The prevalence of diabetes mellitus increased during the last century and it is estimated that 45% of the patients are not diagnosed. In South America the prevalence of diabetes and chronic kidney disease (CKD) increased, with a great disparity among the countries with respect to access to dialysis. In Ecuador it is one of the main causes of mortality, principally in the provinces located on the coast of the Pacific Ocean. The greatest single cause of beginning dialysis is diabetic nephropathy (DN). Even using the best therapeutic options for DN, the residual risk of proteinuria and of terminal CKD remains high. In this review we indicate the importance of the problem globally and in our region. We analyse relevant cellular and molecular studies that illustrate the crucial significance of glomerular events in DN development and evolution and in insulin resistance. We include basic anatomical, pathophysiological and clinical concepts, with special attention to the role of angiogenic factors such as the vascular endothelial growth factor (VEGF-A) and their relationship to the insulin receptor, endothelial isoform of nitric oxide synthase (eNOS) and angiopoietins. We also propose various pathways that have therapeutic potential in our opinion. Greater in-depth study of VEGF-A and angiopoietins, the state of glomerular VEGF resistance, the relationship of VEGF receptor 2/nephrin, VEGF/insulin receptors/nephrin and the relationship of VEGF/eNOS-NO at glomerular level could provide solutions to the pressing world problem of DN and generate new treatment alternatives.
La prevalencia de diabetes mellitus aumentó en el último siglo y se estima que el 45% de los pacientes, no estarían diagnosticados. En Sudamérica la prevalencia de diabetes y de enfermedad renal crónica (ERC) incrementó, existiendo gran disparidad entre los países respecto al acceso a diálisis. En Ecuador es una de las principales causas de mortalidad, principalmente en las provincias ubicadas en la costa del océano Pacífico. La mayor causa aislada de ingreso a diálisis es la nefropatía diabética (ND). Aun utilizando las mejores opciones terapéuticas para la ND, el riesgo residual de proteinuria y de ERC terminal permanece elevado. En esta revisión describimos la importancia del problema en el mundo y en nuestra región. Analizamos estudios moleculares y celulares relevantes que indican la crucial importancia de eventos glomerulares en el desarrollo y en la evolución de la ND y en la insulinorresistencia. Incluimos conceptos anatómicos, fisiopatológicos y clínicos básicos, desarrollando especial énfasis en el rol de factores angiogénicos como el factor de crecimiento vascular endotelial (VEGF-A) y su relación con el receptor de insulina, la sintasa endotelial de óxido nítrico-óxido nítrico (eNOS) y las angiopoietinas. En el transcurso del texto proponemos diversas vías, que a nuestro entender tienen potencial terapéutico. Profundizar en el estudio del VEGF-A y las angiopoietinas, el estado de VEGF resistencia glomerular, la relación del receptor 2 de VEGF/nefrina, VEGF/receptores de insulina/nefrina, la relación VEGF/eNOS-ON a nivel glomerular podría aportar soluciones al acuciante problema de la ND en el mundo y generar nuevas alternativas de tratamiento.
The prevalence of diabetes mellitus has increased worldwide since the last century1. In adults aged between 20 and 79 years of age, its prevalence reaches 8%1. Diabetes spreads through rich and poor countries, but it is prevalent in vulnerable groups and lower-income regions of the world. Territories showing the highest numbers of affected individuals are: China, India, the United States, Brazil and Russia1. This situation is associated with greater urbanisation, low socioeconomic level, inequality, increased life expectancy and population density, ethnic factors, nutrition, physical inactivity, and being overweight1,2. In Spain, a diabetes prevalence rate of 13.8% was reported, while 6.0% had not yet been diagnosed3. Recent estimates suggest that worldwide prevalence will have doubled by 2035, while in our region, South America and Central America, it will have increased to 9.8%1,2. In addition, 45.5% of individuals with diabetes will not be diagnosed with the disease1,2.
