Background and objective: C.E.R.A. (continuous erythropoietin receptor activator, pegilated-rHuEPO ß) corrects and maintains stable hemoglobin levels in once-monthly administration in chronic kidney disease (CKD) patients. The aim of this study was to evaluate the management of anemia with C.E.R.A. in CKD patients not on dialysis in the clinical setting. Methods: Two hundred seventy two anemic CKD patients not on dialysis treated with C.E.R.A. were included in this retrospective, observational, multicentric study during 2010. Demographical characteristics, analytical parameters concerning anemia, treatment data and iron status were recorded. Results: C.E.R.A. achieved a good control of anemia in both naïve patients (mean Hemoglobin 11.6g/dL) and patients converted from a previous ESA (mean Hemoglobin 11.7g/dL). Most naïve patients received C.E.R.A. once monthly during the correction phase and required a low monthly dose (median dose 75µg/month). The same median dose was required in patients converted from a previous ESA, and it was lower than recommended in the Summary of Product Characteristics (SPC). Iron status was adequate in 75% of anemic CKD patients, but only 50% of anemic patients with iron deficiency received iron supplementation. Conclusions: C.E.R.A. corrects and maintains stable hemoglobin levels in anemic CKD patients not on dialysis, requiring conversion doses lower than those recommended by the SPC, and achieving target hemoglobin levels with once-monthly dosing frequency both in naïve and converted patients.
Introducción y objetivo: C.E.R.A. (activador continuo del receptor de la eritropoyetina) administrado una vez al mes corrige y mantiene estables los niveles de hemoglobina en pacientes con enfermedad renal crónica (ERC). Este estudio fue diseñado para evaluar el manejo de la anemia con C.E.R.A. en pacientes con ERC no en diálisis (ERC-ND) en la práctica clínica. Métodos: Estudio observacional, retrospectivo y multicéntrico, llevado a cabo en pacientes con anemia asociada a ERC-ND en tratamiento con C.E.R.A. durante 2010. Se recogieron datos demográficos, parámetros de laboratorio relacionados con la anemia, datos del tratamiento y estado del hierro. Resultados: El tratamiento con C.E.R.A. logró un buen control de la anemia tanto en los pacientes naïve (hemoglobina media 11,6 g/dl) como en aquellos en conversión desde otros agentes estimulantes de la eritropoyesis (hemoglobina media 11,7 g/dl). La mayoría de los pacientes naïve recibieron C.E.R.A. una vez al mes durante el período de corrección y requirieron una dosis mensual baja (dosis mediana 75 µg/mes). Las dosis de C.E.R.A. utilizadas en pacientes en conversión fueron inferiores a las dosis de conversión recomendadas en la ficha técnica. El estatus del hierro fue adecuado en el 75 % de los pacientes con anemia asociada a ERC-ND, y únicamente el 50 % de aquellos con deficiencia de hierro recibieron suplementos de hierro. Conclusiones: En pacientes con anemia asociada a ERC-ND, naïve o en conversión, la administración mensual de C.E.R.A. corrige y mantiene estables los niveles de hemoglobina, incluso en dosis inferiores a las recomendadas en la ficha técnica.
INTRODUCTION
Anemia is a frequent complication of chronic kidney disease (CKD), mainly related to erythropoietin deficiency. Erythropoiesis stimulating agents (ESA) therapy increases hemoglobin levels, reduces the need of blood transfusions, improves quality of life (QoL) and regresses left ventricular hypertrophy (LVH).1,2
Despite the efficacy of ESA in the management of renal anemia, there are several issues not fully solved, such as the appropriate target of hemoglobin level. The European Renal Best Practice guidelines in 2009 recommended a target range of hemoglobin between 11-12g/dL in CKD patients, but individualized according to patients’ age and co-morbidities.3 However, the publication of the CHOIR,4 CREATE5 and TREAT6 studies raised concerns about the safety of targeting to higher hemoglobin levels in CKD patients. In fact, recent meta-analyses have shown that full anemia correction with ESA is associated with an increased risk of hypertension, stroke or vascular access clotting,7 with minor improvements in QoL,8 and no beneficial effects on LVH,9 as compared to partial anemia correction. Several guidelines recommend nowadays a target hemoglobin level of 11-12g/dL with ESA therapy,10,11 whereas the recent KDIGO guidelines advocate for a more restrictive use of ESA and lower hemoglobin targets.12 The aim of anemia treatment in CKD patients is to partially correct and maintain stable hemoglobin levels and within the target range most of the time, avoiding hemoglobin fluctuations, and achieving the target with the lowest ESA dose possible. To reach these objectives, some treatment considerations (frequent anemia monitoring, iron supplementation and avoiding frequent ESA dose changes) are required.
