Clinical guideline of adequacy and prescription of the dialysis peritoneal
More infoIn recent years, the meaning of adequacy in peritoneal dialysis has changed. We have witnessed a transition from an exclusive achievement of specific objectives –namely solute clearances and ultrafiltration– to a more holistic approach more focused to on the quality of life of these patients. The purpose of this document is to provide recommendations, updated and oriented to social and health environment, for the adequacy and prescription of peritoneal dialysis. The document has been divided into three main sections: adequacy, residual kidney function and prescription of continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Recently, a guide on the same topic has been published by a Committee of Experts of the International Society of Peritoneal Dialysis (ISPD 2020). In consideration of the contributions of the group of experts and the quasi-simultaneity of the two projects, references are made to this guide in the relevant sections. We have used a systematic methodology (GRADE), which specifies the level of evidence and the strength of the proposed suggestions and recommendations, facilitating future updates of the document.
En los últimos años se ha modificado el significado de adecuación en diálisis peritoneal. Hemos asistido a una transición desde un concepto de adecuación enfocado esencialmente a la obtención de unos objetivos en determinados parámetros –aclaramiento de solutos y ultrafiltración– a una aproximación más holística centrada en la calidad de vida del paciente. El propósito de este documento es proporcionar a sus destinatarios recomendaciones, actualizadas y centradas en nuestro entorno sociosanitario, para la adecuación y prescripción de la diálisis peritoneal. El documento se ha estructurado en tres grandes apartados: adecuación, función renal residual y prescripción en diálisis peritoneal continua ambulatoria y diálisis peritoneal automática. Recientemente se ha publicado una guía sobre el mismo tema, elaborada por un Comité de Expertos de la Sociedad Internacional de Diálisis Peritoneal (ISPD) (2020). En consideración a las aportaciones del grupo de expertos de la ISPD y a la cuasi simultaneidad de los dos proyectos se hacen referencias a esta guía en los apartados pertinentes. Se ha seguido una metodología sistemática (GRADE), que especifica el nivel de evidencia y la fuerza de las sugerencias y recomendaciones propuestas, y facilita actualizaciones futuras de la guía.
In general, the peritoneal dialysis (PD) working group of the Spanish Society of Nephrology (S.E.N., for its acronym in Spanish) accepts the considerations and recommendations of the recent guidelines issued by the International Society of Peritoneal Dialysis (ISPD)1 regarding PD prescription. However, we consider that renewal of the current PD guides of the S.E.N. is advisable, adapting them to our setting and the current scenario. The main reasons for this revision are:
The wish on the part of the S.E.N to establish a proprietary body of recommendations for the greatest possible number of scenarios in Nephrology. The time elapsed since the introduction of the PD guides in 2005, which were developed on a narrative basis with a non systematic review of the literature that is inconsistent with the current methods for addressing issues of this kind.
This chapter of the PD guide differs in content from the adequacy and prescription of PD in the previous S.E.N.2 guide. This guide dealt with the characteristics that influence prescription, technique-related prescribing factors, PD solutions, and dialysis dose adequacy. The current guideline does not specifically address PD solutions, although some aspects are addressed in the sections on preservation of residual kidney function (RKF) and prescription. This guide also does not address in depth issues related to the peritoneal membrane, these will be the subject of another document.
Overall, we will first analyse the most common parameters for monitoring adequacy, including some methodological aspects, their current significance and the objectives to be achieved. Due to its relevance in PD, we will dedicate a specific section to the preservation of RKF. In a third section, the prescription of Continuous Ambulatory Peritoneal Dialysis (CAPD) will be discussed, focusing especially on the initial phase and the prescription of Automated Peritoneal Dialysis (APD), including the factors that influence it and the results (ultrafiltration, sodium balance, solute clearance) that are obtained with the different prescription modalities. Finally, we will compare both modalities (CAPD and APD) at baseline and during follow-up.
ObjectiveThe aim of this guide is to offer recommendations that are up-to-date and focused on our social and healthcare setting, for the adequacy and prescription of PD.
Target populationThis guide is mainly addressed to:
- none-
Members of the S.E.N in general
- none-
Spanish, Latin American and Spanish-speaking nephrologists in general.
- none-
Residents in training in Nephrology.
- none-
PD nursing staff.
Its training objectives also include:
- none-
Physicians of other specialties with an interest in the field of adequacy and prescription of home treatments in general and PD in particular.
- none-
Professionals, organisations, and people with an interest in the subject, including people with kidney disease and their associations.
The PD Adequacy and Prescribing Guide has been structured in three steps.
- •
The four panellists - named at the beginning of the document- were in charge of the drafting with the fundamental collaboration of Dr Miguel Pérez Fontán as scientific coordinator and Dr Carlos Quereda, on behalf of the Methodological Working Group of the S.E.N.
- •
In a second step, the chapter was submitted to the scrutiny of the PD Clinical Practice Guideline Expert Group.
- •
In a third step, the document was submitted to external scrutiny by a group of professionals, including nephrologists and PD nursing staff, also obtaining the opinion of the main association of people with kidney disease (Alcer).
The methodology used in developing this document was based on the general principles of the GRADE system, summarized in the corresponding document-guide of the S.E.N., and reproduced in other S.E.N. documents of the same nature.3–5
A particular aspect of the Adequacy and Prescription Guide has been the publication, during the writing phase, of a document on the same topic by a Committee of ISPD Experts.1
The methodology and approach of this document are quite different from those of the S.E.N. guide presented here. In consideration of the contributions made by the ISPD panel of experts and the quasi-simultaneity of the two projects, frequent references will be made to this guide in all relevant sections.
In summary, the following steps were followed:
- 1
Development and review of clinical questions. These questions focused on the aspects of adequacy (parameters, methodology, and clinical outcomes), RKF (clinical outcomes and preservation measures), and prescription (CAPD and APD in their different variables).
- 2
Bibliographic search strategy. Once the experts had defined the questions interest on which to subsequently base the recommendations, the following was done:
- a
A systematic literature search on the subject was carried out. In the adequacy section (questions 1–6), the search was carried up until January 2019. In the residual kidney function section (questions 7–11), the systematic search extends until February 2019 and in the prescription section (questions 12–23) until November 2019.
- b
Before the release of the chapter, thorough (non-systematic) searches were made of publications up until December 2020, of potential interest for the guideline.
- c
Tables corresponding to the PICO question3–5for each clinical question were developed in order to define the criteria of the literature strategy, based on the following aspects:
- none-
P = Population studies (adult patients with stage 5d chronic kidney disease treated with PD).
- none-
I = intervention studied.
- none-
C = parameter of comparison.
- none-
O = outcome variables, classified according to clinical relevance (patient survival, mortality rates, technique survival technique, quality of life, hospitalization rates, etc.).
- none-
- a
Adequate methodological designs (systematic reviews-meta-analyses, randomized and controlled clinical trials, prospective or retrospective cohort observational studies, case-control studies) were contemplated in the search strategy. The literature search was carried out by two documentalists in collaboration with the medical committee, designing a search strategy in accordance with the explicit indications of the PICO question, and exploring MEDLINE (PubMed), Cochrane Library, and EMBASE bibliographic bases. The entire process was supervised by experts methodologists in systematic reviews and clinical practice guidelines (InMusc).
- 3
Selection of articles. The identified articles were analyzed independently by two reviewers in order to select those studies that met the clinical criteria referred to inclusion/exclusion, interventions and comparator groups, outcome variables, study methods and methodological quality. Differences between the reviewers were resolved by consensus in those articles in which agreement was initially lacking.
- 4
Estimation of the quality of the evidence. A structured summary was made of the results of the relevant studies addressing each clinical question. For each outcome variable we evaluated the quality of the evidence according to the standard criteria defined by the GRADE system,21 which rates the quality of the evidence as HIGH (A), MODERATE (B), LOW (C) or VERY LOW (D).
Due consideration was made of the following factors capable of modifying confidence in the results: risk of bias, consistency between the results of the available studies, the availability of direct evidence, and precision of the estimators of effect. In the case of observational studies, we took the following into account: size of the effect, dose-response relationship, and the possible impact of confounding factors upon the results.27,28
Each clinical question is accompanied by a summary of the findings from the literature review, stated at the end of each question in a section called “Summary of the evidence”.
The systematic search yielded a total of 6.372 potential publications, but only 198 publications fully met the established criteria. An additional 15 publications were identified on a non-systematic basis between the systematic search and December 2020.
- 5
Structure for edition. This clinical guide is not intended to be a descriptive text of PD adequacy and prescription. Each section has a brief introduction to describe the context. Editing of the results generated in response to the questions raised with the GRADE method has been carried out based on the following narrative scheme, supported by tables: Synthesis or summary of the evidence. This section includes the studies provided by the search with selection and ranking of the papers that answer the questions posed. It is completed with the studies detected through non systematic search. This section includes, if any, the recommendations of ISPD guide.1From evidence to recommendation and definition of recommendations. The team defined the grade of recommendation referred to each question, rated as 1 (strong = we recommend), 2 (weak = we suggest), or opinion (not graduated).3,6,7
- 1
What is the relationship between the parameters of treatment adequacy in CAPD and APD and clinical outcomes?
- 2
What is the minimum target for weekly total Kt/V urea for patients on PD?
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKS as for those without?
- i
- 3
What is the minimum target for weekly total creatinine clearance for patients on PD?
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
- i
- 4
What is the minimum target for daily total fluid removal for patients on PD?
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
- i
- 5
What is the best method to assess hydration status in PD?
- 6
What is the maximum acceptable-tolerable overhydration in PD patients?
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
- i
- 7
Are there differences in mortality, technique survival, or morbidity between patients with preserved RKF and those without?
- 8
Does the use of biocompatible solutions affect RKF?
- 9
Does the use of icodextrin solutions affect RKF?
- 10
Are there differences between CAPD and APD in the preservation of RKF?
- 11
Are there any effective pharmacological measures to preserve RKF?
- 12
Is icodextrin indicated from the beginning of CAPD? Does the use of icodextrin from the beginning of CAPD increase survival?
- 13
Is the use of amino acids indicated from the beginning of CAPD? Does the use of amino acid-based solutions from the beginning of CAPD improve clinical outcomes?
- 14
Is the use of solutions buffered with bicarbonate or low in GDP (biocompatible) indicated from the beginning of CAPD? Does the use of solutions buffered with bicarbonate or low in GDP (biocompatible) from the beginning of CAPD improve clinical outcomes?
- 15
What parameters influence the prescription of nighttime exchanges in APD?
- 16
What factors affect peritoneal permeability in APD?
- 17
What are the factors that affect ultrafiltration and sodium removal during the nighttime session in APD?
- 18
Are there any daytime prescription regimens that optimize ultrafiltration and sodium removal in APD?
