Dear Editor:
Peritonitis is the principal cause of peritoneal dialysis (PD) catheter loss and the primary reason why patients switch from PD to hemodialysis1. It causes death in 6% of patients, particularly when it is caused by Staphylococcus aureus, enteric organisms and fungus2.
Prompt initiation of antibiotics is critical and they should be started as soon as a cloudy effluent is seen, even without confirmation of the cell count from laboratory3. Guidelines recommend empirical treatment with an association of vancomycin or a cephalosporin with aminoglycoside or third-generation cephalosporin3.
Chemical peritonitis, described as peritoneal inflammation caused by a non-infectious agent (such antibiotics and dialysis solutions) is a rarer condition.
Chemical peritonitis induced by vancomycin was first described in 19864 and near 90 similar cases were reported in the 80-90th decade5,6. Since then no other case was noted.
Icodextrin-induced peritonitis has first described in 19997, but its prevalence is not clear. An epidemic outbreak occurred in Europe in 2002, related to solution contamination8. Few cases were reported after improving manufacturing process, all related to “sensibilization” during that period or to other contaminations8.
We reported a case of chemical peritonitis in a patient treated with icodextrin and intraperitoneal vancomycin, in which vancomycin seems to be the offending agent.
A 34-year-old man, with renal failure secondary to diabetic nephropathy, was in PD since 2006. Icodextrin was introduced one year after PD initiation. No peritonitis episodes were detected in the following years.
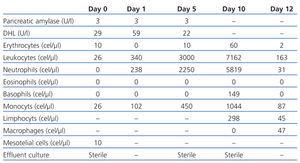
In 2009, he came to hospital with mild abdominal pain with four hours of evolution. He hadn’t other symptoms, exit-site hadn’t inflammatory signs and effluent was clear. Effluent analysis revealed 26 cells/µl (table 1), abdominal radiography and ultrasound were normal.
Intraperitoneal vancomycin (2 g each 5 days) and ceftazidime (1 g each day) were administrated and patient was discarried, maintaining icodextrin.
In the next day, he returned with cloudy effluent (table 1). The same treatment was maintained and cloudy effluent disappeared in the two following days.
At the 5th day, he was asymptomatic and came for second vancomycin administration. Latter in that day, abdominal pain and cloudy effluent reappeared (table 1).
PD was suspended and hemodialysis was started. Vancomycin and ceftazidime were switch to intravenous route and an extra daily dose of intraperitoneal ceftazidime (500 mg) was maintained. He became asymptomatic and cloudy effluent disappeared in the following two days. All cultures, including fungus and Mycobacterium tuberculosis were sterile.
At the 9th day, he reassumed PD (with icodextrin) and at 10th day intraperitoneal vancomycin was delivered. Cloudy effluent reappeared after vancomycin administration. At 12th day, he was asymptomatic and discarried (table 1).
Three months later, he remains asymptomatic, with preserved ultrafiltration and clean effluent.
Patients with peritonitis usually present with cloudy fluid and abdominal pain. Effluent’s leukocytes superior to 100/ml (with 50% polymophonuclear cells) indicate the presence of inflammation, with infectious peritonitis being the most likely cause3. However, in short dwell time leukocytes may not reach 100/ml3 and peritonitis may present with abdominal pain and no cloudy effluent3.
In our patient, other causes of abdominal pain such as gastroenteritis, pancreatitis, appendicitis or pneumoperitoneum were excluded and empirical treatment for infectious peritonitis was started. The cloudy effluent present in the following day was assumed to be a late expression of peritonitis in fluid of a longer dwell time.
Abdominal pain and cloudy effluent reappeared after second vancomycin administration and he was admitted with suspicion of refractory peritonitis. If the suspicion was confirmed, peritoneal catheter should be removed and patient switched to hemodialysis9. However, he didn’t present the typical evolution of a refractory infectious peritonitis and other causes of cloudy effluent, such as hemoperitoneum, malignancy, eosinophilic and chylous effluent were excluded10. Chemical peritonitis related to icodextrin or vancomycin remained a plausible diagnosis5,6.
Icodextrin induced-peritonitis seems to be caused by contamination of solution by peptidoglycans released from bacteria (Alicylobacillus acidocaldarius) during the manufacturing process8. Improvement in the process decreased its frequency from a peak of 0.912% in 2002 to 0.013% in 20038.
Patients present with mild abdominal pain and cloudy effluent, without rebound, fever or rash11,12. Effluent leukocytes vary from 100 to 6,000/µl11,13, with mononuclear predominance11. Culture is always sterile11.
Delay between the initiation of the icodextrin and first symptoms varies from few hours to several years7,12. Clinical course is undulating, with intermittent pain and dialysate cloudiness after each icodextrin dwell, without response to antibiotics12. Discontinuation of icodextrin leads to relief of the symptoms and normalization of leukocytes within 24-48 hours, but relapse is invariably induced after rechallenge12.
In our patient, neither temporal relation with icodextrin administration was detected nor was relapse noted after rechallenge.
A temporal relation with the vancomycin administration supported the diagnosis of vancomycin-induced chemical peritonitis.
The clinical presentation ranges from cloudy effluent alone to severe abdominal pain and fever. It begins 2-12 hours after vancomycin administration5 and resolves within 3 to 4 days after suspension6. There is a predominantly of neutrophils, with eosinophils ranging from 0-10%5,6.
The reported incidence of vancomicyn (Vancoled®)-induced peritonitis was 23%6. The underlying mechanism is unknown5,14. Some patients experience recurrence of abdominal pain and/or effluent leukocytes elevations on re-exposure to intraperitoneal vancomycin, without complains when intravenous or intraperitoneal vancomycin from another manufacturer’s brand is administrated5. These results support the suspicion that inflammation is not completely due to vancomycin itself but to another constituent of its preparation14,15.
Vancomycin include 5.2-16.7% impurities, depending both on the brand and the lot of the preparation14,15. The varying amount of impurities present in individual lots may determine whether inflammatory reaction occurs6.
No fatalities were reported6,7 and no treatment is recommended except for suspension of offending agent7.
Although it is clinically benign with spontaneous resolution, the long-term sequelae are still unknown. Moreover, it could be confused with infectious peritonitis and lead to unnecessary antibiotic prescription or to catheter removal and PD suspension7.
In last 15 years, none case of vancomycin-induced peritonitis was reported, maybe due to progressive improvement in purifications. In a time where generic preparations are in increasing use, our case may alert physicians to the presence of this forgotten adverse effect.
Table 1. Evolution of effluent characteristics