragility. Therefore, bone volume has been included in the new TMV (turnover, mineralization and volume) classification parameters suggested by KDIGO.
• The most accurate way to determine the rate of bone formation, and therefore turnover, is double tetracycline labelling, which also allows calculation of the osteoid mineralization time (the second parameter used for classifying the different types of renal osteodystrophy) (Table 6). In Latin America, there are few laboratories capable of performing histomorphometric studies of bone biopsies. Today, these are limited to Brazil (Sao Paulo State University and the Federal University of Sao Paulo) and Venezuela (Caracas University Hospital).
• The ability of BMD to predict fracture risk or the type of renal osteodystrophy in patients with CKD stage 4-5D is weak and inconsistent.49-52 Therefore, it is not suggested to carry out this study routinely on patients with evidence of CKD-MBD.
• PTH plays an essential role in the pathophysiology of CKD-MBD due to its effect on phosphorus regulation and bone remodelling. Total alkaline phosphatase is an indicator that reflects osteoblastic activity if there is no liver alteration. Abnormal serum levels of these two indicators are related, albeit weakly, to the degree of bone turnover, fracture risk and other clinical events, including mortalilty.53-55 Although bone biopsy remains the gold standard for the diagnosis of the type of renal osteodystrophy, it is not readily accessible for most patients; the determination of bone or total alkaline phosphatase iPTH can be used to estimate bone turnover.50,56-58
• Other markers of bone turnover based on collagen synthesis (C-terminal propeptide of type 1 procollagen) or in its catabolism (such as type 1 collagen telopeptide, pyridinoline or deoxypyridinoline) have not been extensively evaluated in patients with CKD stages 3-5 . Existing studies indicate that these and other biochemical markers provide no more information than PTH or total alkaline phosphatase for predicting biopsy findings of bone or clinical events. Therefore its use is currently not recommended in the evaluation of CKD-MB.
CHAPTER 3. EVALUATION OF VASCULAR CALCIFICATIONS
Guidelines
3.1. In patients with CKD stages 3-5D it is suggested that a lateral abdominal x-ray be performed to detect the presence of vascular calcifications. Echocardiography may also be used to detect valvular calcifications. These diagnostic methods may be reasonable alternatives to other more complex methods that may not be available, such as computed tomography (CT) or electron-beam computed tomography (EBCT) (2C).(KDIGO) It is reasonable to use this information to decide on CKD-MBD treatment (Not graded).(SLANH)
3.2. It is suggested that patients with CKD stages 3-5D with vascular or valvular calcifications be considered at high cardiovascular risk (2A).(KDIGO)
Guideline 3
• Cardiovascular calcifications may occur in the intima or the tunica media of the arteries, and in heart valves, with various consequences including: ischaemic heart disease, stroke, valvular dysfunction, left ventricular hypertrophy and dysfunction. In the general population, the extent of coronary calcification, determined by multi-slice CT or EBCT, is highly predictive of the risk of cardiovascular events.59,60 In patients with CKD, generalised cardiovascular calcification is much more prevalent, more severe and follows a more rapid course compared with the general population.61,62
• The gold standard for the detection of cardiovascular calcification in both the general population and in patients with CKD is the coronary calcification index based on CT. However, other more easily accessible studies, such as the lateral abdominal x-ray, pulse wave velocity measurement and echocardiography may provide comparable information.63-65 The presence and severity of calcifications strongly predicts morbidity and mortality of cardiovascular aetiology in subjects with CKD. Evidence from randomised clinical trials on the impact of interventions in reducing the progression of vascular calcifications on mortality is still limited. Because of all of this, although screening for the random detection of cardiovascular calcification in all patients with CKD cannot currently be recommended, it could be justified in patients with significant hyperphosphataemia, those receiving calcium-based phosphorus binders in high doses, patients on the waiting list for a kidney transplant or in other cases on the doctor’s judgment.
• Lastly, calciphylaxis is a less common but very severe calcification of the tunica media of the small arteries (skin) and it is also known as uraemic arteriopathy. This complication is strongly associated (in approximately a third of the cases) with mineral metabolism alterations related with CKD, including, in a third of cases, secondary hyperparathyroidism. It is characterised by very painful ischaemic ulceration of the skin, followed by superinfection. This disorder is associated with high mortality. Calcification inhibitors (fetuin-A and bone matrix Gla protein) have been involved in the pathogenesis of calciphylaxis, but due to the relatively low incidence of the disease, no conclusive data are available that allow us to comment on the nature of the process and treatment options.
• It has been suggested that statin therapy has a beneficial impact on the atherogenic profile, atheroma progression and cardiovascular events in patients without CKD.66-68 In CKD patients, no data are available on the effects of statins on arterial calcification compared with those of placebo. Furthermore, study 4D69 (Die Dutch Diabetes Dialysis Study) with atorvastatin and the AURORA study70 (A Study to Evaluate the Use of Rosuvastatin in Subjects On Regular Haemodialysis) with rosuvastatin failed to demonstrate the benefit of treatment with the aforementioned drugs in the primary endpoint of death due to cardiovascular causes, non-fatal myocardial infarction or stroke, both in diabetic and non-diabetic patients undergoing chronic haemodialysis. Recently, a study with an ezetimibe and simvastatin combination showed a reduction in LDL cholesterol and the incidence of severe atherosclerotic events in patients with advanced CKD, followed-up for an average of 4.9 years.71 However, the Food and Drug Administration of the United States (FDA)72 did not approve a new indication of these two drugs for advanced kidney disease, since the study was not designed to determine the effect of the two drugs separately.