- 19
Is there any risk for the patient associated with the prescription of tidal PD? Does it improve dialysis adequacy?
- 20
Does adapted APD improve adequacy, ultrafiltration, sodium removal, and blood pressure control?
- 21
Does APD affect survival in patients on PD?
- 22
What are the advantages/disadvantages of initiating with APD rather than CAPD?
- 23
What are the advantages/disadvantages of APD over CAPD during treatment evolution?
Dialysis adequacy is a broad concept that should include the definition of minimum required level of solute clearance and control of fluid balance, but also the proper management of anaemia, bone-mineral metabolic disorder nutritional status and cardiovascular risk. All these elements directly impact in terms of survival and quality of life. As most of these factors are addressed in other documents and the S.E.N. guides, the present guideline will focus on solute clearance and fluid balance. It is essential to emphasize that the evaluation of PD adequacy should consider all aspects related to uremic syndrome. Patients’s quality of life and the impact of PD treatment should also be prioritised. In this line, there has, in recent years, been a shift in the concept of PD adequacy from the merely achieving numerical targets to a more holistic approach8 centered on patient’s quality of life. The latest update of the ISPD guidelines1 suggest changing the concept of «adequate» dialysis to «goal-directed» dialysis, defined as «using shared decision-making between the patient and care team to establish realistic care goals that will allow the patient to meet his/her life goals and allow the physician to provide individualized hight-quality dialysis care».
To ensure the delivery of high-quality PD care within this conceptual framework, the recent ISPD guidelines recommend the assessment of four areas: the patient’s reported outcome measures, hydration status, nutritional status, and toxin clearance.1
This section addresses the clinical significance of commonly used small solute clearance parameters and the recommended targets in clinical practice. Regarding ultrafiltration and fluid status, it discusses ultrafiltration targets, methods for assessing fluid status and acceptable hydration levels in clinical practice.
Parameters and objectivesClearance of small solute.
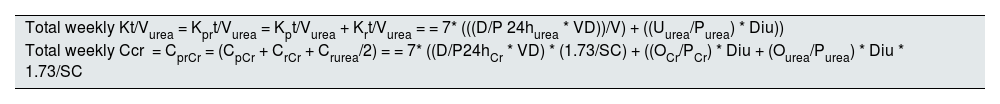
Solute clearance parameters. One of the most commonly used methods to quantify dialysis adequacy involves measuring the clearance of small solute, based on kinetic models for urea and creatinine. Urea kinetics are typically expressed as urea clearance normalized to the patient’s urea distribution volume (Kt/V urea), while creatinine kinetics are expressed as creatinine clearance normalized to the patient’s body surface area. In both cases, results are usually reported on a weekly basis. It should be noted that total Kt/V and total creatinine clearance are calculated by summing the peritoneal and renal clearances. Table 1 outlines how solute clearance calculations are performed and Table 2 shows how the distribution volume and body surface area are estimated. In clinical practice, these calculations are commonly made using software programs.
Calculation of total weekly dose of dialysis.
| Total weekly Kt/Vurea = Kprt/Vurea = Kpt/Vurea + Krt/Vurea = = 7* (((D/P 24hurea * VD))/V) + ((Uurea/Purea) * Diu)) |
| Total weekly Ccr = CprCr = (CpCr + CrCr + Crurea/2) = = 7* ((D/P24hCr * VD) * (1.73/SC) + ((OCr/PCr) * Diu + (Ourea/Purea) * Diu * 1.73/SC |
Cr: creatinine; D/P24h: dialysate versus plasma solute ratio; Diu: 24 -h diuresis in litres; Kprt/Vurea: weekly Kt/V; ureaKpt/V: peritoneal Kt/V; Krt/Vurea: renal Kt/V; U/P: urine/plasma solute ratio; BS: body surface area in m2; V: volume of body water distribution; DV: dialysate drainage volume in 24 horas in litres.
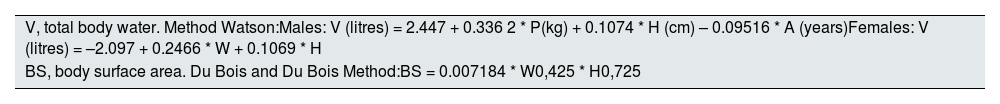
Estimation of total body water (V) and body surface area (BS) in adults.
| V, total body water. Method Watson:Males: V (litres) = 2.447 + 0.336 2 * P(kg) + 0.1074 * H (cm) – 0.09516 * A (years)Females: V (litres) = –2.097 + 0.2466 * W + 0.1069 * H |
| BS, body surface area. Du Bois and Du Bois Method:BS = 0.007184 * W0,425 * H0,725 |
H: height; A: age (V in litres); W: weight.
Priorisation of the use of Kt/V urea is recommended in most of current clinical guidelines such as European Renal Best Practice (ERBP),9 S.E.N.,2Kidney Disease Improving Global Outcomes (KDIGO),10 and Canadian Society of Nephrology (CSN).11Caring for Australians and New Zealanders with Kidney Impairment (CARI12 and United Kingdom Renal Association (UKRA13 recommend both Kt/V urea and creatinine clearance. The ERBP guidelines9 recommend monitoring creatinine clearance in the subgroup of slow transporters on APD and the recent ISPD guidelines suggest using Kt/V urea and/or creatinine clearance.1
Although Kt/V urea is the most recommended parameter, it is far from being an optimal indicator. Conceptually, urea is a relevant toxin, yet it is not the only one, and most uremic toxins have a greater molecular weight than that of urea. Consequently, the estimation of small solute clearance is not very representative of the clearance of toxic products. A further aspect to consider, which complicates the interpretation of the correlation between clinical outcomes and total Kt/V urea, is the likely lack of equivalence between the clearance obtained through the peritoneum and that provided by the RKF.14 Lastly, the calculation of Kt/V assumes stable body composition, which may not be consistently accurate
Beyond conceptual considerations, one of the most significant limitations is the estimation of the urea distribution volume «V», as shown in a recent analysis by Davies et al. in the ISPD guidelines,15 particularly in patients who are not normohydrated. Thus, fluid status (normohydrated, overhydrated, dehydrated) can influence the estimation of Kt/V.
Moreover, methodological doubts persist about which weight (real or ideal) should be used for the calculation. This issue is of interest in obese patients since using actual weight may lead to underestimation of the delivered dialysis dose. It is exacerbated in underweight patients, where the risk of overestimation is more pronounced, potentially leading to more significant adverse outcomes. This is why, despite a lack of evidence, the 2011 Canadian guidelines recommend using ideal weight for the calculation of V. The planning of the present guideline, included a PICO question on the matter: «Should the distribution volume (V) be calculated using ideal weight in obese and underweight patients?» The systematic search did not identify any studies meeting the minimum criteria to answer this question and, consequently, no recommendations are made and no studies are included.
We will now examine the correlation between the current adequacy parameters — Kt/V urea and creatinine clearance— with the clinical results obtained in patients on PD. The clinical outcomes considered include patient survival, technique survival, quality of life, and hospitalizations rates.
Structured Question Q1. What is the relationship between adequacy parameters in CAPD and APD and clinical outcomes?Quality of the evidence: Low.
Strength of the recommendation: Weak.
Evidence synthesisThe recent non-systematic review published in the ISPD guidelines16 confirmed the absence of robust evidence linking peritoneal clearance with mortality. The recommendations made by this committee regarding peritoneal clearance and mortality are based on a literature review, including ten prospective observational studies,14,17–25 six retrospective studies,26–31 and one clinical trial.32 Only one study17 shows that peritoneal Kt/V is an independent factor for patient survival and only in the subgroup of prevalent patients with low RKF.
As reflected in the recent ISPD guidelines,1 there is also no established evidence of an association between peritoneal clearance and technique survival.
The recommendations of the committee on this topic are based on a literature review including two prospective observational studies18,33 and one retrospective observational study.27 One of the prospective studies was performed on anuric patients on PD, and the other shows the association exclusively in the prevalent PD population. The most robust retrospective study identifies peritoneal Kt/V as an independent factor associated with technique survival. For each 0.1 increase in peritoneal Kt/V urea, the relative risk is RR: 0.38 (95% CI: 0.17−0.83).27
Regarding quality of life, the committee’s recommendations are based on two prospective observational studies,18,33 one retrospective study,34 and one clinical trial.35 Only very modest beneficial effects are observed in two low-power observational studies with short follow-ups.
The recommendations formulated by this committee regarding hospitalization rates are based on a literature review including three prospective observational studies17,19,36 one retrospective study,29 and one clinical trial.37
From evidence to recommendationAlthough the adequacy parameters based on small solute clearance have many limitations PD practice has relied on them for decades. Kt/V urea has been more extensively studied than creatinine clearance. However, most studies do not show a consistent relationship between these adequacy markers and patient survival, quality of life, or hospitalization rates. A weak association is observed between peritoneal Kt/V urea and technique survival in anuric patients and, generally, in prevalent PD.
RecommendationsWe suggest using Kt/V urea as the preferred measure of delivered PD dose (opinion). Not Graded.
We suggest not modifying the prescription to increase peritoneal Kt/V urea to achieve better clinical outcomes in asymptomatic patients with a good control of nutritional and hydration status and complications associated with uraemia (2C).
According to the latest updated version of the ISPD guideline on adequacy,1 achieving minimum numerical results, both in terms of uremic toxin elimination and total ultrafiltration value, has become secondary. Undoubtedly, the lack of high-quality scientific evidence supporting one value or another does not favour consensus. However, we believe that within the scope of this guideline, it is possible to recommend minimum values, assuming that the quality of the evidence found is low to moderate, without losing focus on patient quality of life.
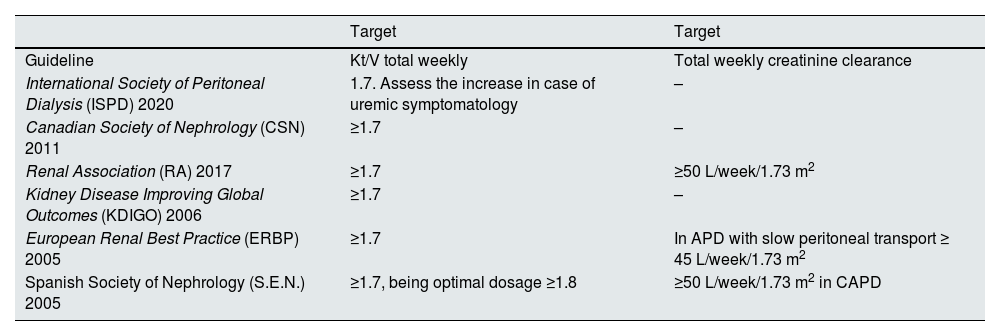
Solute clearance targetsAs detailed in Table 3, which summarises the objectives outlined by different scientific societies, there is no consensus regarding weekly creatinine clearance. Nonetheless, there is broad agreement among all guidelines to use Kt/V urea as a reference parameter, despite a lack of consensus on the specific numerical target.