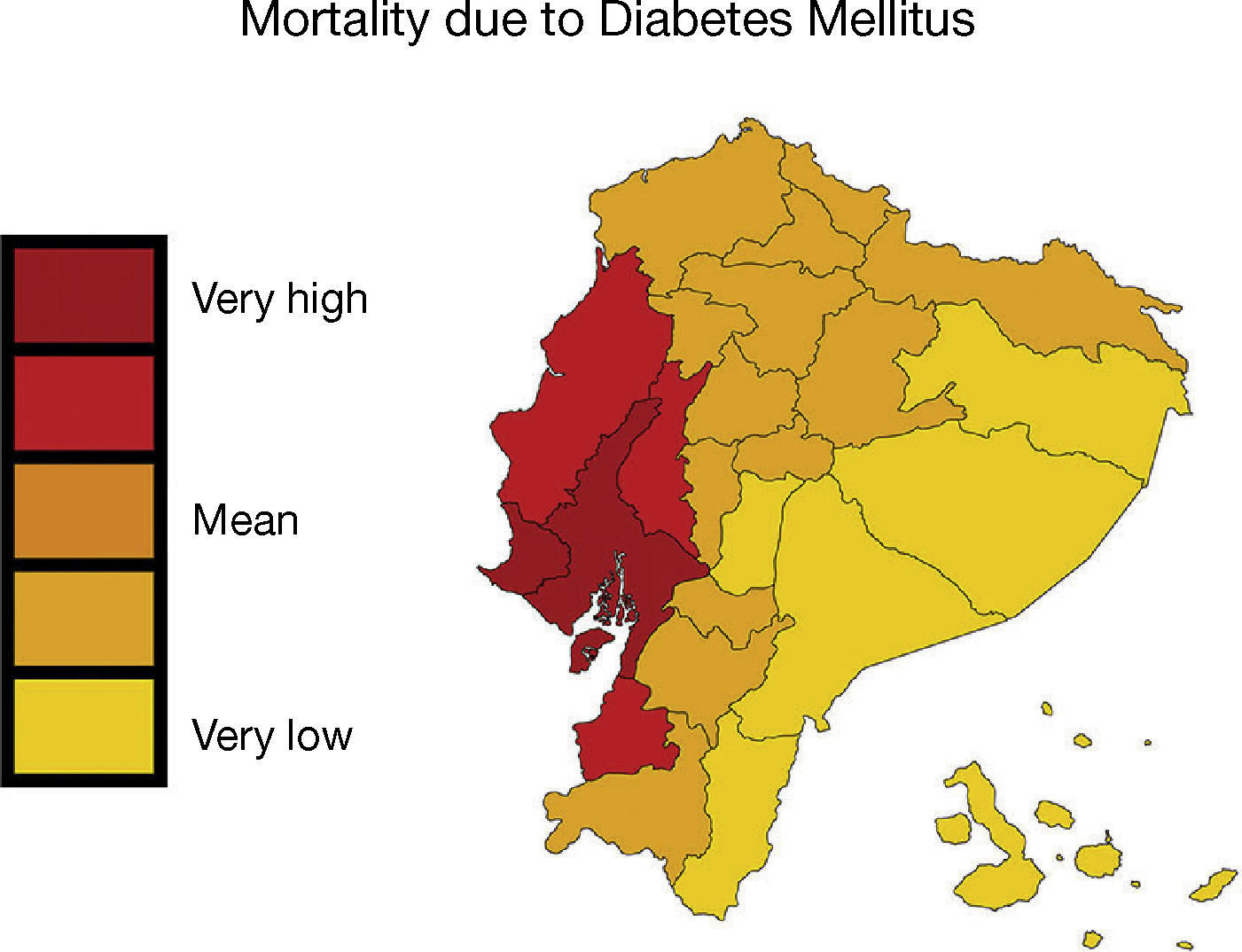
In the urban population located on the coasts of our region, diabetes prevalence is higher than in the mountains or the jungle, and the same happens with people who move from the rural to the urban environment1,2. Moreover, native populations are particularly vulnerable due to the change in lifestyle, marginalisation and lower exposure to health care systems2. In Ecuador, the prevalence of diabetes is 6%, and in 2010 it was the second cause of mortality2,4,5. In the provinces of Guayas, Los Ríos and Manabí, located on the Pacific coast, the mortality rate due to diabetes and industrialised food consumption is higher; meanwhile, in the Amazon, natural food-based nutrition predominates and the rate is lower6 (Figure 1).
– In Ecuador, mortality caused by diabetes mellitus was higher in the provinces of Guayas, Los Ríos and Manabí, located on the Pacific coast. Map shows the mortality rate due to diabetes mellitus (deaths/100,000 individuals per year, INEC [Instituto Nacional de Estadísticas y Censos National Institute of Statistics and Census of Ecuador] 2011). This figure is part of a figure originally published by Neira-Mosquera et al.6, with minor modifications (authorised reproduction).