Despite the similar efficacy and safety of all available ESA, some pharmacokinetic and pharmacodynamic differences, interval and route of administration and hemoglobin stability should be considered while prescribing them to an individual patient.
C.E.R.A. is a continuous erythropoietin receptor activator created by integrating a large methoxy-polyethylene-glycol-polymer chain into the erythropoietin molecule,13,14 providing new properties to the EPO molecule (faster dissociation from the EPO receptor and a longer half-life).15 Since its authorization in Europe in 2007, several studies about C.E.R.A. use in the clinical practice in CKD patients not on dialysis have been published.16-19 The aim of our study was to evaluate the results of anemia treatment with C.E.R.A. in a population of CKD patients not on dialysis in the clinical setting.
METHODS
Study design and data collection
This study was a retrospective, multicentric, observational survey carried out in 19 outpatient Nephrology Units in Catalonia. Patients were recruited from different Nephrology Units in Catalonia during 2010. Data were collected in an electronic CRF. All patients signed an informed consent and the study was approved by the Ethic Committees of all participating Hospitals.
Selection of patients
The study population included adult anemic patients (≥18 years) with CKD stages 3, 4 and 5 (eGFR <60mL/min/1.73m2), not on dialysis and treated with C.E.R.A during at least 6 months, having biochemical and hematological data concerning anemia at the beginning of the treatment with C.E.R.A., and with hemoglobin values at 3 and 6 months after starting the treatment.
Non compliance with the selection criteria and a need of dialysis at the time of the study inclusion were the exclusion criteria.
Each investigator gathered data from medical records of all patients that had been on treatment with C.E.R.A. for at least 6 months and fulfilled the inclusion criteria.
Statistical analysis
For description of continuous variables, mean and standard deviation were used, while categorical variables were described using absolute and relative frequencies. For comparison of quantitative variables, parametric tests (Student t-test or ANOVA) or non parametric tests (Mann-Whitney or Kruskall-Wallis) tests were used, as appropriate. For comparison of categorical variables, the χ2 test was performed, with Fisher’s exact test being used when more than 20% of categories had an expected frequency <5. The level of statistical significance was established at p<0.05. All statistics were performed using the SAS 9.1.3 software.