Summary of Kt/V and weekly creatinine clearance targets proposed by different guidelines.
| Target | Target | |
|---|---|---|
| Guideline | Kt/V total weekly | Total weekly creatinine clearance |
| International Society of Peritoneal Dialysis (ISPD) 2020 | 1.7. Assess the increase in case of uremic symptomatology | – |
| Canadian Society of Nephrology (CSN) 2011 | ≥1.7 | – |
| Renal Association (RA) 2017 | ≥1.7 | ≥50 L/week/1.73 m2 |
| Kidney Disease Improving Global Outcomes (KDIGO) 2006 | ≥1.7 | – |
| European Renal Best Practice (ERBP) 2005 | ≥1.7 | In APD with slow peritoneal transport ≥ 45 L/week/1.73 m2 |
| Spanish Society of Nephrology (S.E.N.) 2005 | ≥1.7, being optimal dosage ≥1.8 | ≥50 L/week/1.73 m2 in CAPD |
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
Quality of the evidence: Moderate.
Evidence synthesisThis issue has been addressed in several practical guidelines, with no substantial changes over the past 15 years. Most current guidelines1,2,9–11,13 have established a minimum weekly total Kt/V urea of 1.7. Despite presenting a holistic approach to the concept of adequacy in PD, the most recent update of the ISPD guidelines1 suggest achieving a weekly total Kt/V urea value of 1.7. With potential increases if uremic symptoms persist. The recommendations made by this committee are founded on a literature review of three prospective observational studies,19,38,39 three retrospective studies,26,28,29 and one clinical trial.40
From evidence to recommendationThis committee endorses the recommendations outlined in the most recent ISPD guidelines 1,16 and underscores the need to maintain a minimum weekly total Kt/V urea level as a target, which should be applicable to the majority of patients.
RecommendationsWe suggest a minimum weekly total Kt/V urea of 1.7, regardless of the PD modality (CAPD or APD) (2B).
We suggest a minimum weekly total Kt/V urea of 1.7, irrespective of whether the patient is anuric or has RKF (2B).
We suggest that weekly Kt/V urea values >2 have not been demonstrated to positively impact patient or technique survival, or quality of life (2B).
We suggest that a weekly total Kt/V urea value between 1.7 and 2 appears to be the most appropriate for ensuring adequate clearance of small solute (2B).
Structured Question Q3. What is the minimum weekly total creatinine clearance target for PD patients?- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
Quality of the evidence: Moderate.
Evidence synthesisFollowing a review of the literature, the recommendations formulated by this committee rest on one clinical trial32 and one prospective observational study.38 The former concluded that increasing the peritoneal clearance of small solutes to 60 L/week/1.73 m2 yields no advantage over a value of 45 L/week/1.73 m2. The latter study assessed the impact of weekly creatinine clearance in a cohort of anuric patients. These authors concluded that a value of less than 40 L/week/1.73 m2 is associated with greater mortality. In the discussion, the authors themselves deem this value to be too low, arguing that the safety margin is small, and they support the value of 45 L/week/1.73 m2 as the minimum required.
From evidence to recommendationThis committee endorses the recommendations of the latest ISPD guidelines update1,16 but emphasis the need to maintain a minimum weekly creatinine clearance target for most patients.
RecommendationsWe suggest that the minimum weekly creatinine clearance should be 45 L/week/1.73 m2, regardless of the PD modality (CAPD or APD) (2B).
We suggest that the minimum weekly creatinine clearance should be 45 L/week/1.73 m2, irrespective of whether the patient is anuric or has RKF (2B).
We suggest that weekly creatinine clearance values above 60 L/week/1.73 m2 have not demonstrated a positive impact on patient survival or quality of life (2B).
We suggest that a weekly creatinine clearance between 45 and 60 L/week/1.73 m2 is the most appropriate range for achieving acceptable clearance of low molecular weight solutes (2B).
Ultrafiltration and hydration statusMonitoring hydration status, and consequently volemia is one of the key factors potentially influencing the development of cardiovascular disease, the leading cause of morbidity and mortality in PD patients.41–43 Thus, all PD clinical guidelines recommend periodic assessments of hydration status to prevent and treat overhydration.44–46
The majority of observational studies indicate a high prevalence of overhydration (OH) in PD patients.47,48 However, consensus has not been reached on the best method to assess and define hydration status. The recent ISPD guidelines recommend monitoring through physical examination and blood pressure measurements. Though they do not specify targed blood pressure levels.45
The section addresses ultrafiltration targets, the main diagnostic methods of hydration status available in clinical practice, and the desirable/acceptable hydration level in PD patients. Other factors that significantly influence volemia, such as sodium balance, are discussed in the prescription section.
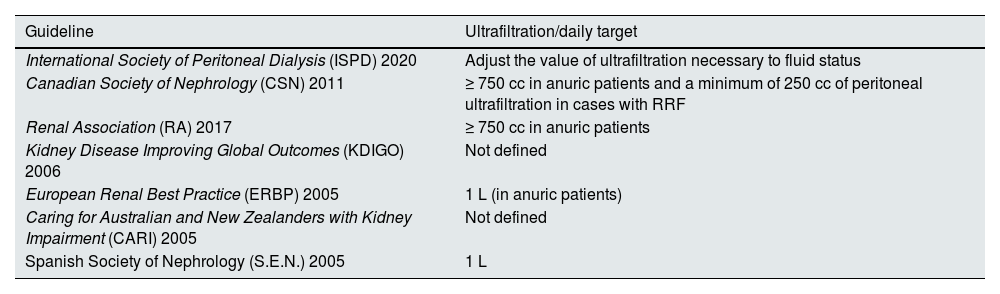
UltrafiltrationUltrafiltration in PD, residual diuresis, and water and salt intake are the three main elements to manage in order to achieve a normohydrated state in PD. The complexity of managing these three factors simultaneously makes it difficult to establish generic ultrafiltration targets in PD, as reflected in the recommendations of most available clinical guidelines (Table 4).
Summary of the targets of minimum ultrafiltration according to the different PDS clinical practice guidelines.
| Guideline | Ultrafiltration/daily target |
|---|---|
| International Society of Peritoneal Dialysis (ISPD) 2020 | Adjust the value of ultrafiltration necessary to fluid status |
| Canadian Society of Nephrology (CSN) 2011 | ≥ 750 cc in anuric patients and a minimum of 250 cc of peritoneal ultrafiltration in cases with RRF |
| Renal Association (RA) 2017 | ≥ 750 cc in anuric patients |
| Kidney Disease Improving Global Outcomes (KDIGO) 2006 | Not defined |
| European Renal Best Practice (ERBP) 2005 | 1 L (in anuric patients) |
| Caring for Australian and New Zealanders with Kidney Impairment (CARI) 2005 | Not defined |
| Spanish Society of Nephrology (S.E.N.) 2005 | 1 L |
- i
Is it the same for patients on CAPD as for those on APD?
- ii
Is it the same for patients with RKF as for those without?
Quality of the evidence: Low.
Evidence synthesisTo date, the published clinical guidelines have not reached a consensus on the total fluid removal target. Some guidelines, such as the ISPD,45 do not set a minimum value, while other recommend a minimum peritoneal ultrafiltration target ranging from 250 to 1.000 mL/day, sometimes distinguishing between peritoneal ultrafiltration and total fluid removal (the sum of peritoneal ultrafiltration and residual diuresis). Many earlier recommendations were based on observational studies, without assessing the impact of biocompatible solutions, icodextrin or the use of bioimpedance devices to objectively individualize each patient’s normohydration status.
The recommendations of this committee are based on two prospective observational studies43,49 and one retrospective study.50
From evidence to recommendationThis committee supports the recommendations from the latest ISPD guideline update1,16 which conclude that ultrafiltration target should be individualised to maintain each patient’s normohydration status.
RecommendationsWe suggest that the minimum daily total fluid removal should be adjusted to maintain the patient’s normohydration status (2C).
There is no evidence to suggest that daily ultrafiltration needs to differ between CAPD and APD patients (opinion). Not graded.
We suggest a minimum daily ultrafiltration of 750 mL in anuric patients (2B).
HydrationstatusSeveral diagnostic methods are currently available to assess the hydration status in patients on PD. The most common methods include physical examination, blood pressure measurement, and bioimpedance analysis (BIA). Biomarkers such as brain natriuretic peptide and the N-terminal fragment of pro-brain natriuretic peptide Type B are also used.51 A more recent technique is lung ultrasound for estimating extravascular water.52 Although potentially useful for evaluating volemia in PD patients, more experience is needed for its widespread use in PD.
In recent years, BIA has become increasingly used as a tool to explore body composition.53 Among the various indices obtained, the most referenced in relation to fluid status are absolute OH measured in litres and relative OH. The latter e is derived from the ratio of absolute OH to total extracellular water (OH/ECW) and is expressed as a percentage. However, there is still no full consensus on its use as a reference, partly due to the existence of different devices and indices.
Additionally, two randomized controlled trials,54,55 – one using a single-frequency device and the other using a multifrequency device - did not demonstrate a clinical benefit of patient monitoring with bioimpedance compared to a clinically managed control group.
This committee has explored the most effective method, applicable in clinical practice, for assessing and monitoring hydrations status in PD patients. A systematic review of studies comparing isotopic methods of body composition assessment, considered the gold standard, with other clinically used assessment methods was conducted.
Structured Question Q5. What is the best method to assess hydration status in PD?Quality of the evidence: Low.
Evidence synthesisA single prospective observational study56 compares commonly used clinical methods (multifrequency BIA) with isotopic methods of body composition assessment, considered the gold standard. This study included 40 PD patients with varying degrees of hydration. The results show a good correlation between isotopic methods and bioimpedance in determining total body water and extracellular water, with correlation coefficients of 0.88 and 0.79, respectively. However, in this study, multifrequency BIA underestimates total body water 2.0 ± 3.0 L (range 9.2–10.7) and overestimates extracellular water –2.7 ± 3.0 L.
The recommendations formulated by this committee are based on the findings of this study.56
From evidence to recommendationThis committee supports the recommendations from the latest update of the ISPD guidelines1,45 regarding the utility of physical examination and clinical blood pressure measurement for assessing volume status. We also suggest incorporation of an additional method, such as BIA.
RecommendationsWe suggest incorporating, whenever possible, a complementary method such as BIA, in addition to physical examination and blood pressure measurement, for the assessment of hydration status in PD patients (2C).
Structured Question Q6. What is the maximum acceptable-tolerable level of overhydration in PD patients?Is it the same for patients on CAPD and APD?Quality of the evidence: Low.