Kidney disease caused by diabetes is called diabetic nephropathy (DN). About 30% of patients with diabetes develop DN7,8. Such disease is the main cause of chronic kidney disease (CKD) and of admission to dialysis7–11. The increase in adult diabetes has been recorded in the last few decades, and CKD affects 10% to 16% of adults, which constitutes a serious worldwide problem7–11. In South America, the prevalence of diabetes and end-stage CKD (ECKD) has increased in recent decades, and access to dialysis varies greatly among these countries9–11. In Ecuador, the prevalence of patients who received renal function replacement therapy in 2010 was 406 individuals per one million inhabitants11. On the other hand, the renin-angiotensin-aldosterone system (RAAS) inhibitors constitute the best therapeutic option for DN, but the residual risk of ECKD continues to be high and the association of these drugs was related to hyperkalemia and acute kidney failure (AKF)12,13. The search for new therapeutic alternatives is necessary.
Population studies raise awareness of the problem, while the knowledge generated in research laboratories expand our understanding of the biological events that occur in individuals. In this review, we will include anatomical and pathophysiological concepts that reveal the crucial importance of events occurring at the glomerular level. In addition, we will analyse the role played by the vascular endothelial growth factor (VEGF-A) and its relationships with nitric oxide (NO), the insulin receptor and angiopoietins. Finally, we will consider basic aspects and the analyses of recently published molecular and cellular studies.
Anatomical and pathophysiological aspects of DNDiabetes involves functional and structural kidney alterations that induce proteinuria at variable magnitudes, ranging from micrograms to several grams per day7,8,13. The risk of developing ECKD is related to albumin urinary excretion, and early treatment with RAAS inhibitors is important due to the beneficial renal and systemic effects7,8,13. DN is accompanied by persistent albumin urinary excretion or microalbuminuria, which is defined as the loss of urinary albumin ranging from 20 to 199μg/min or 30 to 299mg/d on two different occasions and when the albumin/creatinine ratio is 30-299mg/g in an isolated urine sample7,8. In type 1 diabetes, albumin urinary excretion should be quantified on an annual basis, from the fifth year following diagnosis onwards; in type 2 diabetes, given the difficulty to accurately state its onset, measurement is preferable from the moment the disease is diagnosed7,8. In one study, the prevalence of microalbuminuria in patients with type 2 diabetes was 24.9% after a ten-year follow-up14, but 30% of patients with type 2 diabetes and no microalbuminuria developed DN. It is also important to quantify glomerular filtration (GF), since some patients only show renal function impairment with no signs of proteinuria7,8. Considering that 85% of the patients with diabetes have type 2 diabetes, better biomarkers are required7,8,13,14.
Risk factors contributing to the development of DN are hyperglycaemia, hypertension (HTN), dyslipidaemia, age over 65 years, male gender, smoking habit, family history and Hispanic or Afro-American origin7,8. Familial clustering was reported in populations with different ancestors, especially in Pima Indians and Afro-Americans16. Mooyaart et al. found 24 genetic variations associated with DN17. Epigenetic mechanisms were also implied8,18. For example, chronic hyperglycaemia, without altering the nucleotide sequence, may modify DNA or methylate histones associated with DNA18. However, the significance of these findings on the development of DN has not been determined yet.
Many factors were implied in DN pathophysiology, such as: glucose, glucose receptors, VEGF-A, NO, reactive oxygen species (ROS), transforming growth factor beta (TGF-Beta), RAAS, kinin-kallikrein system, mammalian target of rapamycin, inflammation, tumour necrosis factor alpha, adiponectin, advanced glycation end products and receptors thereof, mitochondr ial oxidative stress and micro-RNA7,8,15,19–22.
From the pathologic point of view, type 1 and type 2 diabetes induce common kidney lesions. These lesions were characterised in type 1 diabetes7,8,15,23–26. In type 2 diabetes, the kidney histology and course have special features, associated with comorbidities such as HTN, vascular diseases, ageing and obesity7,8,23,24. Five years after diabetes diagnosis, there is hyperfiltration, microalbuminuria, glomerulomegaly, glomerular basal membrane (GBM) thickening and alteration of podocytes26. Subsequently, the extracellular matrix (ECM) is deposited in the mesangium. Approximately ten years later, proteinuria and HTN are evident, and GF becomes progressively impaired7,23,24,26. Within a period of 20 to 25 years, sclerosis is advanced, there is tubulointerstitial fibrosis and CKD progresses to end-stage phases7,24–26.
