Acute kidney injury (AKI) is common among hospitalized patients with COVID-19 and associated with worse prognosis. The Spanish Society of Nephrology created the AKI- COVID Registry to characterize the population admitted for COVID-19 that developed AKI in Spanish hospitals. The need of renal replacement therapy (RRT) therapeutic modalities, and mortality in these patients were assessed
Material and methodIn a retrospective study, we analyzed data from the AKI-COVID Registry, which included patients hospitalized in 30 Spanish hospitals from May 2020 to November 2021. Clinical and demographic variables, factors related to the severity of COVID-19 and AKI, and survival data were recorded. A multivariate regression analysis was performed to study factors related to RRT and mortality.
ResultsData from 730 patients were recorded. A total of 71.9% were men, with a mean age of 70 years (60–78), 70.1% were hypertensive, 32.9% diabetic, 33.3% with cardiovascular disease and 23.9% had some degree of chronic kidney disease (CKD). Pneumonia was diagnosed in 94.6%, requiring ventilatory support in 54.2% and admission to the ICU in 44.1% of cases.
The median time from the onset of COVID-19 symptoms to the appearance of AKI (37.1% KDIGO I, 18.3% KDIGO II, 44.6% KDIGO III) was 6 days (4–10). A total of 235 (33.9%) patients required RRT: 155 patients with continuous renal replacement therapy, 89 alternate-day dialysis, 36 daily dialysis, 24 extended hemodialysis and 17 patients with hemodiafiltration. Smoking habit (OR 3.41), ventilatory support (OR 20.2), maximum creatinine value (OR 2.41), and time to AKI onset (OR 1.13) were predictors of the need for RRT; age was a protective factor (0.95). The group without RRT was characterized by older age, less severe AKI, and shorter kidney injury onset and recovery time (p < 0.05). 38.6% of patients died during hospitalization; serious AKI and RRT were more frequent in the death group. In the multivariate analysis, age (OR 1.03), previous chronic kidney disease (OR 2.21), development of pneumonia (OR 2.89), ventilatory support (OR 3.34) and RRT (OR 2.28) were predictors of mortality while chronic treatment with ARBs was identified as a protective factor (OR 0.55).
ConclusionsPatients with AKI during hospitalization for COVID-19 had a high mean age, comorbidities and severe infection. We defined two different clinical patterns: an AKI of early onset, in older patients that resolves in a few days without the need for RRT; and another more severe pattern, with greater need for RRT, and late onset, which was related to greater severity of the infectious disease. The severity of the infection, age and the presence of CKD prior to admission were identified as a risk factors for mortality in these patients. In addition chronic treatment with ARBs was identified as a protective factor for mortality
El desarrollo de lesión renal aguda (FRA) durante la hospitalización por SARS-COv2 se ha asociado a elevada morbi-mortalidad. El Registro FRA-COVID 19 SEN ha recogido datos de pacientes con este perfil durante los meses de la pandemia. El objetivo de este trabajo fue caracterizar la población española ingresada por COVID-19 que desarrolló FRA, con/ sin necesidades de tratamiento renal sustitutivo (TRS), modalidades terapéuticas utilizadas y resultados en términos de mortalidad
Material y métodoEstudio retrospectivo de datos procedentes de 30 hospitales españoles, recopilados en el Registro Español FRA COVID-19 desde mayo de 2020 hasta noviembre 2021. Se registraron variables clínicas y demográficas, datos relacionados con la gravedad de la COVID-19, con el FRA y de supervivencia. Mediante estudio de regresión multivariante se analizan los factores relacionados con la necesidad de TRS y con mortalidad.
ResultadosSe registraron datos de 730 pacientes. El 71,9% eran hombres, con edad media 70 años (60–78). El 70,1% eran hipertensos, 32,9% diabéticos, 33,3% con enfermedad cardiovascular y 23,9% presentaban algún grado de enfermedad renal crónica. Desarrollaron neumonía el 94,6%, con necesidad de soporte ventilatorio en el 54,2% e ingreso en UCI en un 44,1% de los casos.
La mediana de tiempo desde el inicio de síntomas COVID hasta la aparición de FRA fue de 6 días (4–10). En cuanto a la gravedad: 37,1% KDIGO I, 18,3% KDIGO II, 44,6% KDIGO III; requirieron TRS 235 pacientes (33,9%). Las técnicas continuas (TCRR) fueron las más empleadas (155 pac), seguidas de hemodiálisis (HD) intermitente (89 pac), HD diaria (36 pacientes), hemodiafiltración (HDF) 17 pac., y HD expandida (HDexp) en 24 casos. El hábito tabáquico (OR 3,41), la necesidad de soporte ventilatorio (OR 20,2), la cifra de creatinina máxima (OR 2,41) y el tiempo transcurrido desde el inicio de los síntomas hasta la aparición del FRA (OR 1,13) se identificaron como predictores de la necesidad de TRS en nuestra cohorte, mientras la edad se comporta como protector (OR: 0,95). El grupo que no requirió TRS se caracterizó por presentar mayor edad, menor gravedad de FRA y un tiempo de aparición y recuperación de la lesión renal más corto (p < 0,05).
El 38,6% de la cohorte falleció durante la hospitalización. La gravedad del FRA y la necesidad de TRS fue más frecuente en el grupo de fallecidos. En el análisis multivariante, edad (OR 1.03), enfermedad renal crónica previa (OR 2,21), desarrollo de neumonía (OR 2,89), soporte ventilatorio (OR 3,34) y TRS (OR: 2,28) se comportaron como factores predictores mientras que el tratamiento crónico con ARAII se comportó como factor protector (OR 0,55).
ConclusionesDel análisis del Registro FRA-COVID 19 SEN se deduce que los pacientes que presentaron FRA durante la hospitalización por COVID-19 tenían una edad media elevada, mayores comorbilidades y presentaron un cuadro de infección grave. Definimos dos patrones clínicos diferentes: un FRA de aparición precoz, en pacientes más ancianos que se resuelve en pocos días sin necesidad de TRS; y otro patrón más grave, con mayor requerimiento de TRS, y aparición tardía en el curso de la enfermedad, que se relacionó con mayor gravedad de la misma. La gravedad de la infección, la edad y la presencia de ERC previa al ingreso fueron factores determinantes en la mortalidad de estos pacientes, identificando el tratamiento crónico con ARA II como un factor protector de mortalidad
Coronavirus disease (COVID-19) has become one of the worst pandemics in recent human history, with dramatic short- and long-term clinical outcomes, more than 250 million people infected, and more than 5 million deaths around the world.1 Over time, the international scientific community has made an unprecedented effort in modern history to understand better the nature and pathophysiology of this disease.
The presentation of COVID-19 varies considerably. Although it manifests primarily as a respiratory disease, it exacerbates and progresses in some individuals due to abnormal immune/inflammatory responses with generalised endothelial damage and alterations in blood coagulation, generating a much more serious spectrum of the disease.2 A substantial proportion of patients with severe COVID-19 show signs of kidney damage. Clinically, renal involvement ranges from mild/moderate proteinuria and haematuria up to acute kidney injury (AKI), with the need for renal replacement therapy (RRT) in some cases. The pathophysiological mechanisms of kidney damage in patients with COVID-19 remain unclear, but they are known to be multifactorial. Current knowledge implies direct effects dependent on SARS-CoV-2 on renal cells (tubular epithelial cells and podocytes) and indirect effects through the systemic impact of the viral infection secondary to critical lung disease and secondary to the management of the disease itself.2,3 Kidney involvement is a determinant factor in higher in-hospital mortality in all published series.4–7
The reported incidence of AKI is highly variable, ranging between 0.5 and 56% depending on the series.8 The population studied, and the region of the world from which the series originates directly impact these differences between the published data. The initial cohorts of Asian origin reported an incidence lower than 7%9,10 while Western series indicate an incidence between 11 and 46%,4–6 reaching up to 76–80% in patients admitted to the ICU.11–13 The factors related to the development of kidney injury during COVID-19 also differ widely between publications, having been related to demographic characteristics (age over 60 years, male gender or African descent), comorbid situations (arterial hypertension (HBP), diabetes mellitus (DM), cardiovascular disease (CVD), obesity, chronic obstructive pulmonary disease (COPD) or chronic kidney disease (CKD), as well as the severity of the patient's condition (haemodynamic instability or need for mechanical ventilation).8,11–15
The mainstay of treatment continues to be the prevention of kidney damage and, if necessary, RRT. In this aspect, the literature reports large fluctuations in the frequency of the need for RRT, ranging from 0.8% to 31%.8 At the time, many doubts arose about the approach to RRT in these patients: whether it should be approached in the same way as in other acute processes or differently, whether any modality of RRT could be superior for the elimination of cytokines, whether adsorptive therapy, repeatedly postulated in the treatment of sepsis, could provide advantages, or whether low-efficiency continuous therapies could be more efficient. Over the course of the pandemic, numerous studies aimed to address concerns that have arisen in the nephrology community in different aspects. However, drawing meaningful conclusions from these studies has often been difficult due to barriers posed by data limitations and the use of uncertain methodology that carries numerous biases.