Evidence synthesisAchieving normohydration is one of the most important therapeutic goals for PD patients due to its clinical significance. Although this is reported in most guidelines and publications, none provided a consensus on the maximum tolerable level of overhydration. Striking a balance between the volume status needed to avoid a negative impact on RKF and that required to prevent cardiovascular events proves challenging. The use of BIA as a tool for objectively assessing patients’ hydration status is increasingly widespread but has not yet been universally adopted or recommended as the method of choice in published guidelines, including the latest ISPD update.1,16 This is partly because most related studies base their values on different indices, depending on the measurement device used.
The recommendations formulated by this committee are based on a review of the literature, including seven prospective observational studies57–63 and one systematic review.64
From evidence to recommendationThis committee shares the recommendations of the ISPD guidelines.1,16 regarding the importance of avoiding overhydratation. However, we consider that, in addition to monitoring blood pressure and physical examination, hydration measurement devices should be incorporated in the follow-up of PD patients to allow for a more objective assessment.
RecommendationsThere is no evidence suggesting that the acceptable-tolerable level of overhydration should be different between CAPD and APD patients (2C).
We suggest using accredited devices that allow for objectively monitoring patients’ hydration status (2C).
We suggest using the absolute OH and/or OH/ECW (relative overhydration) indices, as these are the most commonly reported in the literature (2C).
We suggest not exceeding an OH greater than 2 L and a maximum relative OH (OH/ECW) greater than 15% when monitoring hydration status using multifrequency BIA equipment (2B).
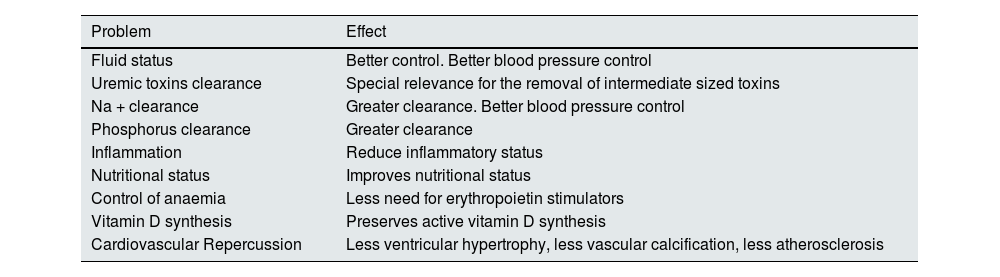
Preservation of residual kidney functionThe importance of preserving RKF is likely one of the most widely agreed-upon aspects among professionals dedicated to PD, a view supported by several observational studies. RKF, defined as the patient’s residual glomerular filtration rate (rGFR) while on dialysis, contributes to more than controlling volume and solute clearance, as highlighted in Table 5.
Beneficial effects of preserving RRF.
| Problem | Effect |
|---|---|
| Fluid status | Better control. Better blood pressure control |
| Uremic toxins clearance | Special relevance for the removal of intermediate sized toxins |
| Na + clearance | Greater clearance. Better blood pressure control |
| Phosphorus clearance | Greater clearance |
| Inflammation | Reduce inflammatory status |
| Nutritional status | Improves nutritional status |
| Control of anaemia | Less need for erythropoietin stimulators |
| Vitamin D synthesis | Preserves active vitamin D synthesis |
| Cardiovascular Repercussion | Less ventricular hypertrophy, less vascular calcification, less atherosclerosis |
The most commonly used method to calculate RKF in PD is the measurement of the average residual clearances of urea and creatinine. This approach counteracts the overestimation of the rGFR that occurs when only creatinine is measured (due to the effect of tubular secretion) and the underestimation of rGFR that occurs when only urea is used (due to the effect of tubular reabsorption).68
Structured Question Q7. Are there any differences in mortality, technique survival, or morbidity between patients who maintain RKF and those who do not?Quality of the evidence: Moderate.
Evidence synthesisMost publications and clinical guidelines addressing this issue emphasize the importance of preserving RKF as an effective measure to reduce mortality and morbidity and improve the technique survival. The majority of the studies supporting this measure are retrospective and very few are prospective. To our knowledge, no clinical trials have examined the prognostic impact of preserving RKF.
The recommendations made by this committee are based on a thorough literature review, which included six retrospective observational studies,69–74 one prospective study,75 and one systematic review.76
Frome evidence to recommendationThis committee endorses the recommendations of the latest update of the ISPD guidelines,1,77 which emphasize the importance of preserving RKF:
RecommendationsWe recommend preserving RKF whenever feasible, to reduce mortality in PD (1B).
We recommend preserving RKF whenever feasible, to reduce morbidity in PD (1B).
We recommend preserving RKF to improve technique survival in PD (1B).
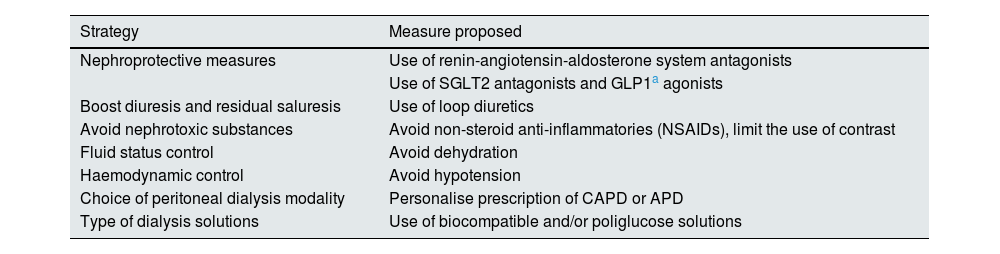
Given the importance of RKF, it seems reasonable to advocate different strategies to preserve, maintain and enhance RKF over time, as outlined in Table 6.
Protective mechanisms of residual renal function.
| Strategy | Measure proposed |
|---|---|
| Nephroprotective measures | Use of renin-angiotensin-aldosterone system antagonists |
| Use of SGLT2 antagonists and GLP1a agonists | |
| Boost diuresis and residual saluresis | Use of loop diuretics |
| Avoid nephrotoxic substances | Avoid non-steroid anti-inflammatories (NSAIDs), limit the use of contrast |
| Fluid status control | Avoid dehydration |
| Haemodynamic control | Avoid hypotension |
| Choice of peritoneal dialysis modality | Personalise prescription of CAPD or APD |
| Type of dialysis solutions | Use of biocompatible and/or poliglucose solutions |
The following questions explore the effectiveness of specific strategies in preserving RKF, focusing on the use of particular dialysis solutions, dialysis modalities, or specific pharmacological treatments.
Structured Question Q8. Does the use of biocompatible solutions affect RKF?Quality of the evidence: High.
Evidence synthesisThe widespread adoption of biocompatible solutions has been one of the most important advances made in field of PD in recent years. Their effects proven beneficial beyond the local protective effect of the peritoneal membrane. Multiple studies, including systematic reviews, clinical trials, and observational studies, have demonstrated the positive impact of these solutions on preserving RKF:
The recommendation made by this committee are based on ten clinical trials79–88 and five systematic reviews.89–93
From evidence to recommendationThis committee agrees with the latest update of the ISPD guidelines,1,77 which emphasize the role of biocompatible solutions in preserving RKF.
RecommendationsWe recommend the use of biocompatible solutions with the goal of preserving RKF and slowing its decline in PD patients (1A).
Structured Question Q9. Does the use of icodextrin solutions affect RKF?Quality of the evidence: Moderate.
Evidence synthesisThe introduction of icodextrin solution was a significant advancement for the prevention and treatment of volume overload in PD, also reducing the need for hypertonic glucose solutions. However, concerns arose regarding whether its use could lead to accelerated loss of RKF due to significant increases in peritoneal ultrafiltration volume. A review of the literature, including observational studies, clinical trials, and systematic reviews, supports the notion that icodextrin does not negatively impact RKF, provided its use does not result in critical volume depletion, The committee’s recommendations are based on a literature review, including five clinical trials94–98 and two systematic reviews.91,92
From evidence to recommendationThis committee agrees with the latest update of the ISPD guidelines1,77 which address the role of icodextrin emphasizing the need to avoid critical volume depletion and to frequently monitor hydration status often when using this solution.
RecommendationsRegular use of icodextrin solution does not impair RKF preservation (1B).
Structured Question Q10. Are there differences between CAPD and APD in maintaining RKF?Quality of the evidence: Low.
Evidence synthesisEvaluating the potential impact of the PD modality (CAPD/APD) on RKF has been one of the most controversial aspects. Most conclusions are based on retrospective observational studies, which have not conclusively established the effect of the dialysis modality on RKF.
The committee’s recommendations are based on a literature review, including six prospective observational studies99–104 and three retrospective studies.105–107
The analysed studies do not all reach the same conclusion. The majority of prospective studies, all including few patients, conclude that APD is associated with a faster decline of RKF. Of the retrospective studies including 494 patients105 reaches the same conclusion, while in the other two, with a total of 897 patients,106,107 no differences are observed.
From evidence to recommendationThis committee agrees with the latest update of the ISPD guidelines1,77 which reinforce the concept that the choice of PD modality (CAPD or APD) does not influence the decline of RKF.
RecommendationsThe available information does not allow concluding that APD is associated with faster rate of decline of RKF than CAPD (3C).
Structured Question Q11. Are there effective pharmacological measures to preserve RKF?Quality of the evidence: Low.
Evidence synthesisThe possibility of pharmacologically modulating RKF is one of the most interesting aspects of nephrological clinical practice. Two groups of drugs have been studied with particular attention: diuretics and, specially, antihypertensive agents that modulate the renin-angiotensin-aldosterone system. Angiotensin II converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) have already demonstrated their effectiveness in slowing the progression of chronic kidney disease in several of the most common chronic nephropathies, particularly diabetic nephropathy or other proteinuric nephropathies.
Regarding the use of ACEI/ARBs, the recommendations made by this team are based on a literature review, including two clinical trials,108,109 one prospective observational study,110 three retrospective observational studies,111–113 and three systematic reviews.114–116
Regarding the use of diuretics, the committee’s recommendations are based on a literature review, including one clinical trial117 and two retrospective observational studies.112,118
From evidence to recommendationThis committee agrees with the recommendations of the latest update of the ISPD guidelines1,77 regarding the beneficial effect of ACEIs/ARBs on preserving RKF and the use of diuretics to optimize volum and hydration status.
RecommendationsACEIs or ARBs are associated with improved preservation of RKF in PD patients (1A).
We recommend ACEsI or ARBs as the preferred antihypertensive medication in PD patients (1A).
We recommend using loop diuretics to optimise volume status and hydration in PD, while taking care of preventing volume depletion (1B).