CHAPTER 4. TREATMENT OF CKD-MBD
Guidelines
General recommendations
4.1. It is recommended that treatment decisions be based on trends rather than individual laboratory results, and all available information on the alterations in bone and mineral metabolism associated with CKD be considered together (1C).(KDIGO)
4.2. It is recommended that CKD-MBD treatment decisions be based on serum calcium and phosphorus concentrations considered individually, instead of the calcium-phosphorus product (Ca x P) (2D).(KDIGO)
4.3. In patients with CKD stages 3-5D, it is recommended that serum calcium concentrations (ionised or total corrected) remain within the normal range for the method used (2C).(KDIGO)
4.4. In patients with CKD stages 3-5, we suggest maintaining serum phosphorus within the normal range (2C). In patients with CKD stage 5D it is suggested a reduction of high phosphorus levels to the normal range (2C).(KDIGO)
4.5. In patients with CKD stage 5D, it is suggested to use a calcium concentration in the haemodialysis fluid between
1.25 and 1.50mmol/l (2.5 and 3.0mEq/l) (2D).(KDIGO)
4.6. In patients with CKD stages 3-5 (2D) and 5D (2B) it is suggested the use of phosphate binders to treat hyperphosphataemia. It is reasonable that the choice of phosphate binders takes into account the stage of CKD, the presence of other CKD-MBD components, concomitant therapies and side effect profile (Not graded).(KDIGO)
4.7. In patients with CKD stages 3-5D and hyperphosphataemia, it is recommended to restrict the dose of calcium-based phosphate binders and/or the dose of calcitriol or vitamin D analogues in the presence of persistent or recurrent hypercalcaemia (1B).(KDIGO)
4.8. In patients with CKD stages 3-5D and hyperphosphataemia, it is suggested to restrict the dose of calcium-based phosphate binders in the presence of arterial calcification (2C) and/or adynamic bone disease (2C) and/or if serum iPTH levels are persistently low (2C).(KDIGO)
4.9. In patients with CKD stage 5D, it is suggested increasing dialytic phosphate removal in the treatment of persistent hyperphosphataemia (2C).(KDIGO)
Treatment aimed at controlling serum phosphorus and calcium concentrations
4.10. Calcium-based phosphate binders are effective at lowering serum phosphorus concentrations. However,
their use may be associated with hypercalcaemia and an increased incidence of vascular calcification (2C).(SLANH)
4.11. It is recommended to restrict the dose of calcium-based phosphate binders up to a maximum of 1500mg of elemental calcium/day (1B).(SLANH) It is also recommended to restrict or prevent the use of calcium-based phosphorus binders in the presence of hypercalcaemia, vascular calcifications, adynamic bone disease or persistently low iPTH levels
(2C).(KDIGO)
4.12. Phosphorus binders containing aluminum should not be used (1C).(SLANH)
4.13. In CKD stages 3-5 patients with normal or high serum phosphorus, we suggest not exceeding dietary phosphorus intake of 800-1,000mg/day, in isolation or in combination with other treatments (2D).(SLANH)
Treatment of abnormal serum iPTH concentrations
4.14. The optimum level of iPTH in patients with CKD stages 3-5 is unknown. Nevertheless, we suggest that patients with iPTH levels above normal for the assay used be evaluated to determine the presence of hyperphosphataemia, hypocalcaemia, and low values (deficiency) of 25(OH)D (2C).(KDIGO) If present, it is reasonable to correct these abnormalities with a low phosphorus diet, phosphate binders, vitamin D and/or calcium supplements (Not graded).(SLANH)
4.15. In patients with CKD stages 3-5D showing a progressive and sustained increase of iPTH levels above the upper limit of the assay used, despite the correction of above-mentioned factors, we suggest starting treatment with calcitriol or vitamin D analogues (2C).(KDIGO)
4.16. In patients with CKD stage 5D, we suggest maintaining iPTH levels at an approximate range between 2 and 9 times the normal upper limit of the assay (Not graded).(KDIGO)
4.17. In patients with stage 5D CKD who show significant alterations in iPTH levels in both directions (upper and lower) within the suggested range (2 to 9 times the normal upper limit of the trial), it is suggested to begin or change treatment in order to prevent the progression of the alterations to levels outside this range (2C).(KDIGO)
4.18. In patients with stage 5D CKD who show a rise or a progressive increase in serum iPTH, we suggest initiating the use of calcitriol, vitamin D analogues, calcimimetics or the combination of these two groups of agents with the aim of reducing the iPTH levels (2B).(KDIGO)
- It is reasonable that the initial drug selection for the control of high iPTH is based on serum calcium and phosphorus concentrations, as well as other aspects of CKD-MBD (no grade).(KDIGO)
- It is reasonable that the doses of calcium and non-calcium phosphorus binders are adjusted in a way that they do not compromise normal serum concentrations of calcium and phosphorus (no grade).(KDIGO)
- It is recommended that, in patients with hypercalcaemia (1B)(KDIGO) or hyperphosphataemia (2D) (KDIGO), the use of calcitriol and/or vitamin D analogues be reduced or omitted.
- It is suggested that in patients with hypocalcaemia, calcimimetics use is reduced or omitted, depending on its severity, concomitant medication and the presence of clinical symptoms and signs (2D).(KDIGO)
- It is suggested that if iPTH levels decrease below twice the normal upper limit of the trial, the use of calcitriol, vitamin D analogues and/or calcimimetics be reduced or omitted (2C).(KDIGO)
4.19. In patients with stage 3-5D CKD and severe hyperparathyroidism unresponsive to medical/pharmacological treatment, we suggest considering parathyroidectomy (2B).(KDIGO)
Guidelines 4
General recommendations
• The measurement of biochemical and hormonal parameters that guide the management of CKD-MBD is subject to variations depending on the type of assay and management and time of sampling (circadian, postprandial, seasonal or post-dialysis fluctuation). Consequently, it is recommended to base treatment decisions on trends rather than on single values of the aforementioned determinations, which should ideally be performed with the same laboratory method and at the same time of day for a certain patient.73
As regards the Ca x P product, it is currently considered to have limited usefulness in clinical practice, since its value is determined primarily by serum phosphorus and it usually does not provide additional information to that provided by the individual values of its two components. Furthermore, there are many situations in which a normal Ca x P product is associated with adverse clinical events and viceversa.74,75
The use of total serum calcium has certain limitations from a clinical point of view, particularly in patients with decreased serum albumin concentration. At physiological pH, albumin binds to approximately 45% of the total calcium. Therefore, variations in albumin concentration alter the total serum calcium concentration even when the ionised calcium remains steady. For these reasons, some formulas have been developed to correct serum calcium values in accordance with albumin levels in order to make an estimate of the concentration of the aforementioned element in patients with hypoalbuminaemia. However, alterations in laboratory techniques for measuring albumin with bromocresol purple (now in use in many laboratories) have questioned the usefulness of making total serum calcium corrected for albumin concentration.76 Moreover, recent evidence indicates that, depending on the agents used, there may be variations as great as 30% to 40% in the corrected calcium value.77 Moreover, serum calcium concentrations are subject to significant variations related to the technique used in the sample. Therefore, for example, using a peripheral vein, the tourniquet and the practice of facilitating the venous filling by intermittently clenching the fist may change calcium values by up to 10%. In addition to this is the effect of the type of anticoagulant used for the sample preservation.77 Serum calcium concentrations are also subject to significant variations related to the technique used in the sample. For example, using a peripheral vein, the tourniquet and the practice of facilitating the venous filling by intermittently clenching the fist may change calcium values by up to 10%. In addition to this is the effect of the type of anticoagulant used for the sample preservation.77 Consequently, many authors suggest abandoning the practice of adjusting calcium values for serum albumin, since, except under special conditions, it may lead to erroneous and potentially dangerous decisions regarding the patient's medication. For these reasons, we consider it reasonable not to use the formulas available for serum calcium corrected for albumin, but insist on improving conditions for determination of total calcium and, whenever possible, use ionised calcium determinations.