Since the beginning of the pandemic, the Spanish Society of Nephrology (SEN) was very sensitive to the situation, actively participating in different initiatives that helped in clinical decision-making. The SEN has repeatedly highlighted that kidney injury can be a severe complication of COVID-19, highlighting the importance of providing more in-depth knowledge to this entity, as well as establishing the necessary interventions to improve the prognosis of patients with COVID-19. In this context, the SEN COVID-AKI Registry emerged, where clinical data of patients with AKI secondary to COVID-19 were collected from May 2020 to November 2021, including variables related to SARS-Cov-2 infection, kidney involvement and the need for RRT in our country. A committee of SEN experts chose a minimum set of variables that would give us a perspective of the pandemic's impact and the need for RRT in acute patients throughout our country. Given the large discrepancies found in the available studies, the exploitation of the SEN COVID-AKI Registry represents an opportunity to characterise patients with this injury in our environment within the context of the great heterogeneity of the published cohorts, as well as to know how these patients were managed in our environment, identify the profile of patients who have required RRT, the replacement treatment modalities used and the results obtained in terms of recovery of kidney function and mortality.
Material and methodThis is a retrospective observational cohort study in which all data collected in the Spanish COVID-AKI Registry from May 2020 to November 2021 was included. The COVID-AKI Registry presented an online structure with access through a website (SEN website: www.senefro.org) where the members of this society, after identifying themselves through a previously registered user, were able to add hospitalised patients with AKI and SARS-CoV-2 infection confirmed by real-time polymerase chain reaction.
The study complied with the guidelines established by the Declaration of Helsinki and the Declaration of Istanbul. The research was approved by the Regional Institutional Ethics Committee of Andalusia. Obtaining informed consent was not necessary given the retrospective nature of the study.
ObjectivesThe objectives of this work were to characterise the Spanish population admitted for COVID-19 that has developed AKI, identify the profile of patients who have required RRT, as well as the replacement treatment modalities used in our environment and the results obtained in terms of kidney function recovery and mortality.
Variables included in the registryThe recorded variables can be classified into the following subgroups:
- none-
Demographic and clinical variables at the time of admission: sex, age, smoking habit, comorbidity (HBP, DM, CVD, CKD, history of oncology, obesity, COPD and immunosuppression), home treatment prior to admission with enzyme-converting angiotensin converting enzyme inhibitors (ACEI) and/or angiotensin receptor antagonists (ARB).
- none-
Clinical presentation of COVID-19: “typical clinical presentation” defined as the presence of respiratory symptoms (dyspnoea, cough, expectoration and rhinorrhoea). The detection of other symptoms was defined as atypical clinical presentation.
- none-
Variables related to the severity of the infection: development of pneumonia, need for ventilatory support, admission to the intensive care unit (ICU), APACHE II score upon admission to the ICU, need for mechanical ventilation and use of extracorporeal membrane oxygenation (ECMO).
- none-
Variables related to acute kidney injury: maximum creatinine, AKI KDIGO classification, use of radiological contrast, need for RRT (time in days and therapeutic modality), presence of albuminuria and/or haematuria during admission (not quantified, only presence or absence was recorded in some of the follow-up tests during admission)
- none-
The patients were treated according to the standard of care in each centre, so therapeutic decisions, as well as the performance of renal replacement therapy, were made based on the assessement of the nephrologist and infectious disease specialists responsible for the patient.
- none-
Analytical parameters on the seventh day after the onset of COVID-19 symptoms: lymphocytes (absolute number), CRP (mg/l), D-dimer (ng/mL), interleukin-6 (pg/mL) and ferritin (ng/mL).
- none-
Time (days) from the onset of symptoms to the diagnosis of AKI and time (days) to recovery of kidney function.
- none-
Condition of patients at the end of hospitalisation: death during admission, recovery of renal function or permanence on RRT upon discharge.
To define AKI, as well as to establish the severity of the episode, the Kidney Disease: Improving Global Outcomes (KDIGO) criteria were used. AKI was defined as the increase in serum creatinine concentration ≥0.3 mg/dl (26.5 μmol/l) during an interval of 48 h or increase ≥1.5 times in the last 7 days, or diuresis <0.5 ml/kg/h for 6 h; and its severity was categorised according to KDIGO stages I, II and III.16
Recovery of baseline creatinine prior to admission or exit from RRT in a patient who would have required replacement therapies was considered recovery from AKI.
Chronic kidney disease was defined as estimated glomerular filtration rate <60 ml/min/1.73 m2, corresponding to a G3 stage or higher according to the KDIGO classification.17 The highest creatinine value and lowest eGFR (CKD EPI) recorded in the patient's history during the year prior to admission were considered baseline renal function.
Statistical analysisA descriptive statistical analysis was performed, and quantitative data were summarised with measures of central tendency: mean and standard deviation, or median and interquartile range for asymmetrical data distributions. For categorical variables, frequencies and percentages were used. For the bivariate analysis between categorical variables, the Chi square test or Fisher's exact test was used as appropriate. For quantitative variables, Student's "t" test or the Mann–Whitney U test was used, depending on whether distributions were normal or not normal, respectively. 2 multivariate regression models were conducted. Binomial logistic regression technique was used, backward stepwise and adjusted by the Wald statistic to study the variables independently related to mortality during hospital admission and the need for renal replacement treatment. The goodness of fit of the model was checked with Hosmer–Lemeshow statistics, and those variables that were clinically relevant and those that presented significant differences with the p-value associated with the test were considered <0.05 in the previous bivariate analysis.
For all hypothesis tests, an alpha error of 0.05 was considered the limit of statistical significance. Data analysis was performed with the IBM SPSS statistical package, version 20.0.
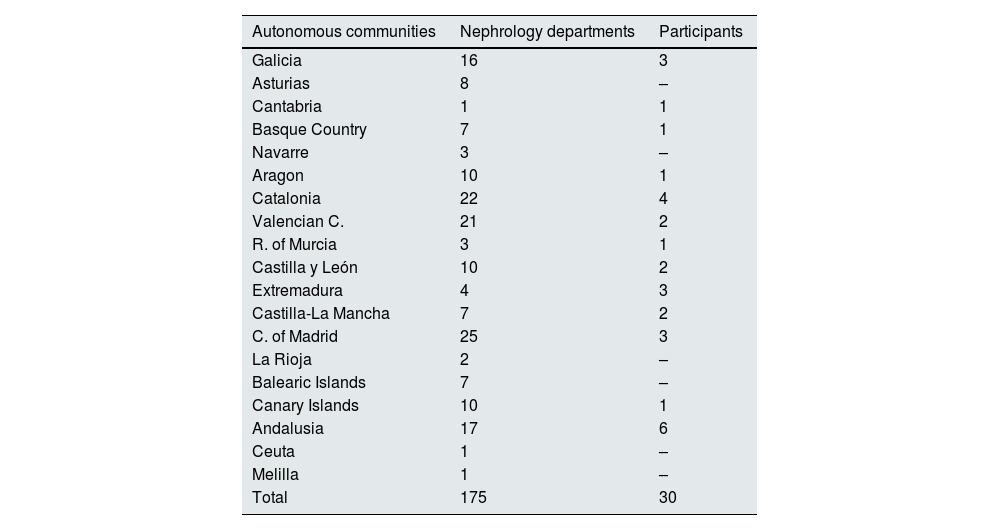
ResultsData were collected from 730 patients with documented SARS-CoV-2 infection, who were hospitalised as a consequence of COVID-19 and who developed an episode of AKI during admission. The patients came from 30 hospitals distributed throughout Spain. In Appendix B, Annexes 1 and 2 detail all the nephrology units that reported cases, as well as the geographic coverage by the autonomous community.