We suggest using ACEIs or ARBs even in normotensive PD patients, with careful dose titration to avoid hypotension, which could have the opposite effect on RKF preservation (opinion). Not graded.
Prescription of peritoneal dialysisIntroductionThe recently published Guideline for Prescription of PD by the ISPD1 recommends considering not just the uremic manifestations, but also the implications and burdens that the prescription schedules may bear on the quality of life of the patients. Previous documents (Lo et al., 2006)41 focused on small solute removal (Kt/V and creatinine clearance) and ultrafiltration. This approach resulted in PD prescriptions which neglected a potential negative impact of long therapies with many exchanges on the quality of life and rehabilitation of the patients. Changes in the clinical profile of PD patients during the last years (higher proportion of diabetic and/or elderly individuals) have made more evident this limitation. These new considerations should not distract from the well-known relevance of traditional targets during PD prescription, including toxin and fluid removal, preservation of RKF, and prevention and management of malnutrition and overhydration, all questions treated in the present guideline.
The publication of the SONG-PD119 study, by focusing on the main health results as reported by patients, caregivers and health professionals confirmed how relevant are peritoneal infections, cardiovascular disease, mortality, PD technique failure and impact on daily life, for these groups. On the contrary, small solute removal was not a significant consideration for patients, except for those who have to be switched to hemodialysis therapy.
The general contentions by the KDOQI Group documents (Teitelbaum et al120) lend support to the lines of action adopted by the ISPD Guidelines, underlining the convenience that PD prescriptions should be oriented to keep the best possible quality of life of the patients. In other words, prescription must adapt to the lifestyle of the patient, rather than the opposite approach. Fortunately, the versatility of PD permits a wide, flexible spectrum of prescriptions.
Under the previous premises, we shall analyze how to prescribe the different PD solutions in current use (glucose-based biocompatible solutions, aminoacid-based solutions and icodextrin), and to describe the essentials of CAPD and APD prescriptions in separate, given their well differentiated characteristics.
Prescription of CAPDPrescription of capd at baselineThe factors to be considered for baseline CAPD prescription include the body size (estimated from the body surface area or the body mass index), RKF, the presence of diabetes mellitus and, only after they can be assessed, the peritoneal membrane characteristics of the patient. Regarding the number and volume of exchanges, the most usual schedules include 3–4 exchanges, with a volume of 1,5−2 L per exchange. In our setting, glucose-based PD solutions belong to the group of so-called biocompatible solutions, with a relatively physiologic pH and a low content of glucose degradation products (GDPs). Even under these conditions, the lowest necessary glucose load to reach target ultrafiltration rates should be applied. The use of icodextrin-based solutions facilitates both optimized ultrafiltration and glucose sparing. Aminoacid-based solutions are also helpful to minimize the peritoneal glucose load,
Icodextrin-based solutions. Icodextrin solution has been available in our country over 25 years. It is glucose-deriver heteropolymer that acts as a slowly absorbed colloid osmotic agent that enables effective ultrafiltration during long dwells. Clinically, it is particularly beneficial during long dwells (over 8 hours), daytime use in APD and night-time dwells in CAPD and for patients with fast and medium-fast peritoneal transport rates. The reduction or even elimination of solutions with high glucose concentrations (3.86–4.25 %), allows a significant decrease in glucose exposure and absorption by the peritoneal membrane. This reduction can potentially enhance the durability of the peritoneal membrane and the patients’ metabolic profile.121,122In vitro and ex vivo studies suggest that icodextrin is less favourable for the preservation of peritoneal membrane integrity than non-biocompatible solutions.121 Due to its low pH (lactate), icodextrin cannot be classified as a biocompatible solution, although it is very low in GDP and remains iso-osmolar with plasma.
Structured Question Q12. Is icodextrin indicated from the onset of CAPD? Does the use of icodextrin from the beginning of CAPD increase survival?Quality of the evidence: Moderate.
Evidence synthesisThe recommendations made by this committee are based on an extensive literature review, including four systematic reviews,123–126 one non-systematic review,121 four clinical trials,85,94,95,127 six observational studies, of which two reprospective studies,122,128 and four retrospective studies,129–132 as well as the recently published ISPD guideline.1
The use of icodextrin may be related to better patient survival according to one systematic review,126 while two others found no differences.125,133 In four observational studies, one prospective study,128 and three retrospective studies,129,130,132 lower mortality was observed with the use of icodextrin. Similarly, it correlated with better survival of the technique according to one clinical trial,95 as well as three observational studies, one prospective study,128 and two retrospective studies.129,130 The use of icodextrin appears to exert no negative effect on RKF or on the rate of peritoneal infections.121,123,125,126,133
No studies were found with respect to quality of life or the relative costs of icodextrin use.
From evidence to recommendationThe use of icodextrin in subjects initiation CAPD is safe, without adverse effects such as increased loss of RKF or higher rates of peritoneal infection. It is effective at achieving good ultrafiltration, especially in fast transporters.
However, the studies published to date do not provide high-quality evidence that using icodextrin from the start of CAPD is associated with reduced mortality and better technique survival. This committee endorses the recommendations of the ISPD adequacy guideline1 which suggest using icodextrin in cases where normohydration or adequate ultrafiltration cannot be achieved, considering the individual characteristics of peritoneal transport.
RecommendationsThis committee considers that there is no evidence to support the routine use of icodextrin solution in patients initiating CAPD (2B).
We recommend that patients on CAPD with fast peritoneal transport, low ultrafiltration capacity, and/or with difficulties in maintaining normohydration use icodextrin solution for the long exchange from the very start of treatment with CAPD (1B).
We suggest that patients who could potentially benefit the most from glucose-sparing strategies use icodextrin solution for the long dwell from the onset of CAPD treatment (2B).
Use of amino acid solutionsThe only available amino acid-based solution (1.1%) has an ultrafiltration capacity similar to that of 1.36% glucose solutions. It was originally developed to improve the nutritional status of patients on PD, with inconclusive results. Therefore, its primary current application is related to glucose-sparing strategies, while also providing high nutritional value amino acids.
Structured Question Q13. Is the use of amino acids indicated from CAPD initiation? Does the use of amino acid-based solutions from the beginning of CAPD improve clinical outcomes?Quality of the evidence: Low.
Evidence synthesisThe recommendations of this committee were formulated after an extensive review of the literature that included only one clinical trial127 with incident PD patients. The recently published ISPD Guideline on Adequacy1 do not mention the use of amino acid-based solutions. The systematic search failed to find any studies with incident PD patients regarding its effects on mortality, technique survival, peritoneal infection, quality of life, or treatment costs.
From evidence to recommendationThe use of amino acid-based solutions in patients who are beginning CAPD helps reduce peritoneal glucose load. To date, no studies have demonstrated that using amino acid-based solutions from the onset of CAPD affects mortality or increases technique survival.
RecommendationsWe suggest there is no basis upon which to support the systematic use of amino acid-based solutions in patients who are starting CAPD (2C).
We suggest including amino acid-based solutions as part of glucose-sparing strategies when applicable (2B).
Use of bicarbonate or low-GDP solutionsDespite overwhelming in vitro and ex vivo evidence suggesting better conservation of the peritoneal membrane, clinical studies have consistently failed to demonstrate significant benefits on peritoneal function, and patients or technique survival in PD. Numerous randomised trials have addressed this issue but they are generally underpowered and have relatively short follow-up periods. Associated meta-analyses have also been unable to reach more convincing conclusions.
It has been suggested that biocompatible solutions result in lower ultrafiltration compared to conventional solutions.92
Structured Question Q14. Is the use of bicarbonate-buffered solutions or low GDP (biocompatible) solutions indicated from the start of CAPD? Can it improve clinical outcomes?Quality of the evidence: Moderate.
Evidence synthesisThe recommendations formulated by this committee are based on 23 studies: five systematic reviews,90–93,133 eleven clinical trials,80,81,83,85,134–140 six observational studies, one prospective141 and five retrospective studies,142–146 as well as the recently published ISPD guideline on adequacy.1
No clinical trials have consistently assessed the effect of using biocompatible solutions from the beginning of PD on patient’ or technique survival. Some observational studies, of varying quality, suggest that biocompatible solutions are associated with lower mortality rates.142–146 Only the study by Quirós et al.145 indicates that the use of these solutions is linked to improved technique survival. Likewise, no clinical advantages have been observed with biocompatible solutions concerning peritoneal infection rates. Conversely, there is substantial evidence supporting their ability to preserve RKF and volume of diuresis.90,92,133
From evidence to recommendationThe use of biocompatible solutions is fully integrated in clinical practice. The review of the literature highlights that preserving RKF is the strongest and most convincing argument for using these solutions from the start of CAPD. In contrast, evidence related to the preservation of the peritoneal membrane is limited to in vitro and ex vivo studies, with no strong clinical evidence supporting benefits on patientsor PD technique survival.
Observational studies show a trend toward better patient survival with biocompatible solutions, but this should be interpreted cautiously due to their retrospective and observational design. In some areas, such as Korea143,144 Australia and New Zeland142 the analyses include low percentages of biocompatible solutions. In our country, an analysis of the Andalusian registry145 also suggests better patient and technique survival with biocompatible solutions.
This committee shares the recommendations of the latest updated ISPD guidelines1,77 which emphasise the role of biocompatible solutions in preserving RKF and their zero effect on patients and technique survival, highlighted in the 2018 Cochrane review.92
RecommendationsThe use of biocompatible solutions helps to preserve RKF and the volume of residual diuresis (1A).
We recommend the systematic use of biocompatible solutions from the start of CAPD in patients with significant RKF to facilitate its preservation (1A).
The use of biocompatible solutions is recommended in all patients initiating CAPD, on the basis of their potential beneficial effects on preserving the peritoneal membrane (1B).
Incremental peritoneal dialysisThe ISPD guideline on adequacy define incremental PD as a prescription of less than the standard dose or «full dose» at the beginning of PD that reaches the adequacy targets established by clinical guidelines. The dialysis dose is subsequently increased as RKF decreases or according to the patient’s needs. The term does not include CAPD regimens that are lower than standard for economic reasons or in cases of palliative PD. The definition has limitations that include the lack of specificity of terms such as «standard regimen» or «full dose». The term «full dose» of CAPD usually refers to four 2-litre exchanges (for an average patient) per day, so that a prescription of three exchanges per day would also be included in definition of incremental CAPD (iCAPD), although this latter regimen may be standard for others. Thus, iCAPD can comprise: (1) CAPD with fewer than four exchanges per day (between one and three) and/or less than two litres per exchange or (2) CAPD with fewer than three exchanges per day (one or two) and/or less than 2 L per exchange.
The concept of iCAPD emerged in the 1990s in a context that would foster the so-called early beginning of dialysis. In most centres, CAPD is started with three exchanges per day, especially in advanced chronic kidney disease units.