RESULTS
Demographics and baseline characteristics
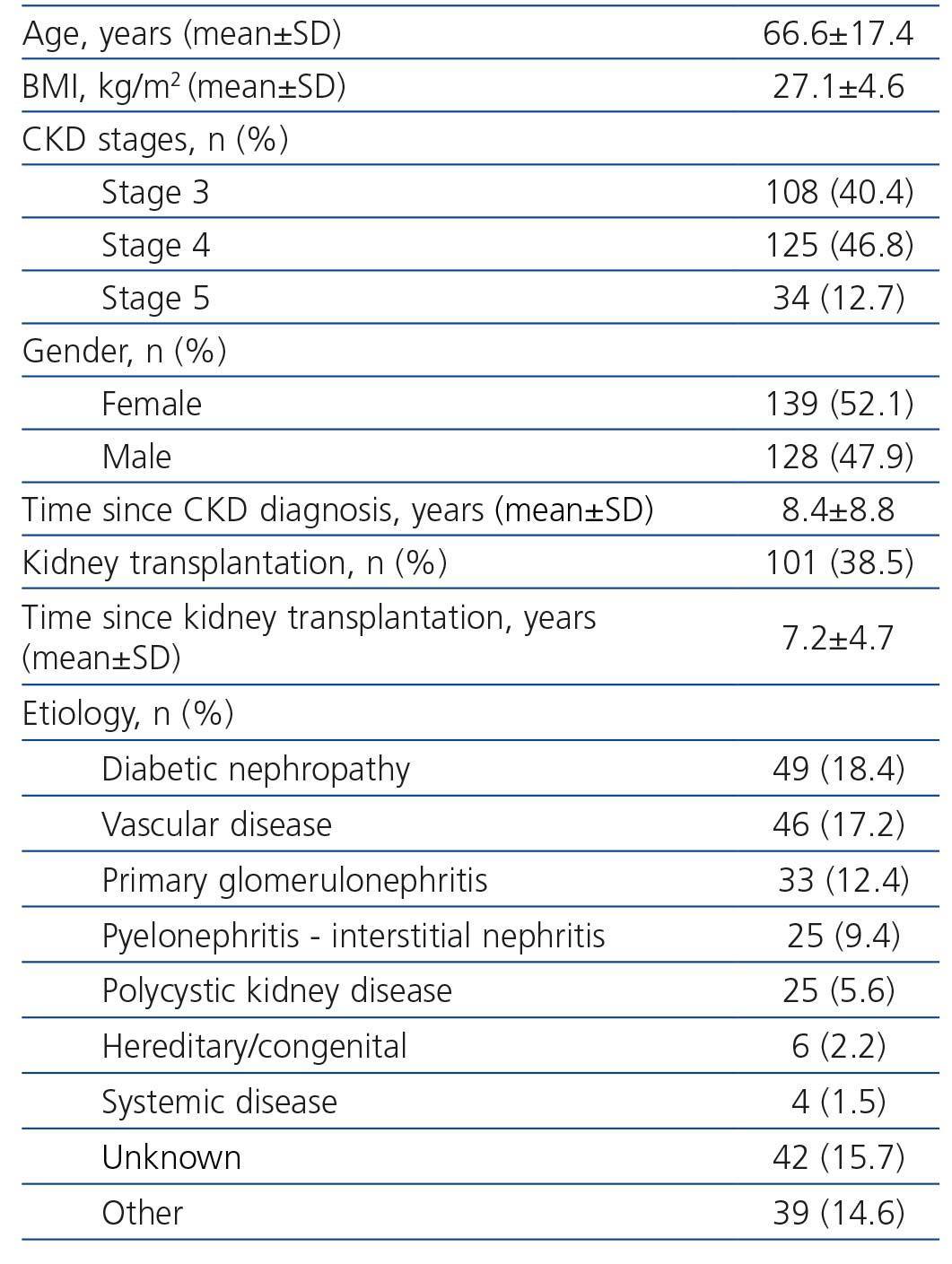
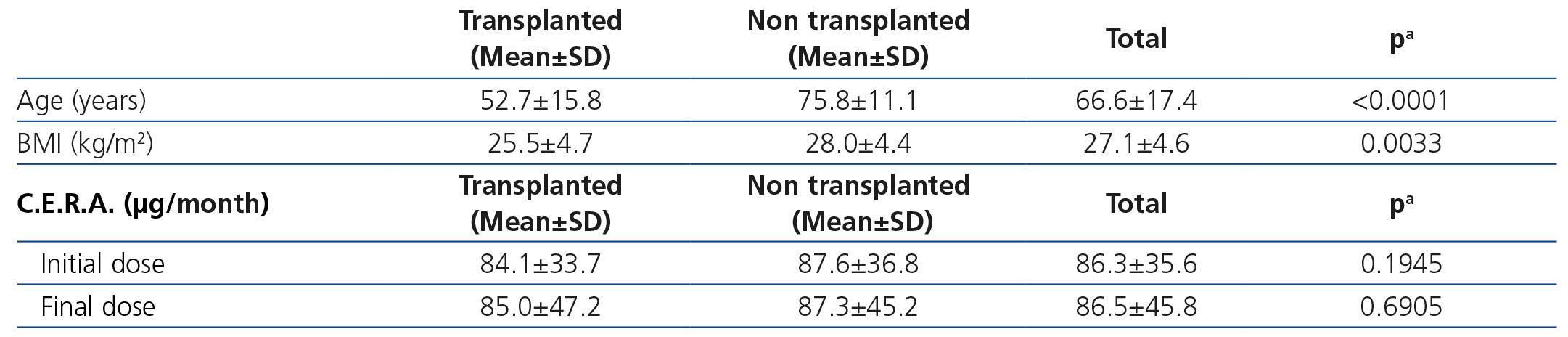
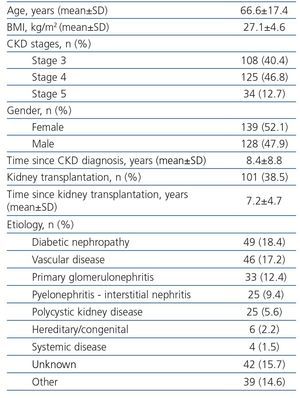
Initially, 331 patients were recruited, but 64 patients were excluded because of not meeting the inclusion criteria (different CKD stage, no anemia or no treated with C.E.R.A., no available data of hemoglobin at the beginning of the treatment with C.E.R.A., and in two follow-up visits at least). Finally, 267 patients were analyzed (Table 1), most of them were in CKD stages 3 and 4. There were no differences in gender across the CKD stages, but patients in CKD stage 4 were older. Diabetes and