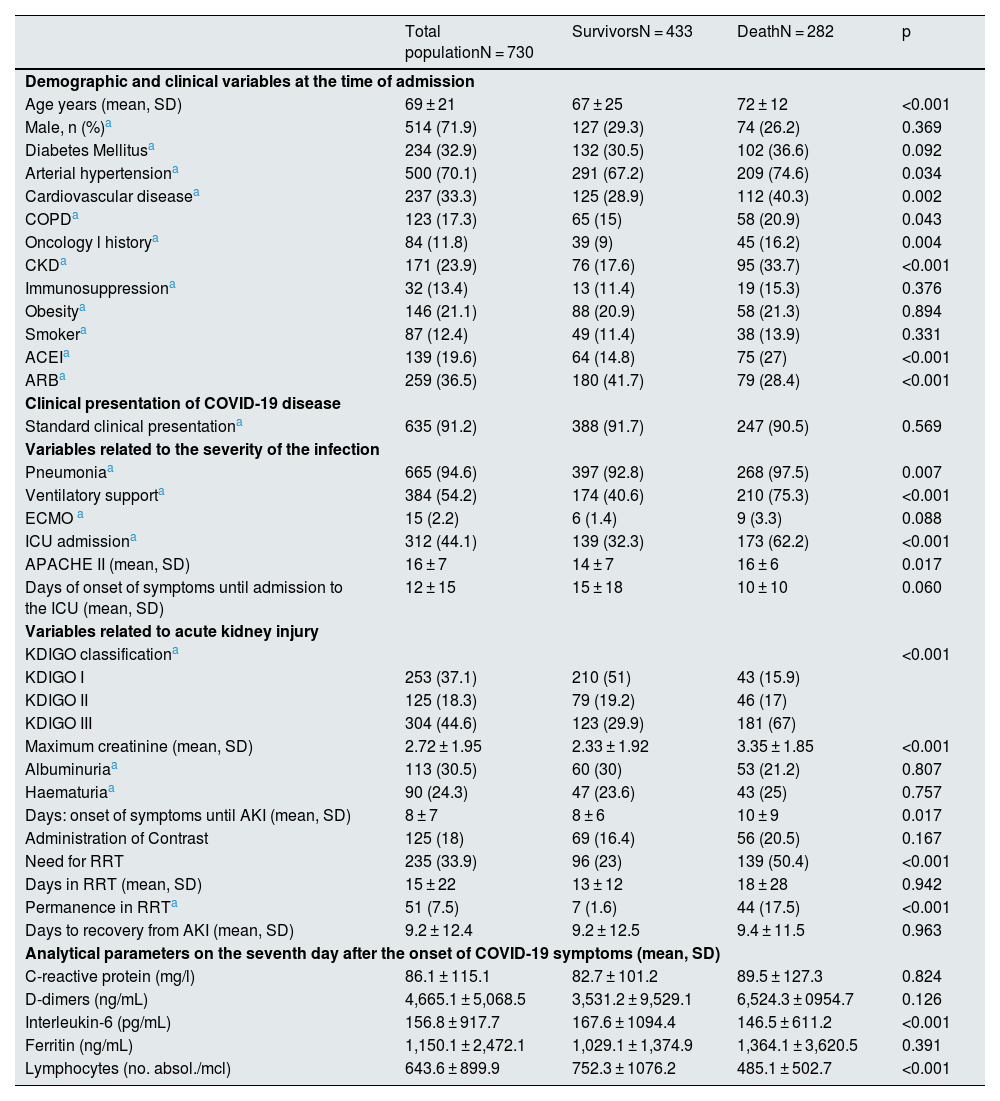
The average age of these patients was 69 years, 71.9% were men, 70.1% had hypertension, 32.9% had diabetes, 33.3% had a history of CVD, 11.8% had a history of oncology, 17.3% COPD, 13.4% immunosuppressed, 21.1% obese, and 12.4% were smokers. 23.9% of the patients (171 patients) had some degree of CKD prior to admission (Table 1). Most patients with CKD had stage G3 (135 patients), with advanced stages of disease being less common (G4: 30 patients; G5: 6 patients).
Baseline characteristics of the patients included in the study, variables related to COVID-19 infection and acute kidney injury.
| Total populationN = 730 | SurvivorsN = 433 | DeathN = 282 | p | |
|---|---|---|---|---|
| Demographic and clinical variables at the time of admission | ||||
| Age years (mean, SD) | 69 ± 21 | 67 ± 25 | 72 ± 12 | <0.001 |
| Male, n (%)a | 514 (71.9) | 127 (29.3) | 74 (26.2) | 0.369 |
| Diabetes Mellitusa | 234 (32.9) | 132 (30.5) | 102 (36.6) | 0.092 |
| Arterial hypertensiona | 500 (70.1) | 291 (67.2) | 209 (74.6) | 0.034 |
| Cardiovascular diseasea | 237 (33.3) | 125 (28.9) | 112 (40.3) | 0.002 |
| COPDa | 123 (17.3) | 65 (15) | 58 (20.9) | 0.043 |
| Oncology l historya | 84 (11.8) | 39 (9) | 45 (16.2) | 0.004 |
| CKDa | 171 (23.9) | 76 (17.6) | 95 (33.7) | <0.001 |
| Immunosuppressiona | 32 (13.4) | 13 (11.4) | 19 (15.3) | 0.376 |
| Obesitya | 146 (21.1) | 88 (20.9) | 58 (21.3) | 0.894 |
| Smokera | 87 (12.4) | 49 (11.4) | 38 (13.9) | 0.331 |
| ACEIa | 139 (19.6) | 64 (14.8) | 75 (27) | <0.001 |
| ARBa | 259 (36.5) | 180 (41.7) | 79 (28.4) | <0.001 |
| Clinical presentation of COVID-19 disease | ||||
| Standard clinical presentationa | 635 (91.2) | 388 (91.7) | 247 (90.5) | 0.569 |
| Variables related to the severity of the infection | ||||
| Pneumoniaa | 665 (94.6) | 397 (92.8) | 268 (97.5) | 0.007 |
| Ventilatory supporta | 384 (54.2) | 174 (40.6) | 210 (75.3) | <0.001 |
| ECMO a | 15 (2.2) | 6 (1.4) | 9 (3.3) | 0.088 |
| ICU admissiona | 312 (44.1) | 139 (32.3) | 173 (62.2) | <0.001 |
| APACHE II (mean, SD) | 16 ± 7 | 14 ± 7 | 16 ± 6 | 0.017 |
| Days of onset of symptoms until admission to the ICU (mean, SD) | 12 ± 15 | 15 ± 18 | 10 ± 10 | 0.060 |
| Variables related to acute kidney injury | ||||
| KDIGO classificationa | <0.001 | |||
| KDIGO I | 253 (37.1) | 210 (51) | 43 (15.9) | |
| KDIGO II | 125 (18.3) | 79 (19.2) | 46 (17) | |
| KDIGO III | 304 (44.6) | 123 (29.9) | 181 (67) | |
| Maximum creatinine (mean, SD) | 2.72 ± 1.95 | 2.33 ± 1.92 | 3.35 ± 1.85 | <0.001 |
| Albuminuriaa | 113 (30.5) | 60 (30) | 53 (21.2) | 0.807 |
| Haematuriaa | 90 (24.3) | 47 (23.6) | 43 (25) | 0.757 |
| Days: onset of symptoms until AKI (mean, SD) | 8 ± 7 | 8 ± 6 | 10 ± 9 | 0.017 |
| Administration of Contrast | 125 (18) | 69 (16.4) | 56 (20.5) | 0.167 |
| Need for RRT | 235 (33.9) | 96 (23) | 139 (50.4) | <0.001 |
| Days in RRT (mean, SD) | 15 ± 22 | 13 ± 12 | 18 ± 28 | 0.942 |
| Permanence in RRTa | 51 (7.5) | 7 (1.6) | 44 (17.5) | <0.001 |
| Days to recovery from AKI (mean, SD) | 9.2 ± 12.4 | 9.2 ± 12.5 | 9.4 ± 11.5 | 0.963 |
| Analytical parameters on the seventh day after the onset of COVID-19 symptoms (mean, SD) | ||||
| C-reactive protein (mg/l) | 86.1 ± 115.1 | 82.7 ± 101.2 | 89.5 ± 127.3 | 0.824 |
| D-dimers (ng/mL) | 4,665.1 ± 5,068.5 | 3,531.2 ± 9,529.1 | 6,524.3 ± 0954.7 | 0.126 |
| Interleukin-6 (pg/mL) | 156.8 ± 917.7 | 167.6 ± 1094.4 | 146.5 ± 611.2 | <0.001 |
| Ferritin (ng/mL) | 1,150.1 ± 2,472.1 | 1,029.1 ± 1,374.9 | 1,364.1 ± 3,620.5 | 0.391 |
| Lymphocytes (no. absol./mcl) | 643.6 ± 899.9 | 752.3 ± 1076.2 | 485.1 ± 502.7 | <0.001 |
Explanatory note: 240 patients require RRT. In the table, 235 appear due to 5 missing values (cases with the RRT variable, but not the death variable). Number of patients with C-reactive protein determinations 406, D-dimers 632; Interleukin 6 506, ferritin 646 and lymphocytes 701.