Treatment aimed at controlling phosphorus and calcium levels
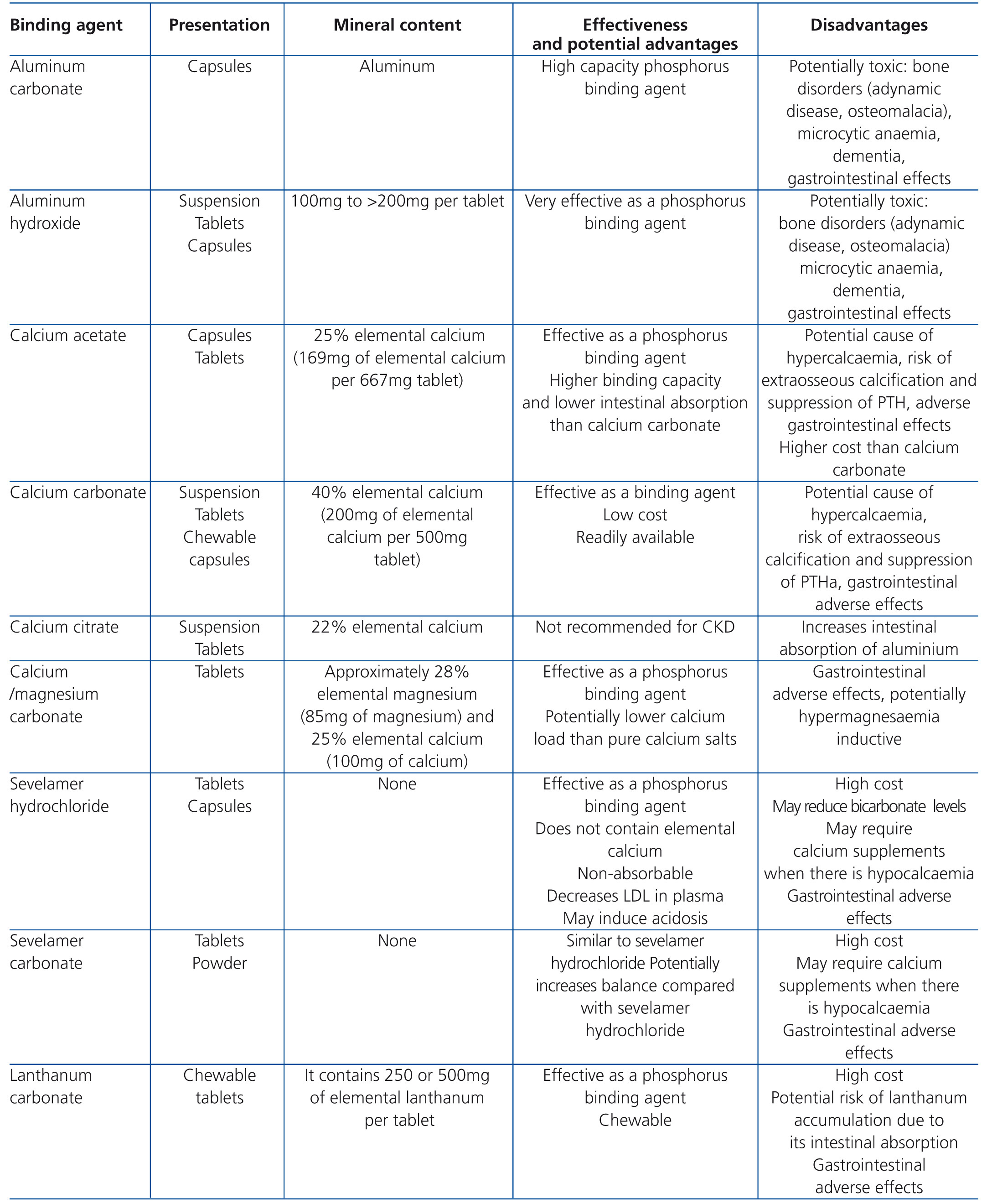
• Despite the fact that phosphorus overload is significant in advanced stages of CKD, few patients with CKD stages 3-5 have high concentrations of phosphorus in the blood in early stages of the disease. Phosphorus overload has been associated with the development and progression of secondary hyperparathyroidism, reduced serum calcitriol levels, abnormal bone remodelling and calcification of soft tissues and arteries. Furthermore, in patients with CKD stage 3-4, serum phosphorus levels at the upper limit of normal have been associated with an increased risk of mortality.78,79 Although there are no randomised clinical trials that demonstrate the impact of reduced phosphorus overload in these stages, maintaining serum concentrations within normal values is suggested. Suggested measures for this control include restriction of dietary phosphorus (ensuring adequate protein intake) and possibly the use of phosphorus binders. The choice of the binder should be individualised and it is suggested to take into account the adverse effect profile of each binder. Table 7 summarises the essential information of the main phosphorus binders currently in use.
• In multiple studies carried out in different parts of the world, a link between a rise in serum phosphorus and an increase in the RR of death has been demonstrated. In most of these studies, the links found were consistent with and dependent on the dose, with progressive increases in risk being observed on increasing serum phosphorus levels. The inflexion point, or range in which the phosphorus concentration is significantly associated with higher mortality from all causes, varies between different studies due to the abovementioned reasons, 5.0-5.5mg/dl (1.6-1.8mmol/l), >5.5mg/dl (>1.8mmol/l) 6.07.0mg/dl (1.9-2.3mmol/l) and >6.5mg/dl (>2.1mmol/l).34.80-82 The DOPPS study showed that the relationship between the rise in serum phosphorus and the RR of mortality is consistent across all countries analysed.34 The Noordzij et al.80 study also found similar relationships in patients on (PD) and HD. These observational data are consistent with data from experimental studies and those on animals, making this link biologically plausible. Based on these observations, it is recommended to carry out interventions that reduce the level of phosphorus to the normal range.