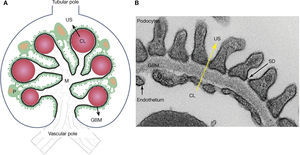
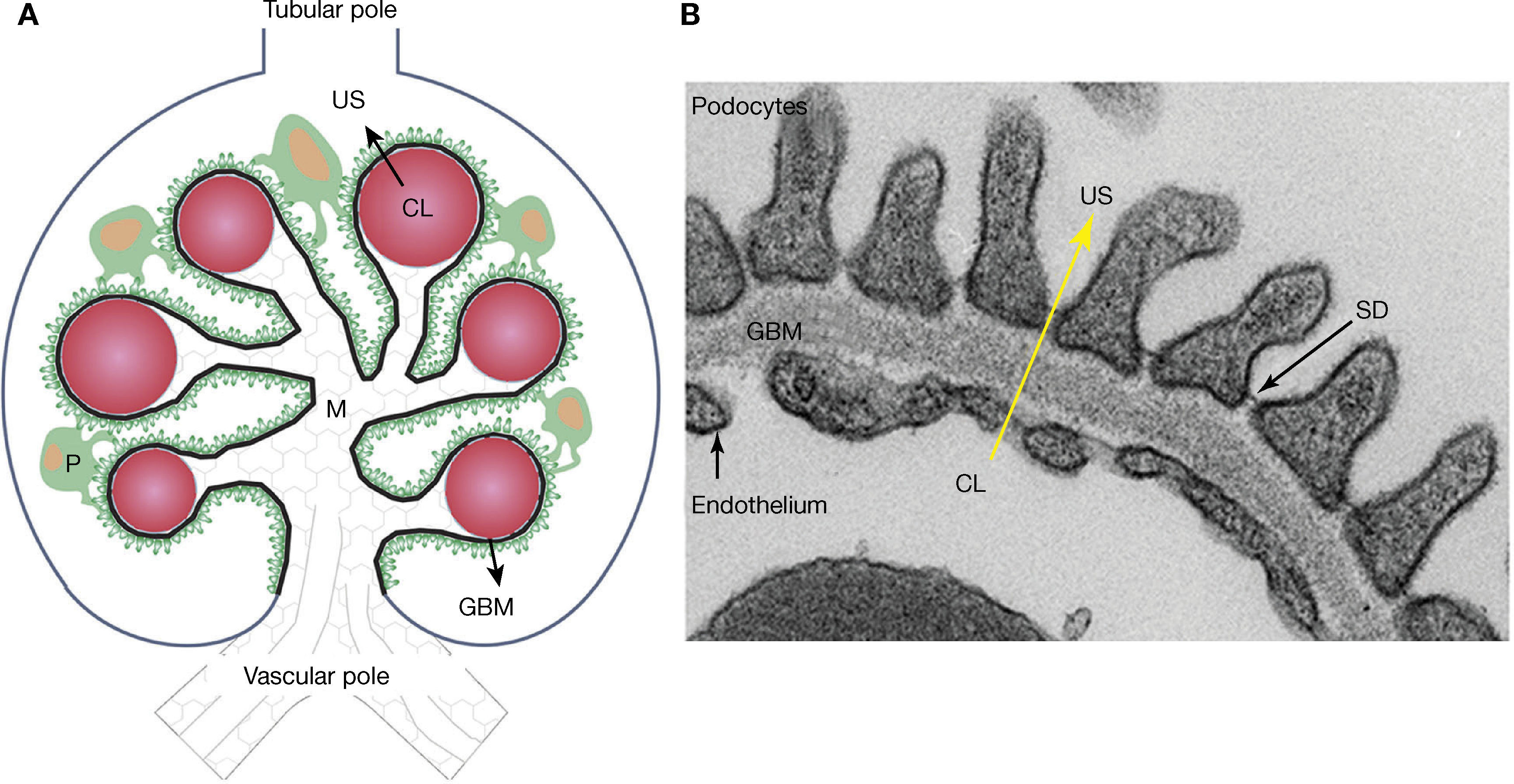
Meanwhile, the glomeruli, tubules, interstitium and renal arteries are modified by the diabetic environment. Glomerular changes involve the glomerular filtration barrier (GFB), ECM, and the main cells composing it (podocytes, endothelial cells and mesangial cells)7,16,19–21. In addition, it prevents the abnormal passage of plasma protein based on size and load, and its alteration was associated with proteinuria7,15,19,20,25. The GFB is composed of podocytes, GBM, and the endothelium (Figure 2). Podocytes are markedly differentiated epithelial cells, with a large cell body, and primary and secondary extensions connected by slit diaphragms (SD)15,19,20. The SD is permeable to water and small solutes, but it is selective to large molecule passage, which is a key factor in GFB permeability25. Moreover, it is composed of a protein complex, where nephrin plays an important role7,15,19,20. On the apical side, podocytes float within the urinary space, while on the basolateral side, they make contact with the GBM. Podocyte cytoskeleton proteins are related to GBM proteins through integrins and dystroglycans15,20,25. The GBM is mainly composed of proteins, such as collagen IV and laminins15,25. The fenestrated endothelium, covered by glycocalyx, is the inner most layer of the GFB7,15,21,25. Diabetes alters the three layers that make up the GFB. Among the early changes, neoangiogenesis in the glomerular vascular pole and loss of endothelial fenestrations have been described7,16,22,23. The GBM shows an increased thickness due to protein exchange alterations7,15,19,20,25. In podocytes, f lattening, hypertrophy, detachment and apoptosis are observed in the early stages, while later podocytopenia is observed7,15,19,20.