ARB: angiotensin II receptor antagonist drugs; SD: standard deviation; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; AKI: acute renal failure; ACEI: angiotensin-aldosterone inhibitors; RRT: renal replacement therapy; ICU: intensive care unit.
In 91.2% of the cases, the form of presentation of the infection was typical of respiratory semiology, and up to 94.6% of the reported patients developed pneumonia. Three hundred eighty-four patients (54.2%) required ventilatory support, 312 (44.1%) were admitted to the ICU and 15 required ECMO. The median time from the onset of COVID symptoms until the patient required admission to the ICU was 8 days (IQR: 5–12).
A 44.6% of the reported cases developed KDIGO III, followed in frequency by KDIGO I, which was reported in 37.1% of the cases. The remaining 18.3% presented grade II. Renal biopsy was performed in only 4 patients, so the data are not included in this study. At the time of admission, there is only data regarding urinary parameters in approximately half of the cases (373 for albuminuria and 374 for haematuria), with the presence of albuminuria positive in 30.5% of the cases collected (113 patients) and haematuria in 24.3% (90 patients).
The analytical values 7 days after the onset of symptoms are shown in Table 1, presenting very high mean levels, above the normal range in all the parameters analysed (CPR; D-dimers, IL6, ferritin), as well as lymphopaenia.
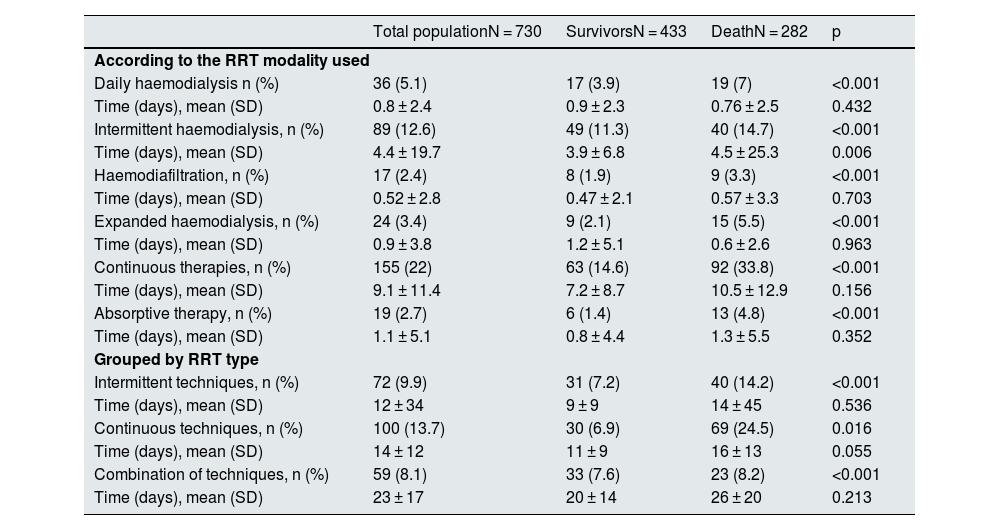
Renal replacement therapy240 patients required RRT during admission. The therapeutic modalities used were daily haemodialysis (HD) in 36 patients, intermittent HD in 89 patients, haemodiafiltration (HDF) in 17 patients, expanded haemodialysis (HDexp) in 24 cases, and continuous therapies (CRRT) in 155 patients (Table 2). In many cases, more than one therapy was used in the same patient: CRRT and intermittent HD were combined in 49 patients, CRRT and daily HD in 16 patients, HDexp and CRRT in 6 patients, 11 patients combined intermittent and daily HD, 6 patients HD intermittent and HDF, and finally in 14 patients intermittent HD and HDexp were combined. The average time of RRT was 15 ± 22 days.
Modalities of renal replacement treatment: clinical outcome.
| Total populationN = 730 | SurvivorsN = 433 | DeathN = 282 | p | |
|---|---|---|---|---|
| According to the RRT modality used | ||||
| Daily haemodialysis n (%) | 36 (5.1) | 17 (3.9) | 19 (7) | <0.001 |
| Time (days), mean (SD) | 0.8 ± 2.4 | 0.9 ± 2.3 | 0.76 ± 2.5 | 0.432 |
| Intermittent haemodialysis, n (%) | 89 (12.6) | 49 (11.3) | 40 (14.7) | <0.001 |
| Time (days), mean (SD) | 4.4 ± 19.7 | 3.9 ± 6.8 | 4.5 ± 25.3 | 0.006 |
| Haemodiafiltration, n (%) | 17 (2.4) | 8 (1.9) | 9 (3.3) | <0.001 |
| Time (days), mean (SD) | 0.52 ± 2.8 | 0.47 ± 2.1 | 0.57 ± 3.3 | 0.703 |
| Expanded haemodialysis, n (%) | 24 (3.4) | 9 (2.1) | 15 (5.5) | <0.001 |
| Time (days), mean (SD) | 0.9 ± 3.8 | 1.2 ± 5.1 | 0.6 ± 2.6 | 0.963 |
| Continuous therapies, n (%) | 155 (22) | 63 (14.6) | 92 (33.8) | <0.001 |
| Time (days), mean (SD) | 9.1 ± 11.4 | 7.2 ± 8.7 | 10.5 ± 12.9 | 0.156 |
| Absorptive therapy, n (%) | 19 (2.7) | 6 (1.4) | 13 (4.8) | <0.001 |
| Time (days), mean (SD) | 1.1 ± 5.1 | 0.8 ± 4.4 | 1.3 ± 5.5 | 0.352 |
| Grouped by RRT type | ||||
| Intermittent techniques, n (%) | 72 (9.9) | 31 (7.2) | 40 (14.2) | <0.001 |
| Time (days), mean (SD) | 12 ± 34 | 9 ± 9 | 14 ± 45 | 0.536 |
| Continuous techniques, n (%) | 100 (13.7) | 30 (6.9) | 69 (24.5) | 0.016 |
| Time (days), mean (SD) | 14 ± 12 | 11 ± 9 | 16 ± 13 | 0.055 |
| Combination of techniques, n (%) | 59 (8.1) | 33 (7.6) | 23 (8.2) | <0.001 |
| Time (days), mean (SD) | 23 ± 17 | 20 ± 14 | 26 ± 20 | 0.213 |
(%): percentage per column; SD: standard deviation.
There were 19 cases reported in which adsorptive therapies were used. In 18 of these, this therapeutic alternative was combined with CRRT.
Table 2 shows the data on survival and treatment time in each of the modalities, independently, considering in each modality the patients who had used each of them (Table 2 ["According to the RRT modality used"]), and grouped according to whether intermittent, continuous or mixed therapy was used (Table 2 ["Grouped by type of RRT"]). It is confirmed that the percentage of patients who required RRT was significantly higher in the group of deceased patients in all therapeutic modalities and groupings.