• Hypophosphataemia can also cause bone metabolism complications because it causes a mineralisation disorder that in extreme cases can bring about osteomalacia deficiency and at a systemic level, it is generally associated with malnutrition. In the DOPPS series there was an evident increase in the risk of mortality in patients with CKD stage 5D with phosphorus levels below 2.0mg/dl (0.65mmol/l), although fewer than 5% of patients fall into this risk category.
In short, even when no studies have been carried out on the benefits of lowering serum phosphorus levels in clinical outcomes at patient level (e.g., hospitalisation, bone fractures, cardiovascular events, and mortality), numerous epidemiological data show a positive link, although not a causal relationship, between high levels of serum phosphorus and an increased RR of mortality, regardless of the stage of CKD. There are experimental data which show a direct effect of phosphorus on PTH secretion in parathyroid cell proliferation and vascular calcification.83-85 Although there are no randomised and controlled prospective studies that show the benefits in the results at any level of phosphate control in patients with CKD stages 3-5D, it is recommended to lower the high serum phosphorus levels to the normal range in an effort to control CKD-MBD complications. Treatment should always be individualised.
Calcium balance during HD is important in determining the short-term cardiovascular function, as the latter influences the haemodynamic tolerability of dialysis. In the long term, calcium flow during HD is an important determinant of total calcium balance. The dialysate calcium concentration should be adjusted to optimise total load body calcium.86 Theoretically, this strategy should help improve bone health by determining a negative calcium balance in patients with adynamic bone disease and calcification, and inducing a positive flow of calcium during dialysis in patients with hypocalcaemia. However, these possibilities have not been tested prospectively. The dialyzable percentage of body calcium is small and studies evaluating calcium balance are limited. The total amount of calcium eliminated with each dialysis treatment will depend not only on its concentration but also on the level of serum ionised calcium, the interdialytic interval and the ultrafiltration rate.87 Studies that have measured the net flow of calcium during haemodialysis have shown that the latter is almost nil in patients dialysed with a calcium concentration of 1.25mmol/l (2.5mEq/l) in the dialysate.88,89 A more recent study used more frequent evaluations of drained dialysate and an average flow of calcium in each dialysis session of -187±232mg (-46±58mmol) was found when using a calcium concentration of 1.25mmol/l (2.5mEq/l). However, 6 of the 52 patients had a positive calcium balance, supporting the fact that the flow of calcium in haemodialysis is not uniform in all patients.90 As a result, it is considered that, in general, the dialysate with a calcium concentration of 1.25mmol/l (2.5mEq/l) may allow an almost neutral calcium balance for most patients. However, it is important to highlight that a low concentration of calcium in the dialysate may also result in a predisposition to cardiac arrhythmias and haemodynamic instability during dialysis sessions.91,92 Maintaining flexibility in dialysate calcium concentrations is suggested, and they should be individualised as much as possible in accordance with the patient’s characteristics.
• Similar considerations apply to PD, in which the calcium concentration of the dialysate must be adjusted, as far as possible, to the individual needs of each patient. In comparison with patients receiving HD, those receiving PD are exposed to a calcium concentration in the dialysate for longer periods. Therefore, calcium concentrations in the peritoneal dialysis fluid greater than 3.5mEq/l (1.75mmol/l) should be avoided in order to prevent calcium overload and induced adynamic bone disease. Concentrations of between 2.5 and 3.0mEq/l (1.25 and 1.50mmol/l) are recommended.
• In a systematic review of controlled randomised studies examining phosphate binders,93 it was demonstrated that all medications currently used as phosphate binders (calcium salts, aluminum salts, sevelamer and lanthanum carbonate) are effective in reducing concentrations of serum phosphorus.
• In three randomised clinical trials, one in patients with CKD stages 3-5 and two in patients with CKD stage 5D,94-96 it was found that sevelamer may weaken the progression of arterial calcification in comparison with calcium-based binders. However, this effect was not observed in more recent clinical trials comparing sevelamer with calcium acetate.97,98 It should be noted that differences in the study design may cause some of the disparities observed between them. Even so, and given the high cardiovascular risk of these patients, it is suggested to limit the dose of calcium-containing binders in subjects with evidence of vascular calcification and in those with persistently low PTH or adynamic bone disease, as this seems to encourage the progression of vascular calcification.
• The use of lanthanum carbonate and sevelamer HCl97,98 did not adversely affect bone histology in short-term studies and when compared with calcium-based binders, it may be less likely that they lead to adynamic bone disease. Comparative studies with different phosphate binders have shown differences in the effects on biochemical parameters of CKD-MBD. For example, use of calcium salts is generally associated with higher levels of serum calcium (and more episodes of hypercalcaemia) and lower serum PTH levels than when sevelamer HCl or lanthanum carbonate is used. In summary, there are insufficient effective comparative data on clinical results that allow recommendation of the use of a specific binder in all patients. However, there are consistent data with respect to the risk of inducing hypercalcaemia and calcium overload using calcium-containing binders, and as such, in this case a reduction in dose would be required. Moreover, available data on bone biopsies suggest that patients receiving binders that contain calcium are more susceptible to develop adynamic bone disease.
• The evidence of the possible role of calcium-based binders compared with those not containing calcium in the pathogenesis of vascular calcification is currently inconclusive, so further research is required to clarify this issue. The Clinical Dialysis Outcomes Revisited DCOR99 study was conducted in 2,103 patients with CKD stage 5D, randomised to receive sevelamer HCl or calcium binders (70% with calcium acetate and 30% with calcium carbonate), followed up for approximately 20 months. Only 1,068 patients completed the study and there were no differences in mortality from all causes or specific mortality when comparing the patients treated with sevelamer hydrochloride with calcium binders. No differences in cardiovascular mortality and hospitalisation were observed, although sevelamer was associated with improved survival in patients over 65 years of age.99 The study has been questioned, however, for its high rate of discontinuation of treatment: the total percentage of patients withdrawn from the study was 47% in the sevelamer HCl branch and 51% in the calcium binder branch. A second analysis of the DCOR100 study, using Medicare claims data, showed no effect on total mortality, cause-specific mortality, morbidity, first hospitalisation or causespecific hospitalisation. However, this study showed a beneficial effect of sevelamer-HCl on the secondary outcomes of all cause multiple hospitalisations and days in the hospital.100 However, the information of both analyses has not been considered of sufficient quality to catalogue it as conclusive.