– Schematic representation of the glomerulus, the glomerular filtration barrier (GFB) composed of podocytes (P), the glomerular basal membrane (GBM), and the endothelium. Plasma ultrafiltration passes through the GFB (black arrow) to reach the urinary space (US). Podocytes (green) make contact with several glomerular capillaries (represented as red circles) and the intraglomerular mesangium (M). The GBM (black line) wraps the capillaries and surrounds the mesangium. The glomerular endothelium is represented by a discontinuous light-blue line, located between the capillary lumen (CL) and the GBM, the vascular pole in the lower part of the glomerulus, the tubular pole in the upper part. B: Ultrastructure of the GFB observed with an electronic microscope: podocytes, GBM, slit diaphragm (SD) and fenestrated endothelium. Plasma ultrafiltration passes through the GFB (yellow arrow) from the capillary lumen (CL) towards the urinary space (US).
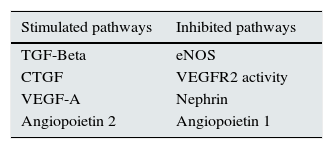
Recently published articles that relate VEGF-A to glomerular proteins involved in human and experimental DN pathophysiogenesis are analysed below. Throughout the text, we will suggest several pathways which may be used to generate new therapeutic tools (Table 1).
– Glomerular pathways with therapeutic potential for DN.
| Stimulated pathways | Inhibited pathways |
|---|---|
| TGF-Beta | eNOS |
| CTGF | VEGFR2 activity |
| VEGF-A | Nephrin |
| Angiopoietin 2 | Angiopoietin 1 |
Legend: TGF-Beta (transforming growth factor), CTGF (connective tissue growth factor), VEGF-A (vascular endothelial growth factor), eNOS (endothelial nitric oxide synthase)
VEGF-A is a potent angiogenic factor related to normal and pathological angiogenesis. It promotes the proliferation, differentiation and migration of endothelial cells; it induces vasodilation and increases vascular permeability15,19,20,27. It plays an important role in kidney development; in the adult kidney, it is secreted by podocytes and is essential for the maintenance of the GFB15. It acts through tyrosine-kinase receptors, which are known as VEGF receptor 1 and 2 (VEGFR1 and 2)15,27. VEGFR2 is expressed in endothelial cells and podocytes; it is related to the most important signals of VEGF-A15,27. Two co-receptors called neuropilins 1 and 2 amplify the VEGFR2 signal15,27.
There is evidence that glucose directly and indirectly stimulates VEGF-A expression in podocytes through angiotensin II and TGF-Beta15,19,20. Glucose plays a very important role in DN pathophysiogenesis. Glycaemic control reduces DN progression and induces reversion of proteinuria and advanced histological lesions28–32. In a 30-year follow-up study, proteinuria, GF and HTN showed an improvement in patients with type 1 diabetes when there was better glycaemic control28. With a higher control of hyperglycaemia, GBM has shown less thickening29. Histological changes of advanced DN reverted 10 years after pancreas transplantation30. Haraguchi et al. were able to revert nephrotic-range proteinuria and histological lesions compatible with advanced DN after five years of intensive treatment of hyperglycaemia31. Treatment with bariatric surgeries administered to patients with type 2 diabetes and obesity improved GF and proteinuria, which was related to weight loss and decreased hyperglycaemia32.
Hyperglycaemia increases renin and angiotensinogen expression in mesangial cells20. Mesangial cells and podocytes synthesise angiotensin II and express angiotensin receptors19,20. The increase in angiotensin II stimulates the expression of TGF-Beta, VEGF-A, connective tissue growth factor (CTGF), interleukin 6 and chemoattractant protein for monocytes-1 inducing expansion of the ECM and podocyte apoptosis7,15,19,20.