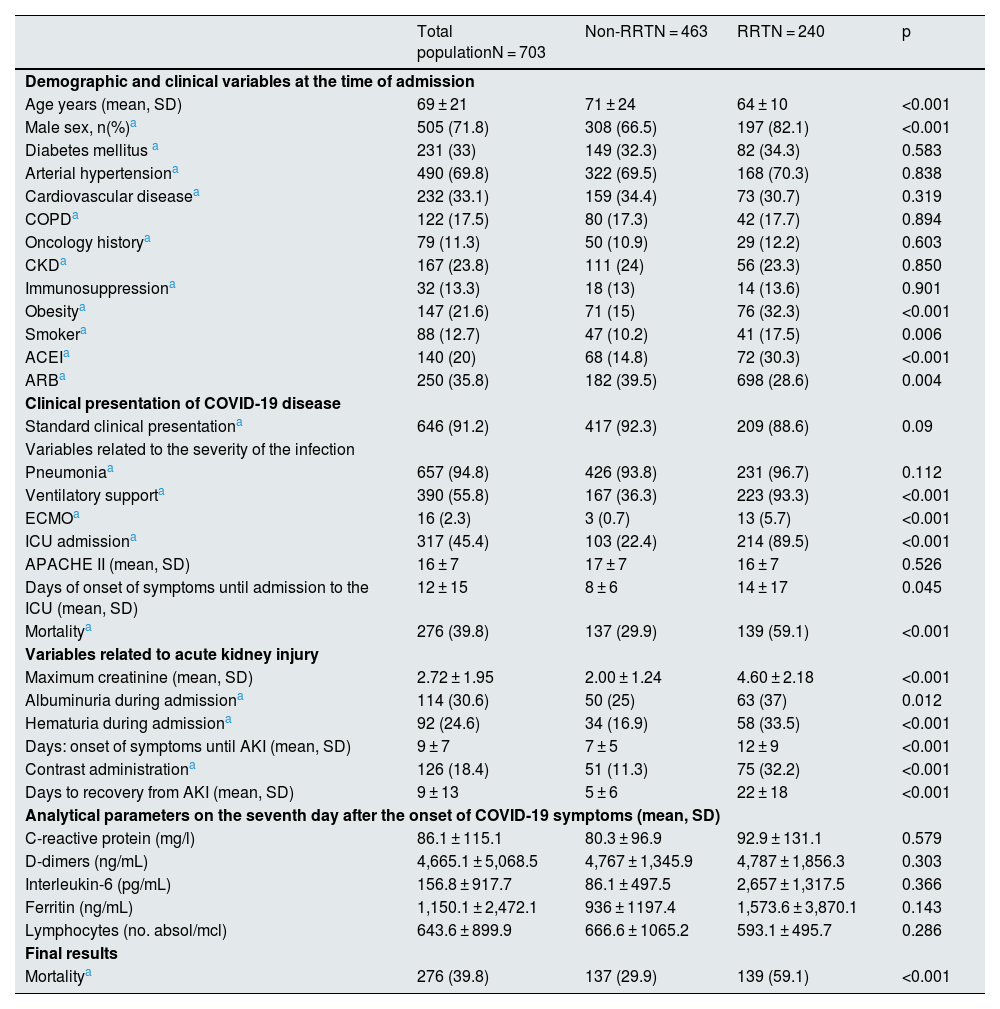
The patients who required RRT were characterised by being mostly male and younger than the group that did not require treatment. We did not find significant differences regarding the prevalence of comorbidities between both groups, except for obesity and a history of smoking, which were more frequent among patients who required dialysis (32.3% vs. 15.9% and 17.5% vs. 10.2%, respectively). The previous degree of renal dysfunction was not related to the need for RRT (Table 3).
Baseline characteristics of the patients, variables related to COVID-19 infection, and acute kidney injury according to the need for renal replacement treatment.
| Total populationN = 703 | Non-RRTN = 463 | RRTN = 240 | p | |
|---|---|---|---|---|
| Demographic and clinical variables at the time of admission | ||||
| Age years (mean, SD) | 69 ± 21 | 71 ± 24 | 64 ± 10 | <0.001 |
| Male sex, n(%)a | 505 (71.8) | 308 (66.5) | 197 (82.1) | <0.001 |
| Diabetes mellitus a | 231 (33) | 149 (32.3) | 82 (34.3) | 0.583 |
| Arterial hypertensiona | 490 (69.8) | 322 (69.5) | 168 (70.3) | 0.838 |
| Cardiovascular diseasea | 232 (33.1) | 159 (34.4) | 73 (30.7) | 0.319 |
| COPDa | 122 (17.5) | 80 (17.3) | 42 (17.7) | 0.894 |
| Oncology historya | 79 (11.3) | 50 (10.9) | 29 (12.2) | 0.603 |
| CKDa | 167 (23.8) | 111 (24) | 56 (23.3) | 0.850 |
| Immunosuppressiona | 32 (13.3) | 18 (13) | 14 (13.6) | 0.901 |
| Obesitya | 147 (21.6) | 71 (15) | 76 (32.3) | <0.001 |
| Smokera | 88 (12.7) | 47 (10.2) | 41 (17.5) | 0.006 |
| ACEIa | 140 (20) | 68 (14.8) | 72 (30.3) | <0.001 |
| ARBa | 250 (35.8) | 182 (39.5) | 698 (28.6) | 0.004 |
| Clinical presentation of COVID-19 disease | ||||
| Standard clinical presentationa | 646 (91.2) | 417 (92.3) | 209 (88.6) | 0.09 |
| Variables related to the severity of the infection | ||||
| Pneumoniaa | 657 (94.8) | 426 (93.8) | 231 (96.7) | 0.112 |
| Ventilatory supporta | 390 (55.8) | 167 (36.3) | 223 (93.3) | <0.001 |
| ECMOa | 16 (2.3) | 3 (0.7) | 13 (5.7) | <0.001 |
| ICU admissiona | 317 (45.4) | 103 (22.4) | 214 (89.5) | <0.001 |
| APACHE II (mean, SD) | 16 ± 7 | 17 ± 7 | 16 ± 7 | 0.526 |
| Days of onset of symptoms until admission to the ICU (mean, SD) | 12 ± 15 | 8 ± 6 | 14 ± 17 | 0.045 |
| Mortalitya | 276 (39.8) | 137 (29.9) | 139 (59.1) | <0.001 |
| Variables related to acute kidney injury | ||||
| Maximum creatinine (mean, SD) | 2.72 ± 1.95 | 2.00 ± 1.24 | 4.60 ± 2.18 | <0.001 |
| Albuminuria during admissiona | 114 (30.6) | 50 (25) | 63 (37) | 0.012 |
| Hematuria during admissiona | 92 (24.6) | 34 (16.9) | 58 (33.5) | <0.001 |
| Days: onset of symptoms until AKI (mean, SD) | 9 ± 7 | 7 ± 5 | 12 ± 9 | <0.001 |
| Contrast administrationa | 126 (18.4) | 51 (11.3) | 75 (32.2) | <0.001 |
| Days to recovery from AKI (mean, SD) | 9 ± 13 | 5 ± 6 | 22 ± 18 | <0.001 |
| Analytical parameters on the seventh day after the onset of COVID-19 symptoms (mean, SD) | ||||
| C-reactive protein (mg/l) | 86.1 ± 115.1 | 80.3 ± 96.9 | 92.9 ± 131.1 | 0.579 |
| D-dimers (ng/mL) | 4,665.1 ± 5,068.5 | 4,767 ± 1,345.9 | 4,787 ± 1,856.3 | 0.303 |
| Interleukin-6 (pg/mL) | 156.8 ± 917.7 | 86.1 ± 497.5 | 2,657 ± 1,317.5 | 0.366 |
| Ferritin (ng/mL) | 1,150.1 ± 2,472.1 | 936 ± 1197.4 | 1,573.6 ± 3,870.1 | 0.143 |
| Lymphocytes (no. absol/mcl) | 643.6 ± 899.9 | 666.6 ± 1065.2 | 593.1 ± 495.7 | 0.286 |
| Final results | ||||
| Mortalitya | 276 (39.8) | 137 (29.9) | 139 (59.1) | <0.001 |
Explanatory note: 27 missing values for the RRT variable. Number of patients with determinations of C-reactive protein 406, D-dimers 632, interleukin 6 506, ferritin 646, lymphocytes 701.