vascular disease were the most common causes of CKD; and 38.5% of patients had a functioning kidney graft. These patients being younger and presenting lower body mass index than non-transplant CKD patients (Table 2).
Mean time since CKD diagnosis was 8.4±8.9 years among non-transplanted CKD patients, and mean time since kidney transplantation was 7.1±4.7 years among kidney transplant recipients.
Anemia data
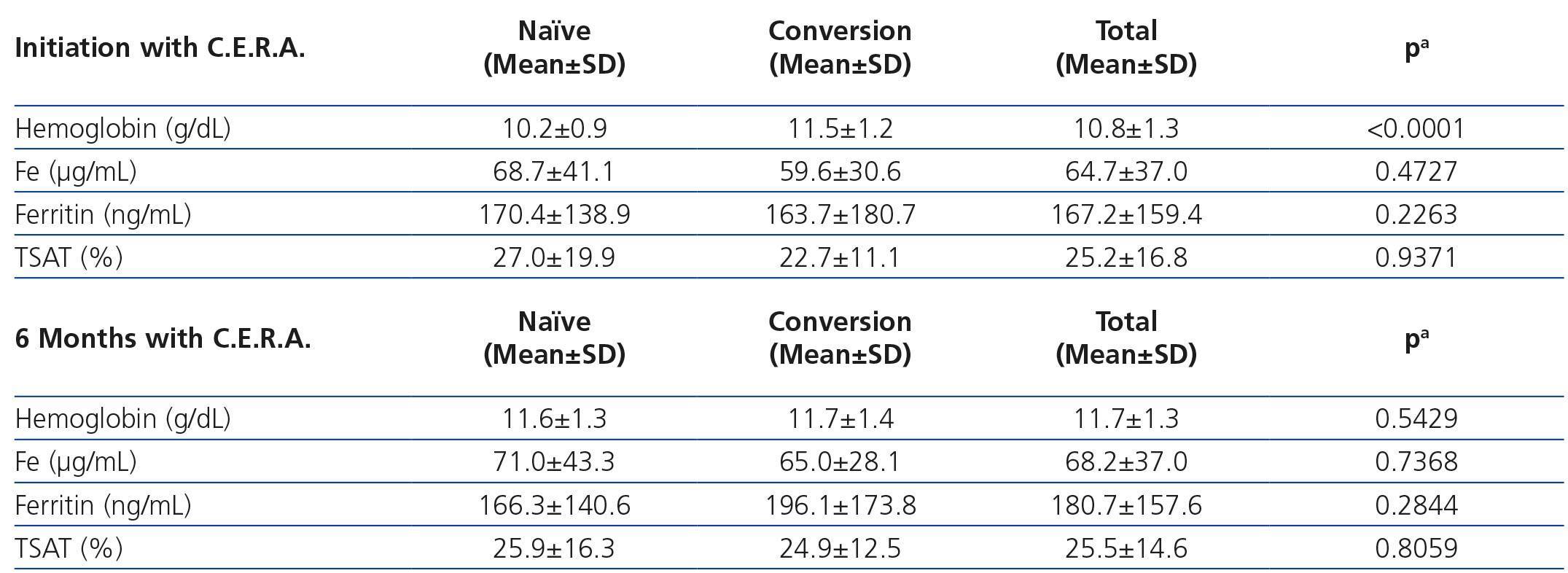
Mean time since anemia diagnosis was 4.3±4.1 years and mean time since the beginning of ESA treatment was 3±2.4 years. Mean hemoglobin levels before starting ESA treatment was 10.2±0.9g/dL. While 48.2% of the patients were naïve, 