In addition, glucose increases TGF-Beta expression in mesangial cells and podocytes19. Active TGF-Beta induces GBM thickening and glomerulosclerosis through the VEGF and CTGF; the increase in VEGF-A inhibits TGF-Beta expression, in a negative feedback mechanism15,19,20. In contrast, the increase in VEGF-A in diabetes is associated with elevated TGF-Beta and CTGF, proliferation and build-up of proteins in the glomerular ECM15,19,20. TGF-Beta has been related to the proliferation of mesangial cells, diffuse nodular glomerulosclerosis and also fibrosis15,19,20. In transgenic mice with no TGF-Beta type 2 receptor and the administration of anti-TGF-Beta antibodies prevented mesangial build-up and kidney function impairment19,20. These antibodies represent a therapeutic hope for DN, but they are not available for human use yet19.
Glomerular VEGF-A modifications in DNStarting from early DN stages, systemic and renal VEGF-A are elevated in humans and mice, VEGF-A has been associated with neoangigenesis15,21,22. RAAS, VEGF-A and nephrinuria were seen to be involved in this process15,19,20,22,33–38. Cultured podocytes and endothelial cells increased VEGF-A and VEGFR2 expression in response to the increase in glucose39–41. We showed that glomerular VEGF is a key factor for DN development and progress33,34. Normoglycaemic mice with VEGF overexpression in podocytes developed glomerulomegaly, hyperfiltration, GBM thickening and podocyte lesion, which are changes similar to early DN33. In these transgenic mice, diabetes caused massive proteinuria, advanced nodular glomerulosclerosis and less nephrin expression34. Diabetic mice with no VEGF overexpression only showed mild diffuse glomerulosclerosis34. These experiments demoatrate that the increase in glomerular VEGF, irrespective of the diabetic environment, generates identical changes to the early DN and that increasing glomerular VEGF speeds up DN progress to more advanced stages. In the absence of diabetes, the urinary VEGF-A was reported to be a good marker of VEGF glomerular expression and it correlated with proteinuria33. Contrarily, in to the diabetes context, VEGF-A has not been observed to be a good marker of glomerular expression or DN severity. Urine and systemic VEGF-A levels were high in diabetic mice with and without glomerular VEGF overexpression34. Probably, within the diabetes context, urinary excretion of VEGF-A ref lects systemic levels, while hiding VEGF glomerular changes34. In short, these experiments suggest that glomerular VEGF-A is a determining factor in DN, that VEGF overexpression in podocytes is dangerous, and that glucose directly and indirectly stimulates the VEGF-A signalling cascade in podocytes. In diabetes, urinary and systemic VEGF-A did not correlate with either glomerular VEGF expression or with the severity of glomerular lesions, which brings into question the use of VEGF-A as a DN biomarker.
Glomerular VEGF-A reduction was shown to generate GFB lesions, proteinuria and kidney failure in animals and humans42,43. Transgenic mice with silencing of VEGF-A in podocytes showed AKF, alteration of the three GFB layers and reduced integrin expression43. Some patients treated with anti-VEGF-A antibodies showed proteinuria, endothelial lesions and thrombotic microangiopathy42. This evidence suggests that VEGF-A released by podocytes is important for the maintenance of the function and the glomerular structure in the adult kidney. Whether glomerular VEGF-A expression control improves DN has not yet been determined, but there is evidence that shows contradictory results. Administration of anti-VEGF antibodies improved DN in rodents44. In experiments conducted in mice, endostatin and tumstatin prevented the development of DN due to a decrease in VEGF-A and angiopoietin 236. In contrast, diabetic mice with gene deletion of VEGF-A in podocytes showed proteinuria and severe diffuse glomerulosclerosis associated with endothelial injury and apoptosis42.
The evidence described herein suggests that close monitoring of glomerular VEGF-A levels in diabetes is required in order to avoid adding new lesions or worsening DN. Monitoring glomerular VEGF-A expression within very close margins may have a therapeutic potential, but the optimal concentrations and the right moment to perform such manipulation have not yet been defined.
VEGF-A relationships with insulin receptors, nephrin and ROS in DNIn DN, glomeruli with different lesion degrees coexist; VEGF-A expression and its signalling cascade have been related to glomerular changes37. In biopsies of patients with DN, there has been evidence of a higher VEGF expression in the glomeruli with lesions due to diabetes than in intact glomeruli37. However, VEGF-bound receptor expression was seen to be elevated in glomeruli with mild lesions and decreased in glomeruli with moderate or severe compromise37. A similar behaviour was observed with phosphorylation of serine/threonine protein kinase, a protein located in the VEGF signalling cascade, which suggested that other factors would modulate VEGF/VEGFR activity37.