• A second study96 randomised 148 incident patients (new patients) on haemodialysis who received sevelamer HCl or calcium binders and were followed up for an average period of 44 months. Only 127 of these patients had an EBCT and patients withdrawn from the study reached 26% in the group treated with sevelamer HCl and 27% in the group with calcium binders. The univariate analysis found a marginally higher unadjusted mortality rate in patients assigned to binders containing calcium, compared to patients treated with sevelamer HCl. However, in the multivariate analysis, the difference between groups was significant, suggesting an imbalance of covariates that were part of the model, increasing the possibility of an unsuccessful randomisation. As a result, the methodological quality of this study was considered to be moderate. Previous studies did not provide data on cardiovascular events, apart from death rates, fractures and parathyroidectomy, making it impossible to draw conclusions in this regard. Moreover, no studies have examined the effects of lanthanum carbonate or any other phosphate binder (including compounds containing calcium or aluminium) in the results at the patient level. Given the findings of the studies analysed, we believe it is justified to limit consumption of calcium in the form of phosphate binders, until further information is available in patients with CKD.
Treatment of abnormal iPTH levels
Treatment options
• Therapeutic forms of vitamin D sterols, available for use in patients with CKD in our region include: ergocalciferol, cholecalciferol and calcitriol. There are also active vitamin D analogues, synthetic derivatives of vitamin D2 (paricalcitol and doxercalciferol) and vitamin D3 (alfacalcidol). Doxercalciferol and alfacalcidol require 25hydroxylation in the liver to become active analogues.
Vitamin D and its analogues have been used in patients with CKD stages 3-5D in order to improve abnormalities in mineral homeostasis and reduce the development or progression of secondary hyperparathyroidism. The use of ergocalciferol and cholecalciferol has received little attention to present since it was considered that the levels of 25(OH)D were not very important in patients with CKD, given their limited conversion to calcitriol by renal 1-α-hydroxylase. However, the prevalence of deficient (<15ng/ml) or insufficient (15-30ng/ml) levels of 25(OH)D is high in patients in all stages of CKD.101-103 Alternatively, some recent studies suggest a significant local hydroxylation of vitamin D in various tissues (due to the presence of 1-α-hydroxylase), independently of the kidney. Calcimimetics (cinacalcet is the only one currently available in some of our countries) are allosteric modulators of the calcium receptor in parathyroids that cause a decrease in the synthesis and release of PTH.104 However, its use is not sufficiently documented in predialysis stages.
Treatment recommendations
• Hyperparathyroidism accompanying CKD is an adaptive response to alterations in mineral metabolism associated with decreased glomerular filtration. This response allows temporary maintenance of a state of homeostasis. However, when the response of the parathyroids is excessive or inappropriate, it has adverse clinical consequences. Currently, it is unclear how to accurately differentiate a compensatory increase in PTH levels from an excessive response, and there are no clinical trials that evaluate the benefits and risks of suppressing moderately elevated levels of this hormone in patients with moderate or severe CKD who have not yet started dialysis. Therefore, there are no data that establish an optimum level of PTH in these patients.
• Although the evidence is limited, on the basis of the pathophysiology, it is reasonable to suggest the search and correction of modifiable factors that may contribute to secondary hyperparathyroidism (hypocalcaemia, hyperphosphataemia and vitamin D deficiency) in patients with PTH levels above the upper normal limit for the assay used. Some studies in patients with CKD stages 3-5 suggest that cholecalciferol and calcium supplementation may reduce PTH concentrations.103
• For patients with CKD stages 3-5D and secondary hyperparathyroidism, calcitriol and vitamin D analogues have proven useful for reducing blood levels of PTH and improving bone histology compared with placebo.105-108 Therefore, its use is suggested in subjects who continue to have levels above the upper limit of the method used, in spite of the abovementioned modifiable correction factors.
• Both calcitriol and vitamin D analogues may increase serum calcium and phosphorus levels in patients with CKD,109 and as such, it is appropriate to suspend its use if hypercalcaemia or hyperphosphataemia do not respond to phosphorus binders.
• In experimental studies comparing the use of calcitriol and various synthetic vitamin D analogues, differences were found in the degree of suppression of PTH, bone histology and incidence of hypercalcaemia, hyperphosphataemia and vascular calcifications.110-113 However, there is no evidence in favour of any of these studies.114
Vitamin D, vitamin D analogues and calcimimetics
• Vitamin D: The therapeutic forms of vitamin D sterols available for use in patients with CKD in our region include: ergocalciferol, cholecalciferol and calcitriol. There are also active vitamin D analogues, synthetic derivatives of vitamin D2 (paricalcitol and doxercalciferol) and vitamin D3 (alfacalcidol). Doxercalciferol and alfacalcidol require 25-hydroxylation in the liver to become active analogues, while paricalcitol is considered a selective vitamin D receptor activator (sVDRA) and does not require prior metabolism for its action.