51.8% had been converted from a previous ESA: erythropoietin beta (n=85, mean monthly dose 16,694±11,180 IU) and darbepoetin alfa (n=50, mean monthly dose 90.5±56.1μg). (Table 3).
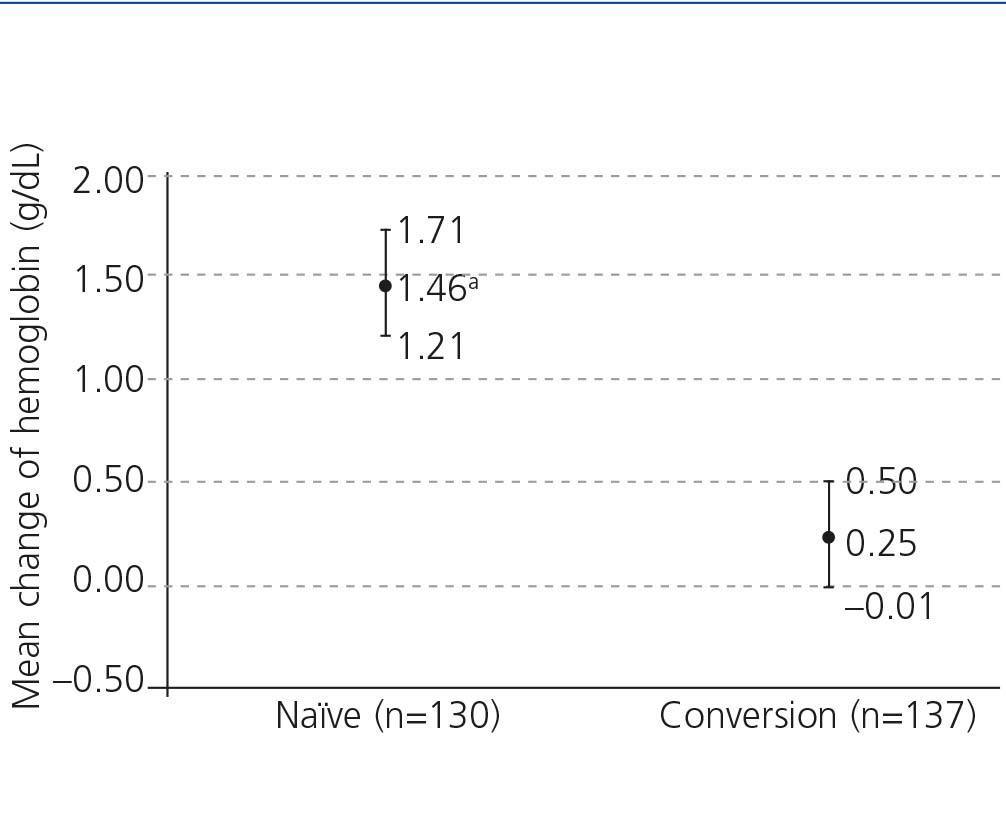
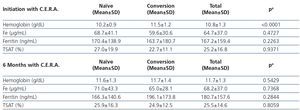
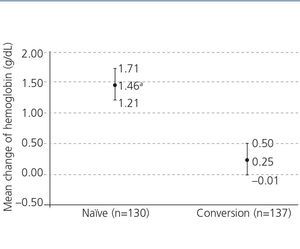
The analytical data concerning anemia during C.E.R.A. treatment are shown in Table 4. Naïve patients had lower hemoglobin levels before starting C.E.R.A. and hemoglobin increased significantly during treatment, while no significant changes in hemoglobin were observed in patients converted from a previous ESA (Figure 1). Iron status remained stable during the study.