Podocytes express insulin receptors, whose activity depends on nephrin expression45,46. Insulin receptors are located in the SD, where podocytes express nephrin and VEGFR233,46. We have characterised the existing interaction between nephrin and VEGFR216. VEGF overexpression in podocytes was found to decrease nephrin expression and phosphorylation16,33. Hale et al. reported that insulin increases VEGF-A production in podocytes, both in humans and mice45. In transgenic mice, this VEGF-A increase was disrupted by insulin resistance, anticipating the development of podocyte lesions secondary to insulin resistance45. In patients with insulin resistance caused by diabetes and by other diseases, kidney alterations, such as hyperfiltration, proteinuria, modifications in FGB and mesangium were described47,48. Jointly, these findings suggest that VEGF, nephrin and insulin receptor may be related to DN and insulin resistance, thus constituting glomerular pathways susceptible to being modified.
Furthermore, oxidative stress secondary to hyperglycaemia may modify glycocalyx, increase ROS and advanced glycation end products, and alter the endothelium. In addition, protein kinase C (PKC) glomerular activation was associated with mesangial expansion, GBM thickening, endothelial dysfunction, cytokine and TGF-Beta activation7,15,21,40,41. Mima et al. described that hyperglycaemia alters nephrin phosphorylation in diabetic rats and cultured podocytes exposed to high concentrations of glucose49. Nephrin phosphorylation interruption was attributed to a “glomerular VEGF resistance” status related to PKC activation49. The VEGF signalling cascade in podocytes and endothelial cells was selectively inhibited by hyperglycaemia49. The increase in glucose and diabetes would cause higher podocyte apoptosis and endothelial dysfunction, partly due to a higher activation of mitogen-activated protein kinase (PKCδ/p38) and SRc homology-2-domain-containing phosphatase-1 (SHP-1) overexpression49. In addition, SHP-1 negatively regulates VEGFR2 and the insulin receptor49.
Warren et al. showed that hyperglycaemia reduces endothelial VEGFR2 activity in diabetes41. ROS generation caused by hyperglycaemia was observed to induce VEGFR2 activation and its subsequent breakdown, notwithstanding the VEGF-A41. This would alter the normal response of endothelial cells to circulating VEGF-A due to lower receptor availability. By blocking ROS production with antioxidants, VEGFR2 availability and the lack of endothelial response to VEGF-A caused by hyperglycaemia were reverted41. These results suggest that the increase in VEGF-A present from early stages of DN may be secondary to “VEGF-resistance” of the VEGFR2 caused by higher receptor breakdown in endothelial cells.
Jointly, these publications indicate that, in DN, VEGF overexpression in podocytes may be stimulated in an autocrine and paracrine way by a “VEGF-resistance” state. VEGF-A connections with oxidative stress at glomerular level may represent pathways with therapeutic potential.
Relationship between angiopoietins and VEGF-A in DNAngiopoietins, which are growth factors involved in angiogenesis, have been related to DN15,36. Plasma levels of angiopoietin 2 are high in diabetic humans and mice, thus altering the angiopoetin-1/angiopoetin-2 ratio. Diabetic mice with lower angiopoietin 1 levels showed aberrant angiogenesis, hyperfiltration, glomerulomegaly and albuminuria, accompanied by VEGF-A and phosphorylated VEGFR2 overexpression. Alterations caused by reduced angiopoietin 1 were seen to be partially prevented by restoring its expression in podocytes of transgenic mice36. These experiments show the importance of angiopoietins and their relationship with VEGF-A in DN pathophysiogenesis. Modification of protein expression at the glomerular level (by manipulating the cells that produce these proteins) is a therapeutic alternative36.
Relationship between VEGF-A and nitric oxide in DNVEGF-A stimulates NO production by means of endothelial NO synthase (eNOS) activation15,35,50. The effects of VEGF-A on vasodilation and on the vascular permeability increase are mediated by the increase in eNOS-dependent NO15,27,35,50. Under normal conditions, VEGF-A induces eNOS activation and an increase in NO; this increase negatively regulates VEGF-A and CTGF, inhibiting ECM build-up15. In diabetes, this relationship changes: the increase in VEGF-A coexists with lower eNOS activity, and there is VEGF-A and NO decoupling50. Along the lines of this theory, eNOS KO diabetic mice increased VEGF-A expression and developed severe DN50. We showed that VEGF overexpression in podocytes of eNOS KO mice, induced indistinguishable changes of the advanced DN35. In the absence of diabetes, these transgenic mice developed proteinuria, kidney failure and nodular glomerulosclerosis35. This evidence suggests that alterations in glomerular VEGF-A/NO-eNOS relationship are critical and very dangerous, highlighting these events and their relationship with VEGF-A as treatment targets at the glomerular level.