• Vitamin D and its analogues have been used in patients with stage 3-5D CKD in order to improve abnormalities in mineral homeostasis and reduce the development or progression of secondary hyperparathyroidism. As was mentioned previously, the prevalence of deficient or insufficient levels of 25(OH)D is high in patients in all stages of CKD.101,115,116
A series of studies shows that in patients with CKD stage 5D, high iPTH levels can be effectively suppressed by calcitriol, compared to placebo.50 However, the development of hypercalcaemia is relatively frequent in patients treated with calcitriol. This problem can be limited with the use of vitamin D analogues. Support for the use of vitamin D analogues (paricalcitol and doxercalciferol) is based on experimental studies that show a dose-equivalent suppression of PTH similar to or higher than calcitriol but with a lower calcaemic response and/or phosphataemic activity.37,117 Paricalcitol is a synthetic analogue of vitamin D with modifications in the side chain (D2) and the A (19-nor) ring that confer greater selectivity by interacting with sVDRA. Paricalcitol suppresses the synthesis and secretion of PTH, but it has been shown to have lower calcaemic and phosphataemic response than calcitriol and therefore has been used with increasing frequency for the control of CKD secondary hyperparathyroidism.37 Doxercalciferol is a synthetic analogue of vitamin D that requires a metabolic activation process by CYP27 in the liver to form 1-α-25(OH)2D2, major metabolite, and 1-α-24(OH)2D2, minor metabolite. Doxercalciferol activation does not require activation by the kidney. 1-α-25(OH)2D2 is capable of suppressing PTH synthesis and secretion by activation of the vitamin D receptor (VDR), with a lower calcaemic and phosphataemic response than calcitriol, the natural compound. For these reasons, it is used to control secondary hyperparathyroidism.38 To present, there has been no general consensus on the initial doses of paricalcitol and doxercalciferol. As regards to paricalcitol, different dose schemes have been used which include calculation in accordance with the patient’s dry weight and formulas based on the initial value of iPTH. Thus, for example, some studies have used the formulas based on the iPTH initial value ratio divided between 80 and up to 120 in order to limit the possible side effects in the development of hypercalcaemia or hyperphosphataemia, or an excessive suppression of iPTH levels.118
• Calcimimetics: Calcimimetics act as allosteric modulators of the calcium receptor, increasing sensitivity of the calcium receptor in the parathyroid cell to extracellular calcium.119 Calcimimetics administration decreases the synthesis and secretion of PTH, it reduces proliferation of the parathyroid gland cells and modulates the regulation of genes involved in calcium receptor and VDR overexpression.39 However, additional studies are required on the impact of calcimimetics use on morbidity and mortality in patients with CKD. Cinacalcet is the only calcimimetic available for clinical use and it does not increase calcium and phosphorus absorption in the intestine. This characteristic distinguishes it from vitamin D sterols, since it may lower PTH levels without increasing concentrations of calcium and phosphorus. Accordingly, the compound may be used in patients with secondary hyperparathyroidism with hypercalcaemia.40
The calcimimetics controlled studies are relatively rare and most published works refer to dialysis patients. A randomised, double-blind, placebo-controlled study evaluated the effect of treatment with cinacalcet in CKD patients not on dialysis.120 However, more studies in populations that are not on dialysis are required before its use in CKD stages 3-5 is suggested. There is only one randomised controlled trial that compares histomorphometric results of one year of treatment with cinacalcet with the standard treatment in patients with CKD stage 5D, using repeated bone biopsies at zero and 12 months.121 In biopsies of the placebo group, 45% showed improved turnover and 23% showed a higher turnover (worsened). None of the patients showed osteomalacia, and the change in the mineralisation lag time (MLT) was the same in the placebo group as in the cinacalcet group. Some of the biopsies showed abnormally high MLT, but details were not provided. The bone volume increased slightly but not significantly in the cinacalcet group and there was no change in the placebo group. In summary, this study did not show significant differences with respect to bone histomorphometry, but was limited due to a small number of patients. The adverse side effects more frequently reported with the use of cinacalcet were nausea and vomiting.122-124 In patients treated with cinacalcet, nausea consistently occurred one and half times more often than usual and vomiting, twice as often. In Lindberg’s study,123 approximately one quarter of patients had some serious adverse events, both in the treatment group and in the placebo group, which may or may not be related to the treatment. In both Block’s study122 (15%) and that of Lindberg123 (9%), approximately twice as many patients suspended treatment in the cinacalcet group due to side effects, mainly vomiting, nausea and other gastrointestinal events. In both studies, 5% of patients treated with cinacalcet and less than 1% of patients in the control groups had serum calcium values below 7.5mg/dl (1.9mmol/l). Hypocalcaemic episodes were transient and rarely associated with symptoms. In another study of effectiveness and safety, lasting from 26 to 52 weeks,125 it was considered that cinacalcet treatment was safe and effective. The adverse side effects (mainly nausea and vomiting) led to discontinuation of therapy in 10% of patients treated with cinacalcet and in none of the control groups, while 3% of the latter had to undergo parathyroidectomy, which did not occur with any of those treated with cinacalcet. After 12 months, no difference was observed between the groups when vitamin D (cinacalcet 64% vs. placebo 63%) or phosphate binders (92% vs. 96%, respectively) were used and there was no difference in the elemental calcium ingested with meals.
• In patients with CKD stages 3-5D with severe hyperparathyroidism not responding to medical/pharmacological treatment, we suggest parathyroidectomy (2B).
There are no studies that evaluate the results of mortality, cardiovascular disease, hospitalisation, fractures, bone disease progression, biochemical parameters or quality of life following parathyroidectomy. However, when parathyroidectomy is performed by an experienced surgeon, it results in a marked and sustained reduction in serum iPTH, calcium and phosphorus levels. A subtotal or total parathyroidectomy with autotransplantation effectively reduces high levels of iPTH, calcium, phosphorus and total alkaline phosphatase. There is no evidence that a total parathyroidectomy with immediate reimplantation of ectopic parathyroid tissue yields better results than the 7/8 subtotal parathyroidectomy, and as such, the latter is suggested. We do not advise total parathyroidectomy without immediate implantation of the parathyroid tissue due to the risk of developing hypoparathyroidism which is very difficult to manage. Most of the patients who underwent parathyroidectomy showed an improvement in biochemical parameters, but it is difficult to evaluate the comparisons between medical and surgical therapies in relation to morbidity and mortality results. In the absence of controlled studies, the observational studies available that compare surgically or medically treated patients are exposed to a selection bias that limits the validity of the findings. Individuals who are candidates for parathyroidectomy differ from those chosen for studies with cinacalcet. The study with the largest sample is that of Kestenbaum et al.126 which showed lower mortality in the long term in patients who were treated with parathyroidectomy compared with a cohort not treated surgically; however, this was a retrospective observational study, where the short-term postoperative mortality was high (3.1%), and the best result obtained in the long term after parathyroidectomy could be due to selection bias, as in the study of Trombetti et al.127 in which patients who had parathyroidectomies were younger and had fewer comorbidities. Due to the lack of randomised controlled studies that allow proper comparison between surgical treatment of secondary hyperparathyroidism, these management strategies are difficult to compare. In patients ineligible for surgery or who are awaiting surgery, medical therapies may be considered, including treatment with cinacalcet. In patients eligible for surgery, parathyroidectomy is considered when hyperparathyroidism is severe and resistant to control by drugs, usually after trial treatment with calcitriol, a vitamin D analogue or cinacalcet.