Data concerning anemia treatment with C.E.R.A.
Most naïve patients (85.1%) started C.E.R.A. treatment in a once-monthly schedule, despite that at the time of recruitment the recommended dose interval for anemia correction was once every two weeks; while almost all converted patients (92.0%) received C.E.R.A. once monthly, as recommended.
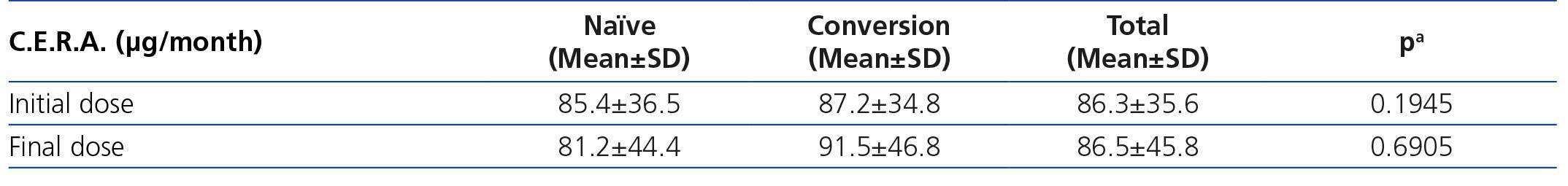
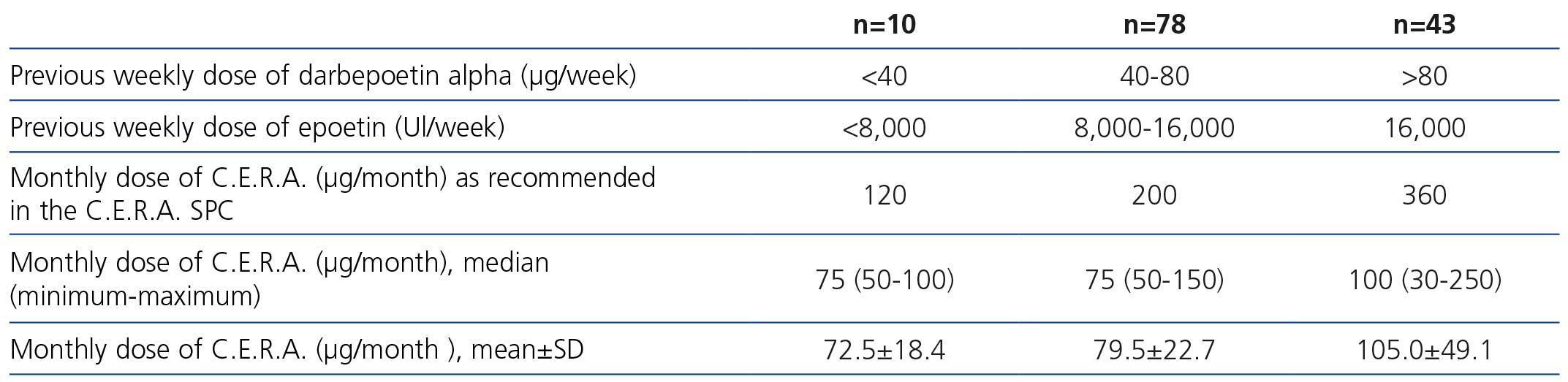
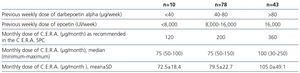
The most common dose of C.E.R.A. at the beginning and at the end of the study was 75μg/month, administered once monthly. The doses prescribed in converted patients were lower than the conversion doses recommended in the Summary of Product Characteristics (SPC) (Table 5).