ARB: angiotensin II receptor antagonist drugs; SD: standard deviation; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; AKI: acute renal failure; ACEI: drugs that inhibit the angiotensin-aldosterone system; RRT: renal replacement therapy; ICU: critical care unit.
Regarding the clinical presentation of the condition, most patients in the RRT group required ventilatory support (93.3% vs. 36.3%; p < 0.001) and ICU admission (89.5% vs. 22.4%; p < 0.001). Mortality during admission was higher in patients with RRT (59.1% vs. 29.9%; p < 0.001), indicating greater severity of the clinical condition. In the analytical parameters collected, these patients had a higher proportion of albuminuria and haematuria on admission and reached higher maximum creatinine levels. We did not find significant differences in the rest of the parameters analysed on the seventh day after the onset of symptoms (Table 3).
It is worth to highlight that the more a frequent use of iodinated contrast in the group that required RRT (32.2% vs. 11.3%; p < 0.001) and the onset and recovery times of AKI between both groups presented significant differences. Patients who required dialysis started AKI later than the rest, 10 vs. 5 days on average from the onset of symptoms, with an average recovery of kidney function of 21 days (Table 3).
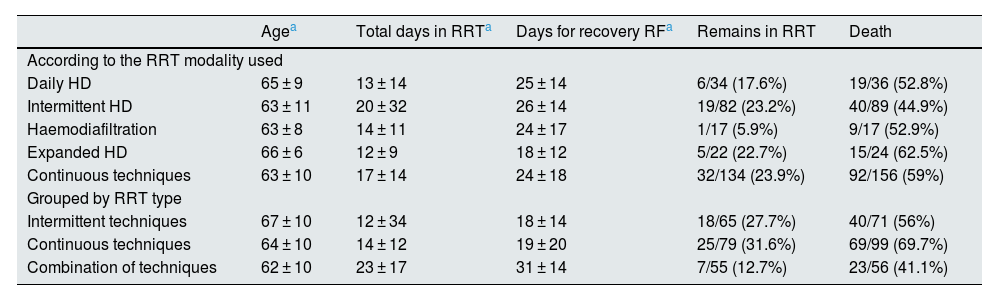
Overall, 17.5% of patients who required RRT remained on dialysis at the time of data collection, and 59.1% died during hospitalisation. Table 4 shows some differential characteristics of the patients and final results according to the different modalities of RRT used.
Differential characteristics according to the RRT modalities used.
| Agea | Total days in RRTa | Days for recovery RFa | Remains in RRT | Death | |
|---|---|---|---|---|---|
| According to the RRT modality used | |||||
| Daily HD | 65 ± 9 | 13 ± 14 | 25 ± 14 | 6/34 (17.6%) | 19/36 (52.8%) |
| Intermittent HD | 63 ± 11 | 20 ± 32 | 26 ± 14 | 19/82 (23.2%) | 40/89 (44.9%) |
| Haemodiafiltration | 63 ± 8 | 14 ± 11 | 24 ± 17 | 1/17 (5.9%) | 9/17 (52.9%) |
| Expanded HD | 66 ± 6 | 12 ± 9 | 18 ± 12 | 5/22 (22.7%) | 15/24 (62.5%) |
| Continuous techniques | 63 ± 10 | 17 ± 14 | 24 ± 18 | 32/134 (23.9%) | 92/156 (59%) |
| Grouped by RRT type | |||||
| Intermittent techniques | 67 ± 10 | 12 ± 34 | 18 ± 14 | 18/65 (27.7%) | 40/71 (56%) |
| Continuous techniques | 64 ± 10 | 14 ± 12 | 19 ± 20 | 25/79 (31.6%) | 69/99 (69.7%) |
| Combination of techniques | 62 ± 10 | 23 ± 17 | 31 ± 14 | 7/55 (12.7%) | 23/56 (41.1%) |
RF: renal function; RRT: renal replacement therapy.
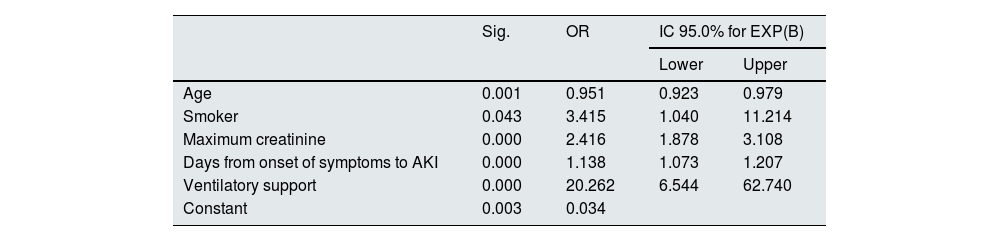
Table 5 shows the results of the multivariate analysis. Smoking habit (OR 3.41; CI 95%: 1.04–11.2), the need for ventilatory support (OR 20.2; CI 95%: 6.5–62.7), the maximum creatinine level (OR 2.41; CI 95%: 1.87–3.10) and the time elapsed from the onset of symptoms to the appearance of AKI (OR 1.13; CI 95%: 1.07–1.20) act as predictors of the need for RRT in our cohort, while age acts as a protector (0.95).
Multivariate logistic regression analysis for the RRT variable.
| Sig. | OR | IC 95.0% for EXP(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.001 | 0.951 | 0.923 | 0.979 |
| Smoker | 0.043 | 3.415 | 1.040 | 11.214 |
| Maximum creatinine | 0.000 | 2.416 | 1.878 | 3.108 |
| Days from onset of symptoms to AKI | 0.000 | 1.138 | 1.073 | 1.207 |
| Ventilatory support | 0.000 | 20.262 | 6.544 | 62.740 |
| Constant | 0.003 | 0.034 | ||
Variables entered: age (years), obesity (dichotomous), smoking habit (dichotomous), haematuria (dichotomous), albuminuria (dichotomous), maximum creatinine (mg/dl), CKD (dichotomous), treatment with ACEI (dichotomous), treatment with ARB (dichotomous), time from onset of COVID symptoms to AKI (days) and ventilatory support (dichotomous).
ARB: angiotensin II receptor antagonist drugs; CKD: chronic kidney disease; AKI: acute renal failure; ACEI: drugs that inhibit the angiotensin-aldosterone system; OR: odds ratio; RRT: renal replacement therapy; ICU: intensive care unit.
A total of 282 patients in the cohort (38.6%) died during hospitalisation. This group was characterised by being older (73 vs. 68 years; p < 0.001) and a higher baseline comorbidity burden. HTN, CVD, COPD, and a history of oncology and immunosuppression were more prevalent among deceased patients. Likewise, there was a greater proportion of patients with CKD prior to admission. Previous treatment with ACEI was more common in the group that died during hospitalisation, while treatment with ARB was more common among survivors (Table 1).
From a clinical point of view, these patients more frequently required ICU admission and ventilatory support (p < 0.001), and the APACHE II score at the time of admission to the ICU was significantly higher. The severity of AKI was greater, with a predominance of KDIGO III patients (p < 0.001) (Table 1). The need for RRT was more frequent in the deceased group, that exceeded the 50%. All RRT therapeutic modalities were associated with higher mortality, and 44 patients died while being dependent on RRT (Table 2).
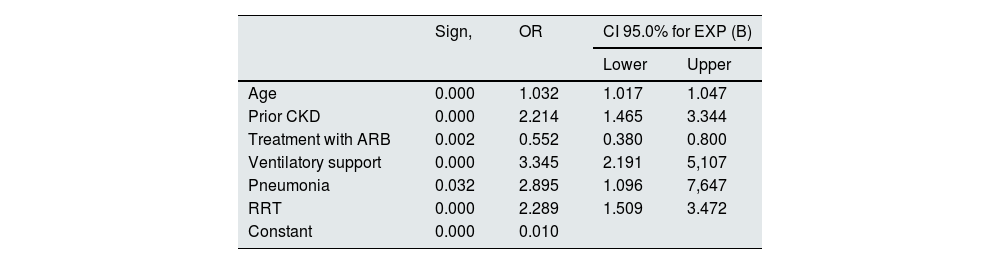
In the multivariate analysis, factors related to the individual themselves and also factors related to the severity of the presentation of COVID-19 were identified as predictors of mortality. Age (OR 1.03; CI 95%: 1.01–1.04), the presence of previous CKD (OR 2.21; CI 95%: 1.46−1.34), the development of pneumonia (OR 2.89; CI 95%: 1.09–1.64), the need for assisted ventilation (OR 3.34; CI 95%: 2.19–5.10), and the need for RRT in the context of AKI (OR: 2.28; CI 95%: 1.50–3.42) acted as predictive factors independently related to mortality during hospital admission. On the contrary, the patient's chronic treatment with ARB acted as a protective factor in the analysis (OR 0.55) (Table 6).