Economic considerations also play a role, as using less dialysis solution reduces costs, which can be relevant depending on the financial agreements in place. This prescribing strategy is also used as a bridging modality toward kidney transplantation. Incremental PD can also be used in the APD modality
iCAPD can be prescribed at the start of PD for patients with preserved RKF and a not very large body surface area. Few observational studies available147–149 and reviews,150 suggest that iCAPD offers good clinical outcomes. It has been hypothesised to delay RKF decline but without sufficient evidence. Additionally, it is a well-accepted initial modality among patients as it helps maintain quality of life. The dose of dialysis should be increased and adjust to dialysis protocols when dialysis adequacy is not achieved, always considering the patient’s clinical status (frailty and palliative dialysis) and quality of life.1 In practice, the leading causes that compel the switch to a standard prescription are the overload volume and loss of RKF.
The recent ISPD guidelines on PD adequacy include a chapter on incremental PD.151 The main conclusion drawn is that incremental PD strategies are supported by low to moderate quality evidence, suggesting that patients on incremental PD are not worse off than those receiving the «full dose».
Adjusting CAPD prescription during follow upThe options for increasing the dialysis dose for CAPD are scarce and include either upping the number of exchanges, typically from three to four per day, or increase the volume of the exchanges. Not all peritoneal solutions are available in the 2.5-litre volume. Five manual exchanges per day as a long-term prescription is uncommon and fairly incompatible with adequate quality of life. Likewise, new solutions can be indicated, especially icodextrin, when they have not been previously used; dwell times can be tweaked to improve ultrafiltration, and the switch to APD can be considered to optimise adequacy.
APD prescriptionAPD follows the general tenets of PD, albeit it is performed with the aid of a cycler that carries out the peritoneal exchanges automatically. Automaticity facilitates that the technique can be made during the night in sessions of varying duration, usually 7−10 hours. Depending on whether the prescription includes or not daytime dwells we will refer to APD with dry day (nocturnal intermittent peritoneal dialysis [NIPD]), APD with wet day (continuous cyclic peritoneal dialysis [CCPD]), or, if more than one daytime exchanges are added, APD with supplementary exchange(s) (or optimised continuous peritoneal dialysis [OCPD]). Partial emptying and replacement of dialysate during nighttime exchanges can be used in any of these modalities, this is called tidal APD.
The previous paragraph underlines the versatility of APD, which makes it possible to adjust the prescription to achieve better overall adequacy. APD enables us to optimize the dose of dialysis and, at the same time, maintain patients’ quality of life, by providing free daytime hours, and the consequent ability to preserve normal life activities.
NIPD is usually performed in cases in which the patient maintains a significant residual kidney function, precluding the need for daytime dwells. For some professionals, this modality may represent a kind of initial prescription of incremental peritoneal dialysis.
From a practical point of view, and in terms of prescription, it is important to individualize nocturnal from daytime prescription because both the dwell volumes and the duration of the exchange are radically different.
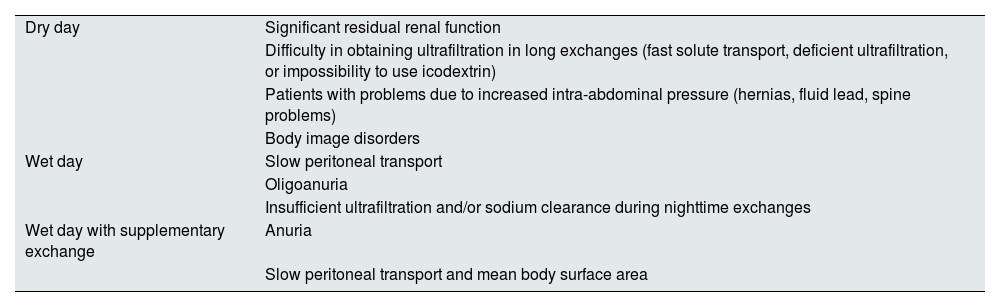
The indications for each automated PD modality can be found in Table 7.
Indications for the different forms of daytime prescription of automated PD.
| Dry day | Significant residual renal function |
| Difficulty in obtaining ultrafiltration in long exchanges (fast solute transport, deficient ultrafiltration, or impossibility to use icodextrin) | |
| Patients with problems due to increased intra-abdominal pressure (hernias, fluid lead, spine problems) | |
| Body image disorders | |
| Wet day | Slow peritoneal transport |
| Oligoanuria | |
| Insufficient ultrafiltration and/or sodium clearance during nighttime exchanges | |
| Wet day with supplementary exchange | Anuria |
| Slow peritoneal transport and mean body surface area |
Nocturnal prescription usually contributes most of the total amount of dialysate infused, including higher volumes per exchange these circumstance provide an advantage in terms of low molecular weight solute removal, offsetting the shorter duration of the exchange (resulting in lower solute saturation rates).
The first point to be defined in nocturnal APD prescription is the volume to be infused during the nighttime exchanges. This volume is better prescribed as a function of the patient’s body surface area, usually between 1,400 and 1,500 ml per square meter of body surface area.152 This theoretical prescription must be personalised to adjust for the intraabdominal pressure generated, which should not exceed 15−18 cm of water, and always be adapted to the patient’s subjective tolerance.153 It must be pointed out that intraperitoneal pressure is highly dependent on body position, being lower while the patient is lying down than while sitting or standing. This circumstance explains why, the nocturnal volume per exchange can be usually increased, when compared the volume typically used in CAPD.154
The dwell time of the nighttime exchanges usually oscillates between 60 and 120 min, which makes small solute saturation possible to around 50% of serum concentration, depending on the peritoneal transport characteristics of the patient.155 Remarkably, the increase in the volume of the exchange made possible by dialysis being performed with the patient in the supine position results in a more efficient peritoneal transport for low weight molecular solutes (urea, creatinine, and glucose), as a consequence of a better distribution of the dialysis solution within the peritoneal cavity, including highly vascularized areas (including the subhepatic peritoneum).156,157 This enhances the removal of low molecular weight solutes, although, as we will see later on, it hinders ultrafiltration during the nocturnal period.
The enhanced ultrafiltration associated with the use of hypertonic glucose solutions (icodextrin is not routinely used for short dwells) boosts the convective transfer of solutes during the nocturnal session; nevertheless, this strategy is regarded as inefficient in that it contributes a slight increase at the expense of greater potential damage to the membrane and worse metabolic consequences of PD.
Structured Question Q15. What parameters influence the prescription of nighttime exchanges in APD?Quality of the evidence: Low.
Evidence synthesisThe bibliographic review has not detected any quality studies that provide information regarding comparative results of different nocturnal APD prescription strategies.
From evidence to recommendationGiven the lack of evidence, this committee suggests that the volume of nocturnal exchanges be optimised, and dwell times modified in accordance with the type of peritoneal transport (varying the number of dwells or the total duration of the session) as the main adjustment strategies when prescribing nocturnal exchanges in the context of APD.
RecommendationsWe suggest optimizing the volume of nighttime exchange according to the patient’s body surface area, within the accepted intraabdominal pressure limits and patient tolerance (opinion). Not graduated.
We suggest adapting the dwell times of nocturnal exchanges in APD to suit each patient’s peritoneal transport characteristics, shortening them (and increasing the number of dwells, when appropriate) in faster than mean transporters, and prolonging them (with fewer dwells and/or increased total duration of the session, as appropriate) in slower than mean transporters (opinion). Not graduated.
In addition to adjusting the volume of the exchange and dwell times to the characteristics of the peritoneal transport, an analysis should be conducted to determine whether there are other factors that might have a bearing on the transfer of solutes.
Structured Question Q16. What factors affect peritoneal permeability in APD?Quality of the evidence: Low.
Evidence synthesisThe search of the literature only identified one clinical trial that examined the impact of different concentrations of glucose and icodextrin on the transport of solutes158 and one prospective observational study that analysed the impact of body position on peritoneal transport.156
The recommendations formulated by this committee are founded on these two studies.
From evidence to recommendationThe weight of the evidence provided by the publications does not make it possible to recommend a specific concentration of glucose for nocturnal exchanges in APD. The increased peritoneal small solute transport attained when performing the session with the patient in the supine position. We favour the feasibility of the regimes based on short exchanges characteristic of APD.
RecommendationsWe suggest the convenience of performing the nocturnal APD session in the supine position (opinion). Not graduated.
The use of solutions having concentrations of glucose exceeding 1.36% or 1.5% dextrose improve the removal of low molecular weight solutes by increasing ultrafiltration (opinion). Not graduated.
Nocturnal ultrafiltrationThe increase in low molecular weight solute transport achieved with the patient being supine results in a significantly reduced concentration of glucose and, consequently, of dialysate osmolarity as the dwell time progresses (a mean increase in glucose transport of 17% has been estimated).156 This potential loss of ultrafiltration capacity is offset by the short duration of the exchanges. This notwithstanding, it is worth remembering that, if the number of nighttime exchanges is increased beyond a certain point, the infusion and dialysate drainage periods will also be increased; consequently, dwell time will be reduced, which affects solute transport. On the other hand, the short duration of the exchanges leads to a lower sodium concentration (a phenomenon known as sodium sieving) in the dialysate. This phenomenon is due to the fact that approximately 40–45% of the water transfer during a short exchange takes place through ultrapores (aquaporins). If no compensatory time is allowed for diffusive transfer, the result is lower sodium removal during the nocturnal session than expected from the ultrafiltration achieved.
Structured Question Q17. What are the factors that affect ultrafiltration and sodium removal during the nighttime session in APD?Quality of the evidence: Moderate.
Evidence synthesisWe have identified several studies that approach the issue of nocturnal ultrafiltration in APD assessing different aspects of the prescription, including tidal volume,159 the number of exchanges in session of a given duration,160 and the use of different concentrations of glucose.161 The first two are clinical trials with small samples (12 and 18, respectively). The third is a prospective observational study and had greater statistical power.
The two clinical trials159,160 cited reveal greater sodium removal with a 70% tidal prescription.
The prospective study by Fischbach et al.162 reported slightly higher sodium removal in a limited number of cases.
From evidence to recommendationThe evidence available allows for generic recommendations about nocturnal ultrafiltration, but it is not possible to make a recommendation regarding sodium removal.
RecommendationsWe recommend using solutions having a higher concentration of glucose as a means by which to enhance ultrafiltration during nocturnal sessions in APD (1B).
For faster than mean transporters, we suggest to increase the number of exchanges, with a consequent decrease in dwell time, as a mean to increase ultrafiltration during the nighttime sessions in APD (2B).