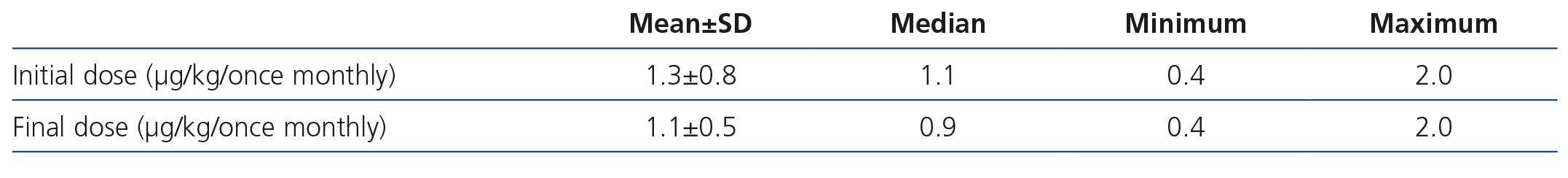
The initial mean monthly dose of C.E.R.A. in naïve patients corrected by weight was 1.3μg/kg/month, whereas the final dose of C.E.R.A. in these patients who achieved hemoglobin levels >11g/dL was 1.1μg/kg/month (Table 6).
Additional treatments for anemia at the start of the study included: iron supplementation (40.7% of patients), folate (12.6%) and vitamin B12 (7.4%). There were no significant changes in these percentages at the end of the study (38.5% vs 14% vs 7.3%, respectively). Iron deficiency was diagnosed in 25% of patients (as defined by a serum ferritin <100μg/L or transferrin saturation index [TSAT] <20%), and 45.6% of them did not receive iron supplementation.
DISCUSSION
This study demonstrates that C.E.R.A. administered once monthly is effective both in correcting and maintaining hemoglobin levels in CKD patients not on dialysis in the clinical setting. C.E.R.A. at a mean initial dose of 1.3μg/kg/month, similar to the starting dose recommended in the SPC, achieved hemoglobin targets in naïve patients. However, the dose required in patients converted from previous ESA was lower than the recommended in the SPC.
The mean hemoglobin achieved with C.E.R.A. in naïve (11.6g/dL) or converted patients (11.7g/dL) was remarkably stable during the observation period, and was maintained within the levels recommended by the European Renal Best Practice Guidelines of 2009 (11-12g/dL).11
Several randomized controlled trials in CKD patients have demonstrated the efficacy and safety of C.E.R.A. in correcting and maintaining appropriate and stable hemoglobin levels.20,21 However, most of these trials were performed in dialysis patients. Thus, the experience with C.E.R.A in CKD patients not on dialysis is more limited both from randomized controlled trials and in the clinical setting.