Multivariate analysis of logistic regression for mortality.
| Sign, | OR | CI 95.0% for EXP (B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.000 | 1.032 | 1.017 | 1.047 |
| Prior CKD | 0.000 | 2.214 | 1.465 | 3.344 |
| Treatment with ARB | 0.002 | 0.552 | 0.380 | 0.800 |
| Ventilatory support | 0.000 | 3.345 | 2.191 | 5,107 |
| Pneumonia | 0.032 | 2.895 | 1.096 | 7,647 |
| RRT | 0.000 | 2.289 | 1.509 | 3.472 |
| Constant | 0.000 | 0.010 | ||
Variables entered: age (years), CKD (dichotomous), treatment with ACEI (dichotomous), treatment with ARB (dichotomous), ventilatory support (dichotomous), pneumonia (dichotomous), RRT (dichotomous), need for ICU (dichotomous).
ARB: angiotensin II receptor antagonist drugs; CKD: chronic kidney disease; AKI: acute renal failure; ACEI: drugs that inhibit the angiotensin-aldosterone system; OR: odds ratio; RRT: renal replacement therapy; ICU: intensive care unit.
We present a descriptive study of the clinical profile of patients who developed AKI during hospitalisation for COVID-19 in our country based on the data collected in the SEN COVID-AKI Registry (May 2020–November 2021). The variables collected did not allow us to calculate the incidence that this entity has presented, but they allow us to adequately characterise this population. Overall, our patients were mostly men, with a high average age (70 years) and a considerable percentage of them had hypertension. (69,8%); DM, CKD, CVD and obesity were frequently observed comorbidities. All these comorbid factors have been previously identified as predisposing factors, both to greater severity of the infection and to the appearance of AKI among patients requiring hospital admission.4,8,11–15 It is striking that the prevalence of CKD prior to admission was 23.9%, much lower than other series collected in our environment. Portolés et al.6 reported a prevalence of CKD in 43.5% of patients admitted for COVID-19 who developed AKI during the first wave of the pandemic in a hospital in Madrid. In our work, data were collected over a long period of time, and this difference in prevalence may reflect the moderation of the pandemic's effect over time among the most fragile patients, on whom the first wave had a major impact.
Previous publications report that the factors with the greatest impact on the risk of acute kidney injury in the course of COVID-19 are related to the severity of the patient's condition, the need to use catecholamines, septic shock and/or the need to use artificial ventilation due to respiratory failure.4,8,13 Our cohort coincided in these terms, with kidney injury appearing in patients with severe COVID and a slow progression of the infectious disease: almost all had pneumonia, half of them required ventilatory support, and the mean numbers of inflammatory markers were very high globally.
A 32.9% of the analysed cohort required RRT. Hassanein et al.,8 in a review of the literature, reports great variation in the frequency of the need for renal replacement therapy in these patients. Without a doubt, differences in the incidence of AKI, clinical management of the patient in each hospital, as well as the saturation of health systems and the availability of technical and human resources, have been determining factors in these oscillations. Several meta-analyses that investigate the overall frequency of need for RRT in this type of patients estimate it varies between 3.6 and 4.3%,18,19 reaching figures of 20–40% in series of patients admitted to the ICU.20 We must consider the selection bias that our Registry presents, due to the fact that the data come from patients evaluated by the nephrology services. It is reasonable to think that we have followed the most serious cases, hence our results are more similar to those obtained in the series of serious patients admitted to intensive care.
The presence of haematuria and proteinuria in hospitalised COVID patients has been identified as predictors of kidney injury and mortality during admission.6,11 In our data these urinary parameters were only recorded in approximately half of the cases. Even so, the detection of haematuria and proteinuria was significantly higher in patients requiring RRT. These urinary alterations probably reflect the development of severe kidney injury. In the literature, the most frequently reported histological lesion was acute tubular necrosis,21 although interstitial nephropathy, thrombotic microangiopathy, collapsing glomerulosclerosis or even direct invasion by SARS-CoV-2 of kidney tissue have also been described in the context of a large viral load2,3,20; in all of them, it is possible to find associated haematuria and proteinuria.
An interesting aspect of this work is that the clinical characteristics of a significant number of patients who developed AKI without needing RRT were collected. In our environment, where access to replacement therapy was practically guaranteed, the fact that these patients were an average 6 years older than those who received RRT is especially striking. Furthermore, milder AKI predominated in this group (KDIGO I 59.8%) and, both the average time of appearance of AKI and recovery was shorter (5 and 4 days, respectively). The need for ICU admission, ventilatory support, and COVID treatment was lower in this group, indicating, overall, a lower severity of the course of the infection. This type of AKI, which appeared early in the course of the infection and resolved without the need for RRT, was the most frequently recorded in our series, constituting 2 out of every 3 reported cases. There are series of patients in our environment that identify the prerenal cause as predominant in patients with COVID-19 who developed AKI.22,23 Although the Registry did not collect the clinical impression of nephrologists on the origin of AKI, the rapid appearance and recovery of the condition leads us to think that the prerenal cause probably justifies renal dysfunction in an elderly, fragile population, with high comorbidity, with greater risk of kidney dysfunction during the first days of infection. We only found a similar description in the literature by Peng et al.24 They described 2 patterns of AKI in a total of 285 cases with COVID: one earlier and another later which occurs when there is already dysfunction of other organs. This later kidney injury was accompanied by significantly higher levels of systemic inflammatory parameters, a higher percentage of proteinuria and glycosuria, lower mean arterial pressure on admission, higher rates of lymphopenia, thrombocytopenia and coagulation disorders on admission, and higher mortality. All of this would coincide with our data: later AKI occurs in the context of greater severity of the infection, reaches greater severity and is more likely to require RRT. The time elapsed from the onset of symptoms to the appearance of AKI was identified in our analysis as an independent predictor of the need for RRT, such that each day of delay in the onset increases the risk by 13%, which would confirm this proposed hypothesis. And, along the same lines, age acts as a protective factor, which would be justified by the fact that older patients are those who have developed milder AKI, as it was previously explained.
Other factors independently related to the need for RRT during hospitalisation for COVID-19 were, in addition to the serum creatinine level, the need for ventilatory support (OR: 20.20) and smoking habit (OR: 3.41). Hypoxaemia has already been related in other studies to RRT in AKI due to COVID-19.25 In fact, hypoxaemia can compromise cardiac output and renal perfusion, as well as stimulate signalling pathways that determine greater thrombogenicity. In turn, aggressive ventilation strategies can lead to neurohormonal alterations and the release of systemic inflammatory mediators, which impact intrarenal blood flow and further deteriorate renal perfusion, inducing more severe kidney injury through all these mechanisms.
Another fact that the data reveal is the absence of clear indications and multiple questions regarding which modality of RRT could be most effective. eEach centre has opted for different treatment modalities depending fundamentally on the experience and availability of resources in each. In this manner, CRRT, intermittent haemodialysis, daily haemodialysis, expanded haemodialysis and haemodiafiltration have been used, with the first two being the most widely represented, in many cases being used in combination. It has been suggested in the literature, although there are no randomised controlled studies, that the use of adsorption membranes, special membranes or convective dialysis modalities could be useful to eliminate circulating inflammatory molecules, as well as SARS-CoV-2 molecules in selected patients.26 All of them are represented in this Registry to a greater or lesser extent, without having found a clear benefit in favour of any of them in terms of results. To date, the data available on the results of RRT in these patients remain limited. The largest series, which included 3,099 critically-ill COVID-19 patients admitted to the ICU in 67 hospitals in the United States, showed that the most commonly used initial modality was CRRT (52.4%), followed by intermittent haemodialysis (30,0%) with wide interhospital variability both in the frequency of use and in the modality used.25
All published works agree that the coexistence of AKI during hospitalisation for COVID-19 behaves as an independent predictor of mortality.14,18,19 Mortality in our series was also high, 38.6% in the entire cohort, reaching 59.1% in patients who required RRT. Factors related to greater severity of infection were those that had the greatest impact on mortality; thus, the development of pneumonia and the need for ventilatory support tripled the risk of death. The development of more severe AKI in the context of infection requiring RRT doubled the risk of mortality. On the other hand, the patient's age and the presence of CKD prior to admission were host-dependent factors, which were associated with a higher risk of mortality in our series. The age of the patients has been a factor clearly related to the worse outcome and higher mortality of COVID-19 in the literature.5,26,27 Frailty and age-related comorbidity make these patients especially vulnerable to COVID-19 infection.