Daytime prescriptionThe most usual daytime prescription in APD includes a single dwell, lasting between 14 and 16 h. Similarly, dry day regimens (NIPD) are also frequent, as is the addition of a supplementary exchange during daytime. In any case, the long duration of the daytime dwells demands the use of solutions that maintain ultrafiltration and enable sodium removal.
Structured Question Q18. Are there any daytime prescription regimens that optimise ultrafiltration and sodium removal in APD?Quality of the evidence: High.
Evidence synthesisThe search for information regarding daytime prescription regimens that optimise ultrafiltration in long dwells yielded three systematic reviews,92,123,124 four clinical trials163–166 and three observational studies.167–169 Six studies analysed only patients on APD, with a total of 1,428 patients,163–165,167–169 whereas the systematic reviews included 2,412 patients on APD. All the studies cited corroborate the efficacy of icodextrin solution to optimise ultrafiltration, maintaining its effect for the daytime periods usually prescribed in APD. Furthermore, better weight and blood pressure control are achieved than with glucose-based solutions, with no apparent negative effects on RKF.
Regarding sodium removal, three clinical trials were identified163,164,166 for a total of 1,284 patients, and one prospective observational study with 12 patients.169 The results of the studies163,164,169 reveal that daytime exchanges with icodextrin increase sodium extraction. Another study166 focused on the efficacy of a mixed solution of glucose and icodextrin with a low concentration of sodium, that this approach results in higher sodium removal rates.
From evidence to recommendationAll the systematic reviews, clinical trials, and observational studies analysed concur that the use of icodextrin during the daytime exchange improves both ultrafiltration as well as sodium removal.
This committee shares the recommendation of the recent ISPD guideline16 concerning the use of icodextrin to improve ultrafiltration.
RecommendationsWe recommend that daytime APD exchanges be performed with an icodextrin-based solution, with the aim of enhancing ultrafiltration (1A).
We recommend daytime APD exchanges with an icodextrin-based solution, with the aim of increasing sodium removal (1A).
We recommend supplementary daytime exchanges, with the aim of optimizing solute clearance, ultrafiltration, and sodium removal, in appropriate cases (1A).
If icodextrin is not available, a dialysis solution should be used with a 2.27% glucose/2.5% dextrose concentration, supplementary exchange, or setting aside some hours without glucose solution in the peritoneum (short exchange followed by some dry day hours).
Tidal APDInitially designed to optimise solute transfer, it was soon apparent that tidal prescription was not efficient for this task, as it failed to produce higher clearance rates than APD prescriptions with wet day and required large volumes of dialysate, with higher economic costs and potential damage to the peritoneal membrane.170
At present, tidal PD is more usually prescribed under high (80–90% of the exchange volume) renewal schedules, with the aim of improving outflow mechanics in patients with malfunction of the peritoneal catheter or pain at the end of inflow or outflow of the dialysate exchanges.170
Incomplete emptying of the fluid introduced during the initial exchange, leaving between 10 and 20% of each exchange, may result in a gradually increasing intraperitoneal volume, which can raise intraabdominal pressure, with a consequent decrease in vital pulmonary capacity and deranged venous return. Some measures which could contribute to prevent this complication includes requiring an outflow of at least 70% of the volume of the daytime exchange, scheduling nocturnal ultrafiltration always exceeding 0, or blocking bypass during nighttime exchanges.
There is a cycling model that switches from outflow to inflow when it detects that the rate at which the solution is draining slows down (the so-called break-point), infusing only the volume of solution drained. The accumulation of fluid during the nighttime session is less likely, using this approach.
Structured Question Q19. Is there any risk for the patient associated with the prescription of tidal PD? Does it improve dialysis adequacy?Quality of the evidence: Low.
Evidence synthesisOnly one study was retrieved that analysed the incidence and the consequences of a gradual accumulation of dialysate in the abdominal cavity with the modality of tidal APD,170 conducted with a specific model of dialysis cycler. This study was based on the systematic analysis of 116,500 treatments, with the objective of ascertaining the factors that led to overfilling of the peritoneal cavity. The study disclosed that most of the cases were secondary to errors in the dialysis schedule prescription, related to insufficient outflow. The results of this study led to modifications in the prescription tolerances aimed at enhancing safety: demanding a 70% minimum outflow of the daytime volume at the beginning of the nocturnal session, programming a minimum acceptable ultrafiltration during the nighttime session (greater than 85%), and not allowing a bypass to be made if the outflow is insufficient.
These recommendations are not applicable to the cycler model that aborts outflow when the rate during drainage declines.
The studies that assessed the possible improvement of adequacy with the use of lower tidal volumes (50–70%) provided no evidence endorsing this indication.
From evidence to recommendationDespite the fact that there is only one study that evaluates risks associated with tidal PD, and given the weight of the evidence, it is advisable that the recommendations given by the authors for cyclers with a set tidal volume be followed.
There is no evidence in the case of cyclers with variable tidal.
This committee considers that there is insufficient evidence upon which to base the indication of tidal APD with the aim of optimising solute clearance or ultrafiltration.
RecommendationsWe suggest adopting the measures needed to prevent overfilling of the abdominal cavity during nocturnal tidal APD sessions (opinion). Not graduated.
Adapted APDThe adapted APD technique modulates the volume and duration of nighttime exchanges. The original model proposed two short, smaller-volume exchanges, followed by three longer exchanges and with a greater volume. However, a variable number of exchanges and types of dwells are used in clinical practice.171
The objective is to maximise solute clearance by taking advantage of the long, high-volume exchanges and the ultrafiltration attained during the short, low-volume exchanges.
Structured Question Q20. Does adapted APD improve adequacy, ultrafiltration, sodium removal, and BP control?Quality of the evidence: Low.
Evidence synthesisOnly one prospective observational study172 could be retrieved that analyses the outcomes achieved by the adapted APD technique. This study shows statistically significant, albeit clinically small improvements insofar as the parameters of small solute clearance, ultrafiltration, and sodium removal are concerned.
From evidence to recommendationDespite achieving statistically significant improvements compared to standard nocturnal schedules, the differences are clinically minor. No clinical practice guideline currently in force contemplates using this modality of APD to enhance adequacy.
RecommendationsThis committee issues no recommendation as to the routine use of the adapted APD technique to optimise solute clearance, ultrafiltration, or sodium removal (opinion). Not graduated.
APD offers prescription versatility and makes it possible to reconcile adequate clearance and ultrafiltration while maintaining quality of life, making it very appealing to many patients. On the other hand, it is more costly in economic terms and more complex. The clinical outcomes [of this technique] have been the subject of many studies compared to the results provided by CAPD.
Structured Question Q21. Does APD affect survival in patients on PD?Quality of the evidence: Moderate.
Evidence synthesisA systematic review173 that included relatively few patients (139 in total and 63 on APD) failed to detect differences in survival between subjects treated with APD and those undergoing CAPD. More recently, a narrative review174 revealed differences only with regard to CAPD in some studies and in some subpopulations. Sun et al.175 observed lower mortality among patients on APD under the age of 65 (risk of mortality 35% lower than in patients of the same age range on CAPD). Johnson et al.176 disclosed better survival for fast transporters on APD, in both the intent-to-treat analysis (risk of mortality five times lower) and as treated (risk of mortality 72% lower).
At least eight prospective observational studies105,107,175–180 (28,824 patients) and four retrospective studies180–183 (451 patients) have examined the issue. Follow up oscillated between 17 months and 5 years. Five studies found no difference in survival between patients treated with APD or DPCA.105,107,179,181,183 In contrast, De Carvalho et al.177 analysed two broad populations on PD (1,445 on CAPD and 1,445 on APD) from the Brazilian registry. Only those patients who did not change modality were selected and an adjusted risk analysis was performed for the main covariables. The study detected a higher mortality risk with CAPD than with APD (overall relative risk 1.47, cardiovascular 1.41).
A recent retrospective study that compared a relatively large number of patients (459 on CAPD and 266 on APD) followed over 12 years185 observed a 49% lower mortality rate with APD versus CAPD, after controlling for age, diabetes, and pre-existing cardiovascular disease.
From evidence to recommendationAlthough some studies show a trend toward lower mortality in patients treated with APD than those on CAPD, the results are inconsistent and the observational and retrospective design of most of the studies make it impossible to draw reliable conclusions. The lower mortality in fast transporters on APD reported by the Australia and New Zealand registry (Anzdata) are appealing in as much as they respond to consistent theoretical bases, but the data presented require confirmation.
RecommendationsWe suggest that APD may offer some advantage over CAPD for fast transporters in terms of patient survival (2B).
Advantages of APDAPD from the beginningThe use of APD is highly variable around the world, depending, to a large extent, on the availability of the technique, but also on clinical practice in each PD unit and whether the patient has free choice. The use of APD makes it possible to optimise the dose of dialysis; personalise ultrafiltration; adapt to employment, school, and social life, and, in some cases, enables management abdominal wall problems.174 APD lends versatility to PD and can contribute to improving the survival of the technique, albeit this aspect is difficult to demonstrate in practice.
There are only two randomised clinical trials, which are now somewhat out of date, in incident patients that compare APD and CAPD. The study by Bro et al.186 carried out in 34 subjects focuses on quality of life and favours APD, whereas the study by De Fijter et al.187 compares patient and technique survival, finding no differences; nevertheless, the latter study was clearly underpowered given the small size of the sample (n = 82) and the short follow up.
The comparative effect of APD and CAPD on the rate of RKF decline is specifically analysed on Structured Question 10.
Several cost analyses have been carried out in prevalent population on PD, consistently showing that APD increases the cost of the technique. No high-quality studies have been performed to assess whether the use of APD is able to enhance technique survival, improving efficiency in terms of costs and healthcare.
Structured Question Q22. What are the advantages and disadvantages of initiating with APD rather than CAPD?Quality of the evidence: Moderate.
Evidence synthesisFollowing an extensive bibliographic review, the recommendations formulated by this committee rest on two randomised studies,185,186 one crossover study,188 one systematic review,173 twenty observational studies (seven of which are prospective102–105,189–191 and thirteen retrospective106,107,175,178,180,183,184,192–197), together with the recent ISPD guideline on adequacy.1 Most of the observational studies were conducted in the general PD incident population, except for two,176,184 that include only fast transporters and one performed with patients on assisted PD.195 The two clinical trials included few patients (116 subjects) and had a follow up of less than one year.
Regarding mortality, greater survival with APD from the beginning was only observed in observational studies such as the Brazilian registry177 and in special populations: assisted PD,196 fast transporters,176 and individuals under 65 years of age.175
Overall, the evidence supporting APD from the beginning for improved technique survival is both weak and low-quality. This includes six observational studies: three single-centre studies;107,176,184 the United States Historical Registry that includes over 30,000 patients;189 the French-language Registry of Assisted PD,196 where APD is performed by a nurse, and the Taiwan Registry of patients under 65 years of age.175
The recent ISPD guideline on adequacy1 does not approach the potential advantages of APD as the initial option.