Parathyroidectomy may also be considered when the medical control to reduce iPTH levels results in an unacceptable increase in serum calcium and/or phosphorus levels (as often occurs when using calcitriol or vitamin D analogues) or when there is no tolerance due to adverse effects. It can be difficult to determine what constitutes “resistant hyperparathyroidism”. It is clear that, the higher the PTH, the less likely it is that the gland regresses in response to medical treatment.124 In short, for secondary hyperparathyroidism in patients with CKD stages 3-5D , management should begin with medical treatment (control of calcium, phosphorus, vitamin D, vitamin D analogues and/or calcimimetics), and only due to the failure of medical treatment or the presence of calciphylaxis, is parathyroidectomy indicated.
Conflicts of interest
In preparing this work, the Latin American Society of Nephrology and Hypertension (SLANH) has taken special care to avoid any influence or interference from industry or private groups in order to prevent potential situations that could be perceived as institutional, personal or commercial conflicts of interest that may occur as a result of SLANH’s normal relationships as a scientific society.
Individually, all members of the Mineral and Bone Metabolism Committee of SLANH who participated in the preparation of these guidelines have made a declaration of conflicts of interest which presents the relationships that could be perceived as conflicts of interest, as is described below.
Dr. Ezequiel Bellorin-Font: Lecturer/consultant: Sanofi, Abbott. Lecturer: Amgen.
Dr. Pablo Ambrosoni: Has no conflicts of interest to declare.
Dr. Raúl G. Carlini: Lecturer/consultant: Laboratories Abbott, Sanofi-Genzyne. Lecturer: Novartis, Laboratory Merck Sharp & Dohme.
Dr. Aluizio B. Carvalho: Advisor/consultant: Sanofi; Laboratories Abbott, Amgen. Lecturer: Abbott, Amgem, Lilly.
Dr. Ricardo Correa-Rotter: Lecturer: Laboratories Abbott, Sanofi-Genzyme, Amgen, Novartis. Member of the executive committee of the Evolve research study, sponsored by Amgen.
Dr. Alfonso Cueto-Manzano: Has no conflicts of interest to declare.
Dr. Aquiles Jara: Advisory: Laboratorios Abbott, Diaverum.
Dra. Vanda Jorgetti: Advisor/consultant: Sanofi-Abbott; Amgem. Lecturer: Sanofi-Genzyme, Laboratorios Abbott, Amgem.
Dr. Armando Negri: Lecturer: Laboratorios Abbott, Sanofi-Genzyme.
Dra. Inés Olaizola: Has no conflicts of interest to declare.
Dr. Isidro Salusky: Research funding: Laboratories Abbott, Sanofi-Genzyme, Amgen. Consultant: Kureha America Inc. and Cytochroma.
Dr. Eduardo Slatopolsky: Lecturer/research funding: Laboratories Abbott, Genzyme-Sanofi.
Dr. José R. Weisinger: Lecturer/advisor: Sanofi-Genzyme, Amgen.
SUMMARY OF RECOMMENDATIONS
1. EVALUATION OF BIOCHEMICAL ALTERATIONS
1.1. It is recommended to measure serum levels of calcium, phosphorus, intact parathyroid hormone (iPTH) and alkaline phosphatase when the glomerular filtration rate (GFR) is <60ml/min (1C).(SLANH)
1.2. The frequency of calcium, phosphorus and iPTH measurement must be based on the presence of abnormalities in the rate of CKD progression (Table 1). In patients who are being treated for CKD-MBD and in those in whom biochemical alterations have been detected, it is reasonable to take measurements more frequently, in order to monitor trends and evaluate the effectiveness of treatment, as well as side effects (no grade).(SLANH)
1.3. In patients with CKD stages 3-5D, it is suggested to measure serum levels of 25-hydroxyvitamin D [25(OH)D] (calcidiol) and repeat in accordance with the baseline value and therapeutic intervention (2C). It is also suggested to treat vitamin D insufficiency and deficiency in accordance with the strategies recommended for the general population (2C).(KDIGO)
1.4. It is recommended that doctors be informed of the methodology and changes in techniques, sample type (plasma or serum) and processing used by laboratories in the biochemical determinations in stages 3-5D CKD-MBD, in order to obtain an adequate interpretation of the results (1B). (KDIGO)
2. EVALUATION OF BONE ALTERATIONS
2.1. The most accurate diagnostic method for determining the type of bone disease associated with CKD (renal osteodystrophy) is bone biopsy with histomorphometric analysis (1A).(SLANH)
2.2. It is reasonable to perform a bone biopsy on patients with CKD stages 3-5D in certain situations, including, amongst others (no grade)(SLANH):
- Fractures or bone pain with no apparent cause.
- Suspected aluminum-associated bone disease.
- Suspected osteomalacia.
- Unexplained hypercalcaemia or hypophosphataemia.
- Before beginning treatment with bisphosphonates.
- Before parathyroidectomy.
2.3. In patients with CKD stages 3-5D with evidence of CKD-MBD, we suggest that bone mineral density (BMD) testing not be performed routinely, because BMD does not predict fracture risk as it does in the general population, and BMD does not predict the type of renal osteodystrophy (2B).(KDIGO)
2.4. In patients with CKD stages 3-5D, the use of serum PTH or bone-specific alkaline phosphatase measurements is suggested to evaluate bone disease, since markedly high or low values predict underlying bone turnover (2B).(KDIGO)
2.5. In patients with CKD stages 3-5D, it is suggested to not routinely measure specific bone turnover markers based on the synthesis of collagen (such as the C-terminal propeptide of type I procollagen) and its catabolism (such as type I collagen telopeptide, pyridinoline and deoxypyridinoline) (2C).(KDIGO)
2.6. In patients with CKD stages 3-5D, the measurement of iPTH and alkaline phosphatase have not consistently demonstrated a correlation with the histological alterations of renal osteodystrophy (2B).(SLANH)
2.7. It is suggested to detect and eventually correct metabolic acidosis in order to prevent loss of bone and muscle mass in patients with CKD stages 3-5 (Not graded)(SLANH).