Unlike other series, we have not found that the presence of CKD is independently related to greater severity of AKI with higher RRT requirements than the rest of the population. However, a direct impact of CKD on mortality was detected (OR: 2.21). CKD has been identified by the ERACODA Group as one of the 4 comorbidities associated with the highest risk of mortality from COVID-19. Specifically, the risk associated with CKD stages 4 and 5 is higher than the risk associated with diabetes mellitus or chronic heart disease.28 The publication Global Burden of Disease identified that, worldwide, CKD has been the most common risk factor for severe COVID-19.29 There is likely a bidirectional relationship between COVID-19 and CKD: CKD increases the risk of severe COVID-19, and the greater severity of COVID-19 leads to greater risk of acute renal dysfunction, in turn leading to greater mortality.
Much has been discussed in recent months about the role played by ACEIs and ARBs in the course of COVID-19 infection. Although most studies have attributed a neutral role to them, in our analysis, chronic treatment with ARB was identified as a protective factor in the mortality of those patients who developed AKI. Given that ARBs act by direct blockade of AT1R and do not competitively inhibit ACE, the use of ARBs could be beneficial in this way. Furthermore, ARBs, by blocking AT1R, can also increase the availability of ACE2 bound to the cell membrane, inhibiting its internalisation, thus also contributing to a lower aggressiveness of SARS-Cov2.30,31
Like any retrospective registry-based study, our study has limitations. Firstly, the entire population that suffered AKI in our country was not included, presenting the bias that the patients registered were those treated by nephrologists, so the cohort may represent a group of patients with a more torpid evolution. Data collection was limited and follow-up data are lacking. Finally, the evolution of the pandemic and the application of vaccines have altered its virulence and transmission, which influences the severity and mortality of the disease, so the results would probably not be applicable today. As strengths, it is worth highlighting that this analysis shows a fairly global view of the evolution of patients admitted for COVID-19 who developed AKI in the early phases of the pandemic in our country, not previously available in subjects with different evolution of both the infection as from the AKI. On the other hand, although the development of AKI has been an important complication of COVID-19, data on RRT in these patients are limited. Fortunately, our health system was not overwhelmed in most cases, and we can evaluate the impact without major resource limitations. We can also review the indication of RRT in a large cohort, not only limited to critically ill patients, but also comparing the different modalities used and the results obtained, an aspect that has been scarcely discussed in the literature to date.
In conclusion, patients who presented AKI during hospitalisation for COVID-19 had a high average age, with associated comorbid conditions, and presented with severe infection. We differentiate 2 clinical patterns of kidney injury in these patients: the first one occurs early in older patients, with a greater burden of comorbidity, and resolves in a few days without the need for RRT; and the second one, is more severe and with greater requirements for RRT, appeared later in the course of the disease and was related to greater severity of the disease. Early and more mild presentation was the most frequent, constituting 2 of every 3 reported cases. It is also reflected in this analysis that different modalities of RRT were used without a clear benefit of any of them over the others. The severity of the infection, age, and presence of CKD prior to admission were determining factors in the mortality of these patients, identifying chronic treatment with ARB as a protective factor for mortality.
Conflicts of interestThe authors declare that they have no conflicts of interest.
| Hospital Universitario Marqués de Valdecilla (Santander): Lara Belmar Vega |
| Cáceres University Hospital Complex (Cáceres): Pedro Jesús Labrador Gómez, Elena Davin, and André Rocha Rodrigues |
| Hospital Universitario Virgen Macarena (Seville): Marina Almenara Tejederas, María de los Ángeles Rodríguez Pérez, Nuria Aresté Fosalba, Javier Burgos Martín, Fabiola Alonso García, Mercedes Salgueira Lazo, María Dolores Salmerón Rodríguez |
| Hospital General Universitario Gregorio Marañón (Madrid): Rosa Melero Martín |
| Hospital Universitario Puerta de Hierro Majadahonda (Madrid): Marisa Serrano Salazar |
| Hospital Clinic Barcelona (Barcelona): Alicia Molina Andújar, Esteban Poch López de Briñas |
| Hospital Vall d’Hebron (Barcelona): Natalia Ramos Terrades |
| Fundació Puigvert [Puigvert Foundation] (Barcelona): María Jesús Lloret |
| Hospital Universitario Infanta Sofía (Madrid): Rocío Echarri Carrillo, Raquel Díaz Mancebo |
| Complejo Hospitalario Universitario de Toledo [Toledo University Hospital] (Toledo): Diego Mauricio González Lara, Francisco Javier Ahijado Hormigos |
| Hospital Clínico Universitario Lozano Blesa (Zaragoza): María Pilar Martín Azara |
| Hospital Nuestra Señora de Candelaria (Tenerife): Orlando Siverio Morales, Desiré Luis Rodríguez, Patricia García García |
| Hospital Universitario Juan Ramón Jiménez (Huelva): Fernando Fernández Girón |
| Complejo Hospitalario de Pontevedra [Pontevedra Hospital] (Pontevedra): María Jesús Castro Vilanova, Luz María Cuiña Barja |
| Complejo Hospitalario Universitario de Albacete [Albacete University Hospital] (Albacete): Sara Piqueras Sánchez |
| Hospital Universitario de Jerez de la Frontera (Cádiz): Gema Velasco Barrero, Marina Almenara Tejederas |
| Hospital General Universitario Reina Sofía (Murcia): Antonio Pérez Pérez, Ana Cristina Ródenas Gálvez |
| Hospital Universitario de Torrevieja (Alicante): Beatriz Díez Ojea |
| Hospital Universitario San Agustín (Jaén): Anna Gallardo Pérez |
| Hospital de Poniente (Almería): Francisco Roca Oporto |
| Hospital Ntra. Sra. de Sonsoles (Ávila): Jesús Martín García |
| Hospital Universitario de Badajoz (Badajoz): María Victoria Martín Hidalgo-Barquero |
| Complejo Hospitalario Universitario de Ferrol [Ferrol University Hospital] (A Coruña): Pablo Bouza Piñeiro |
| Fundació Salut Empordà [Empordà Health Foundation] (Girona): Montserrat Picazo Sánchez |
| Hospital Arquitecto Marcide (A Coruña): Edwin Palomino Guere |
| Hospital General Universitario de Elche (Alicante): Leonidas Cruzado Vega |
| Hospital Universitario Donostia (Donostia): María Teresa Rodrigo de Tomás |
| Hospital Universitario El Bierzo (León): Fernando Simal Blanco |
| Hospital Universitario Reina Sofía (Córdoba): Cristina González Ruiz Moyano |
| Hospital Virgen del Puerto (Cáceres): Miguel Ángel Suárez Santisteban |
| Autonomous communities | Nephrology departments | Participants |
|---|---|---|
| Galicia | 16 | 3 |
| Asturias | 8 | – |
| Cantabria | 1 | 1 |
| Basque Country | 7 | 1 |
| Navarre | 3 | – |
| Aragon | 10 | 1 |
| Catalonia | 22 | 4 |
| Valencian C. | 21 | 2 |
| R. of Murcia | 3 | 1 |
| Castilla y León | 10 | 2 |
| Extremadura | 4 | 3 |
| Castilla-La Mancha | 7 | 2 |
| C. of Madrid | 25 | 3 |
| La Rioja | 2 | – |
| Balearic Islands | 7 | – |
| Canary Islands | 10 | 1 |
| Andalusia | 17 | 6 |
| Ceuta | 1 | – |
| Melilla | 1 | – |
| Total | 175 | 30 |