From evidence to recommendationBased on the evidence available, no differences have been found regarding patients or technique survival when starting treatment with APD or CAPD. The perception of benefits for APD in subpopulations such as high transporters, younger patients, and those requiring assisted PD persists, though it lacks robust supporting evidence.175,176,184,196
RecommendationsWe recommend initiating PD with either CAPD or APD, depending on patient preference, needs, and resources of the centre (1C).
We suggest considering early start of APD in fast transporters, particularly if there is little RKF (2B).
APD and CAPD in continuation therapyAPD offers much greater versatility to dialysis and contributes to technique survival. 174 Clinical practice often suggests that switching from CAPD to APD for any medical or social indication can increase technique survival, although there is no evidence proving that APD as continuation treatment offers patient survival advantages.
A well-prescribed patient-tailored APD, can increase the dose of dialysis, improve ultrafiltration, address issues related to the abdominal wall, enhance quality of life, and minimise the sodium and phosphorus extraction.174
Structured Question Q23. What are the advantages/disadvantages of APD over CAPD during treatment evolution?Quality of the evidence: Low.
Evidence synthesisThe recommendations formulated by this committee are based on three systematic reviews,173,198,199 two non-systematics reviews,174,199 nine observational studies (three of which were prospective102,179,201 and five retrospective180,202–205), one cross-sectional study,206 and the ISPD Guideline on Adequacy in DP.1
A non-systematic review of the advantages/disadvantages of APD174 indicated that there were no differences between APD and CAPD regarding patient and technique survival, rate of peritoneal infection, volume control, preservation of RKF, and quality of life. In near systematic review on peritoneal infection200 in APD and CAPD, concluded that there are no differences. One systematic review199 and one cross-sectional observational study206 found that APD provides better quality of life in some aspects, albeit not overall.
This issue is not specifically addressed in the recent ISPD adequacy guidelines1
From evidence to recommendationThe evidence available does not indicate that APD offers advantages over CAPD in terms of patient survival (except possibly in specific populations; see Structured Question 21), technique survival, rate of peritoneal infections, and quality of life. Clinical practice suggests that APD can prolong the viability of PD in some cases, but no high-quality evidence confirms this perception.
RecommendationsWe suggest that APD offers no general advantages over CAPD as a technique for continuation treatment (2C).
We suggest that continuation APD be indicated based on the need to adjust the dose of dialysis, ultrafiltration, reconciliation of work and social life balance, quality of life, and abdominal wall issues (2B).
We recommend not using APD rather than CAPD with the aim of reducing the rate of peritoneal infection (1C).
Summary of recommendations and suggestions of the guidelineAdequacy- 1
We suggest using Kt/V urea as the preferred measure of delivered PD dose (opinion). Not graded (question 1).
- 2
We suggest not modifying the prescription to increase peritoneal Kt/V urea to achieve better clinical outcomes in asymptomatic patients with a good control of nutritional and hydration status and complications associated with uraemia (2C) (question 1).
- 3
We suggest a minimum weekly total Kt/V urea of 1.7, regardless of the PD modality (CAPD or APD) (2B) (question 2).
- 4
We suggest a minimum weekly total Kt/V urea of 1.7, irrespective of whether the patient is anuric or has RKF (2B) (question 2).
- 5
We suggest that weekly urea Kt/V values of >2 have not demonstrated to positively impact patient or technique survival, or quality of life (2B) (question 2).
- 6
We suggest that a weekly total Kt/V urea value between 1.7 and 2 appears to be the most appropriate for ensuring adequate clearance of small solute (2B) (question 2).
- 7
We suggest that the minimum weekly creatinine clearance should be 45 L/week/1.73 m2, regardless of the PD modality (CAPD or APD) (2B) (question 3).
- 8
We suggest that the minimum weekly creatinine clearance should be 45 L/week/1.73 m2, irrespective of whether the patient is anuric or has RKF. (2B) (question 3).
- 9
We suggest that weekly creatinine clearance values above 60 L/week/1.73 m2 have not demonstrated a positive impact on patient survival or quality of life (2B) (question 3).
- 10
We suggest that a weekly creatinine clearance between 45 and 60 L/week/1.73 m2 is the most appropriate range for ahieving acceptable clearance of low molecular weight solutes (2B) (question 3).
- 11
We suggest that the minimum total daily fluid removal should be adjusted to maintain the patient’s normohydration status (2C) (question 4).
- 12
There is no evidence to suggest that daily ultrafiltration needs to differ between CAPD and APD patients (opinion). Not graded (question 4).
- 13
We suggest a minimum daily ultrafiltration of 750 ml in anuric patients (2B) (question 4).
- 14
We suggest incorporating, whenever possible, a complementary method such as BIA, in addition to physical examination and blood pressure measurement, for the assessment of hydration status in PD patients. (2C) (question 5).
- 15
There is no evidence suggesting that the acceptable-tolerable level of overhydration should be different between CAPD and APD patients (2C) (question 6).
- 16
We suggest using accredited devices that allow for objectively monitoring patients’ hydration status (2C) (question 6).
- 17
We suggest using absolute OH and/or OH/ECW (relative overhydration) indices, as these are the most commonly reported in the literature (2C) (question 6).
- 18
We suggest not exceeding an absolute OH greater than 2 L and a maximum relative OH (OH/ECW) greater than 15% when monitoring hydration status using multifrequency BIA equipment (2B) (question 6).
- 19
We recommend preserving RKF whenever feasible to reduce mortality in PD (1B) (question 7).
- 20
We recommend preserving RKF whenever feasible, to reduce morbidity in PD (1B) (question 7).
- 21
We recommend preserving RKF whenever feasible, to improve technique survival in PD (1B) (question 7).
- 22
We recommend the use of biocompatible solutions with the goal of preserving RKF and slowing its decline in PD patients (1A) (question 8).
- 23
Regular use of icodextrin solution does not impair RKF preservation (1B) (question 9).
- 24
The available information does not allow concluding that APD is associated with faster rate of decline of RKF than CAPD (3C) (question 10).
- 25
ACEsI or ARBs are associated with improved preservation of RKF in PD patients (1A) (question 11).
- 26
We recommend ACEIs or ARBs as the preferred antihypertensive medication in PD patients (1A) (question 11).
- 27
We recommend using loop diuretics to optimise volume status and hydration in PD, while taking care of preventing volume depletion (1B) (question 11).
- 28
We suggest using ACEIs or ARBs even in normotensive PD patients, with careful dose titration to avoid hypotension, which could have the opposite effect on RKF preservation (opinion). Not graded (question 11).
- 29
This committee considers that there is no evidence to support the routine use of icodextrin solution in patients initiating CAPD (2B) (question 12)
- 30
We recommend that patients on CAPD with fast peritoneal transport, low ultrafiltration capacity, and/or with difficulties in maintaining normohydration use icodextrin solution for the long dwell from the very start of CAPD treatment (1B) (question 12)
- 31
We suggest that patients who could potential benefit the most from glucose-sparing strategies use icodextrin solution for the long dwell from the onset of CAPD treatment (2B) (question 12).
- 32
We suggest there is no basis upon which to support the systematic use of amino acid-based solutions in patients who are starting CAPD (2C) (question 13).
- 33
We suggest including amino acid-based solutions as part of the glucose-sparing strategies when applicable (2B) (question 13).
- 34
The use of biocompatible solutions helps to preserve RKF and the volume of residual diuresis (1A) (question 14).
- 35
We recommend the systematic use of biocompatible solutions from the start of CAPD in patients with significant RKF, to facilitate its preservation (1A) (question 14).
- 36
The use of biocompatible solutions is recommended in all patients initiating CAPD, on the basis on their potential beneficial effects in preserving the peritoneal membrane (1B) (question 14).
- 37
We suggest optimizing the volume of the nighttime exchange based on patient’s body surface area, within the accepted limits of intraabdominal pressure and patient tolerance (opinion). Not graduated (question 15).
- 38
We suggest adapting the dwell times of nocturnal exchanges in APD to suit each patient’s peritoneal transport characteristics, shortening them (and increasing the number of dwells, when appropriate) in faster than mean transporters, and prolonging them (with fewer dwells and/or increased total duration of the session, as appropriate) in slower than mean transporters (opinion). Not graduated (question 15).
- 39
We suggest the convenience of performing the nocturnal APD session in the supine position (opinion). Not graduated (question 16).
- 40
The use of solutions having concentration of glucose exceeding 1.36% or 1.5% dextrose improve the removal of low molecular weight solutes by increasing ultrafiltration (opinion). Not graduated (question 16).
- 41
We recommend using solutions having a higher concentration of glucose as a means by which to enhance ultrafiltration during nocturnal sessions in APD (1B) (question 17).
- 42
For faster than mean transporters, we suggest increasing the number of exchanges, with a consequent decrease in dwell times, as a mean to increase ultrafiltration during the nighttime sessions in APD (2B) (question 17).
- 43
We recommend that daytime APD exchanges with icodextrin-based solution, with the aim of increasing ultrafiltration (1A) (question 18).
- 44
We recommend daytime APD exchanges with icodextrin-based solution, with the aim of increasing sodium removal (1A) (question 18).
- 45
We recommend supplementary daytime exchanges, with the aim of optimising solute clearance, ultrafiltration, and sodium removal, in appropriate cases (1A) (question 18).
- 46
We suggest adopting the measures needed to prevent overfilling of the abdominal cavity during nocturnal tidal APD sessions (opinion). Not graduated (question 19).
- 47
This committee issues no recommendation as to the routine use of the adapted APD technique to optimise solute clearance, ultrafiltration, or sodium removal (opinion). Not graduated (question 20).
- 48
We suggest that APD can offer an advantage over CAPD, in terms of patient survival, in fast transporters (2B) (question 21).
- 49
We recommend initiating PD with either CAPD or APD, depending on patient preference, needs, and resources of the centre (1C) (question 22).
- 50
We suggest considering early start of APD in fast transporters, particularly if there is little RKF (2B) (question 22).
- 51
We suggest that APD offers no general advantages over CAPD as a technique of continuation therapy (2C) (question 23).
- 52
We suggest that continuation with APD be indicated based on the need to adjust the dose of dialysis, ultrafiltration, reconciliation of work and social life balance, quality of life, and abdominal wall issues (2B) (question 23).
- 53
We recommend not using APD rather than CAPD with the aim of reducing the rate of peritoneal infections (1C) (question 23).
This paper is part of the supplement entitled «Clinical guideline of adequacy and prescription of peritoneal dialysis », which has been funded by the Spanish Society of Nephrology, with the sponsorship of Fresenius Medical Care and Vantive.
Conflict of interestsThe authors have no conflict of interests to declare.