3. EVALUATION OF VASCULAR CALCIFICATIONS
3.1. In patients with CKD stages 3-5D it is suggested that a lateral abdominal x-ray be performed to detect the presence of vascular calcifications. Echocardiography may also be used to detect valvular calcifications. These diagnostic methods may be reasonable alternatives to other more complex methods that may not be available, such as computed tomography (CT) or electron-beam computed tomography (EBCT) (2C).(KDIGO) It is reasonable to use this information to decide on CKD-MBD treatment (Not graded).(SLANH)
3.2. It is suggested that patients with CKD stages 3-5D with vascular or valvular calcifications be considered at high cardiovascular risk (2A).(KDIGO)
4. TREATMENT OF CKD-MBD
General recommendations
4.1. It is recommended that treatment decisions be based on trends rather than individual laboratory results, and all available information on the alterations in bone and mineral metabolism associated with CKD be considered together (1C).(KDIGO)
4.2. It is recommended that CKD-MBD treatment decisions be based on serum calcium and phosphorus concentrations considered individually, instead of the calcium-phosphorus product (Ca x P) (2D).(KDIGO)
4.3. In patients with CKD stages 3-5D, it is recommended that serum calcium concentrations (ionised or total corrected) remain within the normal range for the method used (2C).(KDIGO)
4.4. In patients with CKD stages 3-5, we suggest maintaining serum phosphorus within the normal range (2C). In patients with CKD stage 5D, it is suggested a reduction of high phosphorus levels to the normal range (2C).(KDIGO)
4.5. In patients with CKD stage 5D, it is suggested to use a calcium concentration in the haemodialysis fluid between 1.25 and 1.50mmol/l (2.5 and 3.0mEq/l) (2D).(KDIGO)
4.6. In patients with stages 3-5 CKD (2D) and 5D (2B) it is suggested the use of phosphate binders to treat hyperphosphataemia. It is reasonable that the choice of phosphate binders takes into account the stage of CKD, the presence of other CKD-MBD components, concomitant therapies and side effect profile (Not graded).(KDIGO)
4.7. In patients with CKD stages 3-5D and hyperphosphataemia, it is recommended to restrict the dose of calcium-based phosphate binders and/or the dose of calcitriol or vitamin D analogues in the presence of persistent or recurrent hypercalcaemia (1B).(KDIGO)
4.8. In patients with CKD stages 3-5D and hyperphosphataemia, it is suggested to restrict the dose of calcium-based phosphate binders in the presence of arterial calcification (2C) and/or dynamic bone disease (2C) and/or if serum iPTH levels are persistently low (2C).(KDIGO)
4.9. In patients with CKD stage 5D, it is suggested to increasing dialytic phosphate removal in the treatment of persistent hyperphosphataemia (2C).(KDIGO)
Treatment aimed at controlling serum phosphorus and calcium concentrations
4.10. Calcium-based phosphate binders are effective at lowering serum phosphorus concentrations. However, their use may be associated with hypercalcaemia and an increased incidence of vascular calcification (2C).(SLANH)
4.11. It is recommended to restrict the the dose of calcium-based phosphate binders up to a maximum of 1500mg of elemental calcium/day (1B).(SLANH) It is also recommended to restrict or prevent the use of calcium-based phosphorus binders in the presence of hypercalcaemia, vascular calcifications, adynamic bone disease or persistently low iPTH levels (2C).(KDIGO)
4.12. Phosphorus binders containing aluminum should not be used (1C).(SLANH)
4.13. In CKD stages 3-5 patients with normal or high serum phosphorus, we suggest not exceeding dietary phosphorus intake of 800-1,000mg/day, in isolation or in combination with other treatments (2D).(SLANH)
Treatment of abnormal serum iPTH concentrations
4.14. The optimum level of iPTH in patients with CKD stages 3-5 is unknown. Nevertheless, we suggest that patients with iPTH levels above normal for the assay used be evaluated to determine the presence of hyperphosphataemia, hypocalcaemia, and low values (deficiency) of 25(OH)D (2C).(KDIGO) If present, it is reasonable to correct these abnormalities with a low phosphorus diet, phosphate binders, vitamin D and/or calcium supplements (Not graded).(SLANH)
4.15. In patients with CKD stages 3-5D showing a progressive and sustained increase of iPTH levels above the upper limit of the assay used, despite the correction of above-mentioned factors, we suggest starting treatment with calcitriol or vitamin D analogues (2C).(KDIGO)
4.16. In patients with CKD stage 5D, we suggest maintaining iPTH levels at an approximate range of between 2 and 9 times the normal upper limit of the assay (Not graded).(KDIGO)
4.17. In patients with CKD stage 5D who show significant alterations in iPTH levels in both directions (upper and lower) within the suggested range (2 to 9 times the normal upper limit of the assay), it is suggested to begin or change treatment in order to prevent the progression of the alterations to levels outside this range (2C).(KDIGO)
4.18. In patients with CKD stage 5D who show a rise or a progressive increase in serum iPTH, we suggest initiating the use of calcitriol, vitamin D analogues, calcimimetics or the combination of these two groups of agents with the aim of reducing the iPTH levels (2B).(KDIGO)
- It is reasonable that the initial drug selection for the control of high iPTH is based on serum calcium and phosphorus concentrations, as well as other aspects of CKD-MBD (Not graded).(KDIGO)
- It is reasonable that the doses of calcium and non-calcium phosphorus binders are adjusted in a way that they do not compromise normal serum concentrations of calcium and phosphorus (Not graded).(KDIGO)
- It is recommended that, in patients with hypercalcaemia (1B)(KDIGO) or hyperphosphataemia (2D)(KDIGO), the use of calcitriol and/or vitamin D analogues be reduced or omitted.
- It is suggested that in patients with hypocalcaemia, calcimimetics use is reduced or omitted, depending on its severity, concomitant medication and the presence of clinical symptoms and signs (2D).(KDIGO)
- It is suggested that if iPTH levels decrease below twice the normal upper limit of the trial, the use of calcitriol, vitamin D analogues and/or calcimimetics be reduced or omitted (2C).(KDIGO)
4.19. In patients with CKD stages 3-5D and severe hyperparathyroidism unresponsive to medical/pharmacological treatment, we suggest considering parathyroidectomy (2B).(KDIGO)
Table 1. Suggested frequency for measuring calcium, phosphorus and the intact parathyroid hormone
Table 2. Origin and extent of the variation in the measurement of serum calcium, phosphorus, intact parathyroid hormone and vitamin D sterols
Table 3. Vitamin D levels in serum and suggested treatment doses
Table 4. Fractures in patients with stage 3-4 chronic kidney disease
Table 5. Fractures in patients with stage 5D chronic kidney disease
Table 6. Characteristics of the bone biopsy in the different types of renal osteodystrophy.
Table 7. Comparison of phosphorus binders currently available
Table A. Chronic kidney disease stages
Table B. Strength of evidence
Table C. Quality of the evidence
Figure 1. Mechanisms of the changes in the metabolism of calcium, phosphorus and parathyroid hormone in chronic kidney disease