Endothelial NO deficiency secondary to reduced eNOS activity may also associate insulin resistance mechanisms with endothelial dysfunction47,48. Endothelial cells express insulin receptors. By means of eNOS activation, these receptors control vascular tone by inducing vasodilation. For example, in patients with diabetes there are alterations in eNOS activation, establishing a relationship between NO and endothelial insulin resistance47–49. These findings suggest that VEGF-A and the glomerular NO/eNOS ratio may be implied in the insulin resistance status associated with prediabetes, diabetes and CKD.
ConclusionsPopulation studies reveal an increasing prevalence of type 2 diabetes worldwide, which suggests that DN will become an even more serious problem. It is imperative to look for alternatives for the diagnosis, prevention and treatment of DN. Going further in the study of molecular pathways with therapeutic potential, such as angiogenic factors, the glomerular VEGF resistance status, insulin resistance in podocytes, the VEGFR2/nephrin relationship, VEGF/insulin receptors/nephrin relationship, and the VEGF/NO-eNOS relationship, may provide solutions to the urgent problem of DN in the world.
Key concepts- 1.
Diabetes mellitus and CKD prevalence have increased in recent decades. The most frequent isolated cause of CKD is DN. Factors related to DN development are: age over 65, uncontrolled hyperglycaemia, hypertension, dyslipidaemia, male gender, smoking habit, family history, and Hispanic or Afro-American origin.
- 2.
Glucose directly and indirectly stimulates VEGF-A cell expression. In DN, there is a systemic and glomerular increase in VEGF-A, but glomerular VEGF-A and the glomerular VEGF-A/NO-eNOS relationship are key factors in DN pathophysiogenesis.
- 3.
Endothelial cells and podocytes express insulin receptors. Nephrin is essential for the action of the insulin receptor in podocytes; its activation is related to VEGF-A. VEGFR2 and nephrin interact in podocytes. Insulin receptors, nephrin and VEGF-A receptors may be mechanistically related to DN and insulin resistance.
- 4.
In DN, VEGF overexpression in podocytes may be stimulated in an autocrine and paracrine way by a “VEGF-resistance” status, in which PKC and ROS would be involved. VEGF-A connections to oxidative stress at the glomerular level may represent pathways with a therapeutic potential for DN.
- 5.
Angiogenic factors, such as VEGF-A and angiopoietins, the relationship of VEGF receptor 2/nephrin, VEGF/insulin receptors/nephrin and the relationship of VEGF/NO-eNOS, VEGF-A/insulin receptors/nephrin, and VEGF/NO-eNOS, represent glomerular pathways that have a crucial significance and may be potential treatment targets for DN.
We would like to thank Maiten Fernández Verón, Facultad de Arquitectura, Diseño y Urbanismo [School of Architecture, Design and Urbanism], Universidad de Buenos Aires [University of Buenos Aires], Argentina for collaborating in the design of the Figures. We would also like to thank Eco. Patricio Álvarez, UNEMI (Universidad Estatal de Milagro [State University of Milagro]) for helping in the publication processes and Gonzalo Fernández Verón for the language review.


![– In Ecuador, mortality caused by diabetes mellitus was higher in the provinces of Guayas, Los Ríos and Manabí, located on the Pacific coast. Map shows the mortality rate due to diabetes mellitus (deaths/100,000 individuals per year, INEC [Instituto Nacional de Estadísticas y Censos National Institute of Statistics and Census of Ecuador] 2011). This figure is part of a figure originally published by Neira-Mosquera et al.6, with minor modifications (authorised reproduction). – In Ecuador, mortality caused by diabetes mellitus was higher in the provinces of Guayas, Los Ríos and Manabí, located on the Pacific coast. Map shows the mortality rate due to diabetes mellitus (deaths/100,000 individuals per year, INEC [Instituto Nacional de Estadísticas y Censos National Institute of Statistics and Census of Ecuador] 2011). This figure is part of a figure originally published by Neira-Mosquera et al.6, with minor modifications (authorised reproduction).](https://static.elsevier.es/multimedia/20132514/0000003500000002/v1_201509100053/S2013251415000048/v1_201509100053/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)