To the Editor:
Acute renal failure (ARF) is a devastating disease with high incidence and no specific curative treatment. Although dialysis therapies are the cornerstone of contemporary treatment, there is no compelling evidence that they reverse established renal damage or prevent the development of chronic renal failure. Currently, the incidence of end-stage kidney disease secondary to ARF is almost 25%, similar to diabetic and hypertensive nephropathy.1
SECONDARY PREVENTION OF ACUTE RENAL FAILURE
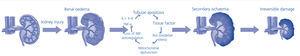
The treatment of ARF is defined as preventive when introduced before a potentially harmful event, or interventional when the injury has occurred and has already induced kidney damage. Primary prevention refers to the identification of subjects at risk. Interventions carried out between the identification of a kidney injury and the development of an established AKI are known as secondary prevention (Figure 1). Thus, secondary prevention is an early form of intervention that decreases the damage or prevents its progression.
In severe cases, the increase in vascular permeability to proteins and the net increase in transcapillary hydrostatic pressure favour the development of oedema, which is a major pathogenic mechanism in the development of multiorgan dysfunction in patients with sepsis. Theoretically, tissue oedema and an increase in renal hydrostatic pressure might directly affect renal function. A marked reduction in corticomedullary blood flow significantly decreases the glomerular filtration rate during the early stages of ARF. Hypoperfusion of the outer medulla is common in many forms of ARF and it can contribute to tubular ischaemia. Attempts have been made to use vasodilators to restore blood flow and stabilise renal function; however, these attempts have been unsuccessful.2,3 Therapeutic manoeuvers capable of increasing renal perfusion might reduce medullary ischaemia and slow down the progression towards dialysis-dependent ARF.4
CAN ACUTE RENAL FAILURE CONSTITUTE A RENAL COMPARTMENT SYNDROME?
Compartment syndrome is the final result of a process that begins with the persistent increase in pressure within a tissue or parenchyma to such an extent that it is capable of altering the regional vascular inflow, which may culminate in local organ failure, and if the damage persists, multiple organ failure may occur, resulting in the death of the patient. This is true for any body compartment surrounded by a rigid or semi-rigid structure such as in intracranial hypertension syndrome or abdominal compartment syndrome. From the theoretical point of view, the “renal compartment”, whose content and structure are the parenchyma and renal capsule, respectively, should not be different from other body compartments, as it is subject to the same physical laws. Viewed from a hydraulic perspective, sudden oedema that occurs in an acutely injured kidney could increase intrarenal pressure exponentially, reducing vascular inflow and inducing ischaemia in the outer medulla, the renal parenchymal area that consumes the most oxygen in relation to mass, a phenomenon that is widely known in ARF.5 This could accelerate the progression of acute kidney injury and even contribute to the total or partial irreversibility of damage (Figure 2).
Renal decapsulation: BACKGROUND IN THE LAST THREE DECADES
Decapsulation of acutely injured kidney was reported between 1901 and 1944 in 2307 patients; a recovery of renal function was achieved in almost 70% of patients, with a poor response being reported in late interventions, i.e. after parenchymal necrosis established itself.6,7 Following the progressive improvement of dialysis therapies, renal decapsulation was abandoned, but its interest was renewed with experimental studies in the last three decades. These studies reported a faster recovery of ischaemic kidney function and an improvement in intrarenal blood flow redistribution.8-10 Thus, the relative rigidity of the renal capsule and increased renal compliance after decapsulation imply the possibility of compartment pressure developing during renal oedema and decapsulation releasing this pressure, which reinforces the suspicion of a hydraulic component during ARF.
Based on the background set out herein, the application of renal decapsulation, after development of acute kidney injury, could have beneficial hydraulic effects and improve renal perfusion pressure by reducing intrarenal pressure and thus decreasing outer medullary ischaemia and preventing progression towards an irreversible “renal compartment syndrome”, a hypothesis which has not been studied in depth to date.
This becomes more important if we consider the usual approach to the renal parenchyma when this organ is obtained for renal transplantation; decapsulation would not increase the difficulty of this process. However, it is imperative to deepen our understanding of the benefits, risks and potential indications of this intervention.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Potential therapeutic options in accordance with the progression of an acute kidney damage
Figure 2. Hypothetical progression sequence of an acute kidney damage towards irreversible damage.