Mean hemoglobin levels in naïve patients significantly increased by 1.46g/dL from baseline, reaching mean hemoglobin levels of 11.6g/dL; whereas a minimal change of 0.25g/dL was observed in converted patients (from mean hemoglobin levels of 11.5g/dL to 11.7g/dL). The median dose of C.E.R.A. required to reach and maintain the target hemoglobin levels was 75µg/month, both in naïve and converted CKD patients not on dialysis. Doses that are remarkable similar to those reported in the study OASIS in the same population.22
Our results in naïve patients are similar to the results of the ARCTOS (dose interval once every 2 weeks),20 CORDATUS (once monthly)21 and SUPRA (once monthly) studies,17 and the dose required was similar to the recommended in the SPC, supporting its suitability also in the clinical setting. It should be noticed that in our study most CKD naïve patients started C.E.R.A. on a once-monthly basis, even before the results of the CORDATUS and SUPRA studies were available.17,21
Furthermore, in converted patients the dose of C.E.R.A. required was lower than the dose recommended in the SPC, in agreement with previous studies,16,18,22confirming that the C.E.R.A. dose required to maintain stable hemoglobin levels after conversion from shorter acting ESA in non-dialysis CKD patients is lower than 120μg/month.18 These results deserve further investigation, since the new guidelines recommend the use of the lowest ESA dose possible to reach the target hemoglobin levels, in order to minimize the risk of adverse events.11,12,23 Thus, this finding has potentially relevant clinical and economical implications. The OCEANE study also confirmed the efficacy and safety of C.E.R.A. in anaemia management in CKD patients not on dialysis, with or without a previous kidney transplant16. Contrarily to the OCEANE study, in our study the doses of C.E.R.A. required were similar in CKD patients not on dialysis and kidney transplant recipients, despite achieving similar hemoglobin levels and iron status.16 Yet, the doses in our kidney transplant recipients were similar to those prescribed in the Anemia Trans study in Spanish kidney transplant recipients.19
CKD is associated with reduced intestinal iron absorption and poor gastrointestinal tolerance to oral iron, thus frequently requiring iron supplementation.24 An adequate iron status in order to optimize ESA response is mandatory in anemic CKD patients, in order to optimize erythropoiesis and achieve an adequate response to ESA therapy.11,25 In iron-deficient patients, therapy with oral or intravenous iron reduces ESA dose requirements and increases the probability of maintaining hemoglobin levels within the desired target range.26 In our study, 75% of patients had an adequate iron status, but less than half of patients with iron deficiency received iron supplementation. Despite the recent advances in iron handling in CKD anemic patients, there is still room for improvement in anemia management in this population.
Finally, our study included CKD patients in the clinical setting, making our findings broadly applicable to usual management of non-dialysis patients.
We recognize the several limitations of this study (its retrospective and observational nature, no record of several parameters influencing hemoglobin level and ESA dose requirements, such as co-morbidities, inflammation, malnutrition, iPTH level, use of ACE inhibitors and angiotensin II receptor blockers).
Our main conclusion is an adequate management of anemia with C.E.R.A. in catalonian CKD patients not on dialysis, hemoglobin targets were achieved with doses lower than the recommended in the SPC in patients converted from shorter-acting ESA, and the once-monthly dosing schedule was appropriate both in naïve and converted patients.
Acknowledgements
The study was sponsored by the Societat Catalana de Nefrologia with an unrestricted grant from Roche Pharma in Spain.
Investigators of the MICENAS II study: The following investigators also participated in the present study: Torguet P (Hospital Universitari Josep Trueta, Girona, Spain), Lauzurica R (Hospital Germans Trias i Pujol, Badalona, Spain), García R (Hospital de Palamós, Girona, Spain), Llibre J (Hospital Mútua Terrassa, Barcelona, Spain), Fulladosa X (Hospital de Bellvitge, Barcelona, Spain), Cañas L (Hospital Germans Trias i Pujol, Badalona, Spain), Bayés B (Hospital Germans Trias i Pujol, Badalona, Spain).
Conflicts of interest
Alberto Martínez Castelao have received lecture fees from Abbvie, Amgen, Boëhringer_I, Novartis and Roche and have participated in advisory boards of Abbvie, Amgen and Roche.
Aleix Cases have received lecture fees from Amgen, Roche, Fresenius, Bristol Myers Squibb, Novartis, Pfizer, Almirall, Esteve, Siemens and Astra Zeneca and have participated in advisory boards for Amgen, Roche, Astra Zeneca, Abbvie, Novartis and Esteve.
The other authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographical and clinical characteristics of patients
Table 4. Hemoglobin and iron status parameters according to previous use of ESA or not
Table 3. Treatment with C.E.R.A. in naïve and converted patients
Table 2. Main characteristics of kidney transplant recipients vs. non-transplanted CKD patients
Table 5. Treatment with C.E.R.A. according to the Summary of Product Characteristics (SPC)
Table 6. Weight-adjusted dose of C.E.R.A. in naïve patients at the beginning and at the end of the study
Figure 1. Mean change in hemoglobin levels (hemoglobin at the end minus hemoglobin at the beginning) after 6 months of treatment according to patients&